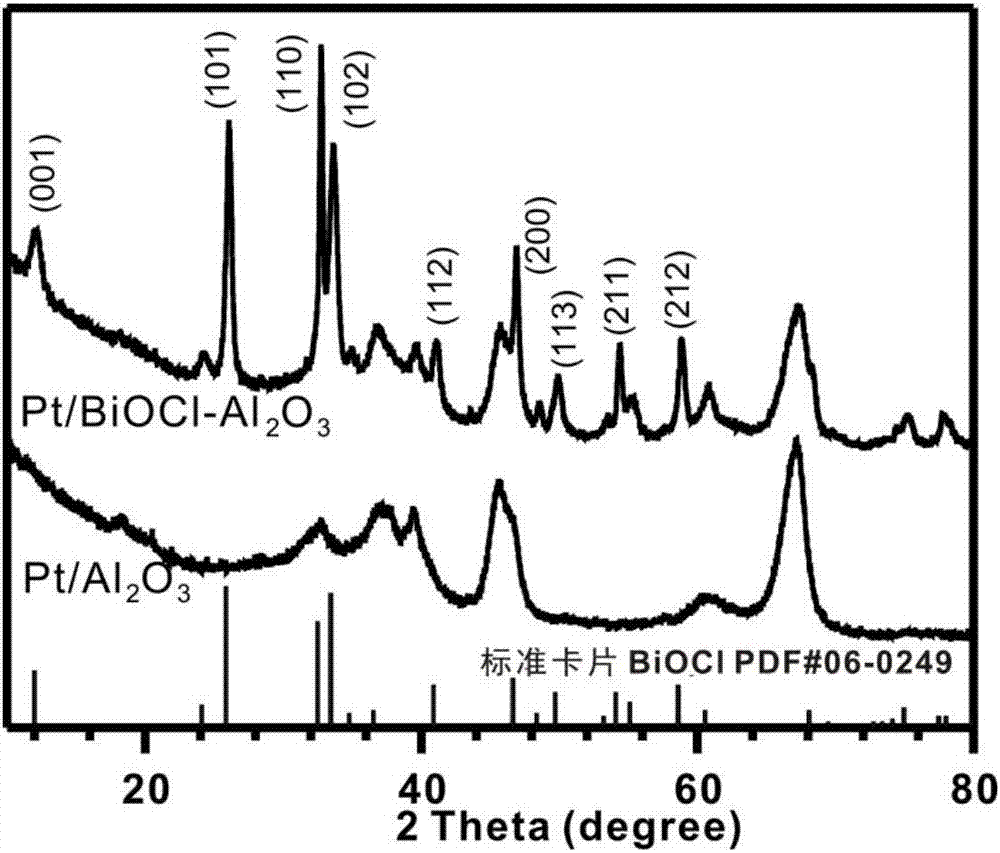
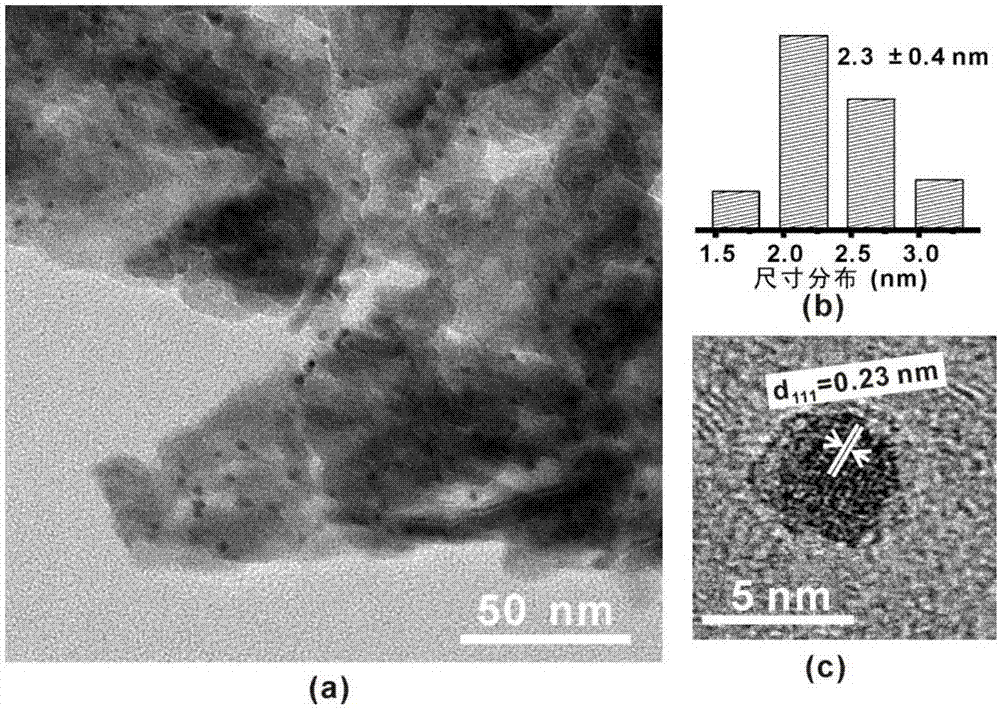
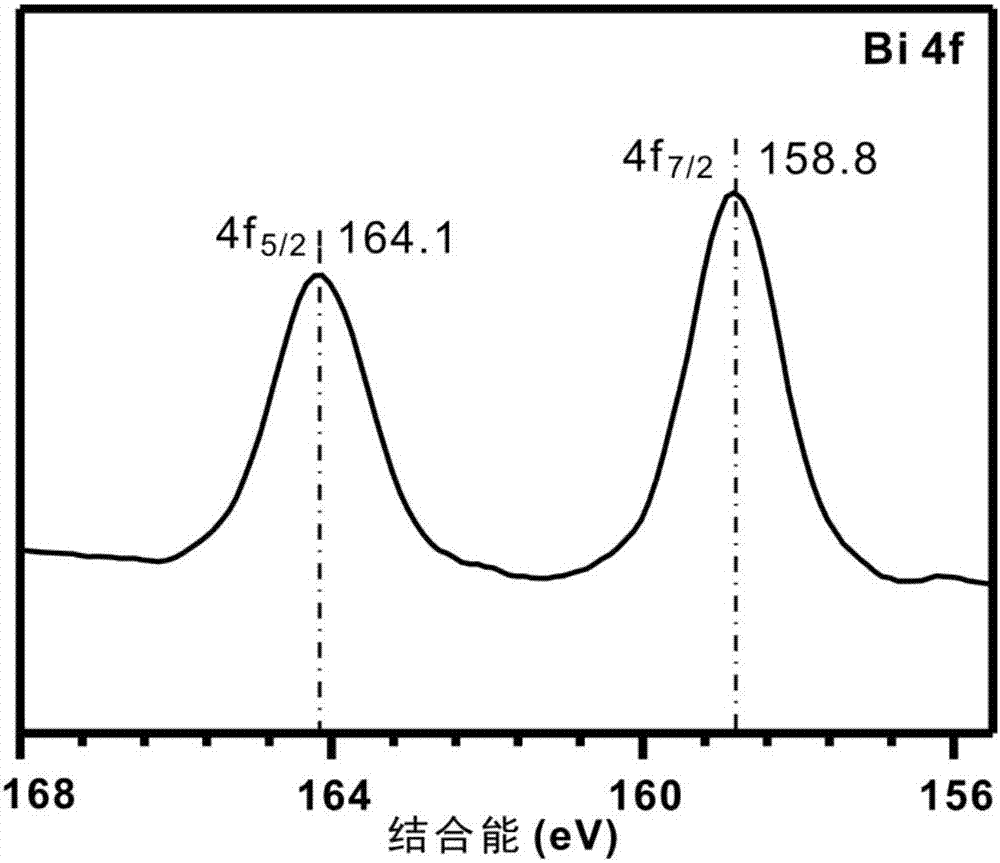
Method for preparing benzaldehyde by catalytically oxidizing phenylcarbinol through Pt/BiOCl-metal oxide serving as catalyst
A technology for catalytic oxidation and oxides, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, carbon-based compound preparation, etc., can solve problems such as low efficiency, and achieve high application prospects, reaction Mild conditions, selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Dissolve chloroplatinic acid hexahydrate in deionized water so that the concentration of the Pt element is 19.2 mM, and sonicate for 5 minutes until the Pt salt is completely dissolved to obtain a yellow transparent solution A;
[0043] (2) Dissolve bismuth nitrate in deionized water so that the mass volume ratio of bismuth element to solvent b is 0.025g:10mL, and ultrasonically dissolve the soluble bismuth salt completely for 5 minutes to obtain a colorless and transparent solution B;
[0044] (3) 0.5g of Al 2 o 3 Disperse into 10mL solution B, sonicate for 5min to make Al 2 o 3 Uniformly disperse to obtain suspension C;
[0045] (4) Under stirring conditions, add 1.66mL of A solution to C to obtain a mixture D, in which Pt and Al 2 o 3 The mass ratio is 1:100;
[0046] (5) Dissolve 30 mg of sodium borohydride in 6 mL of ice water, and sonicate for 5 minutes to form a colorless and transparent solution E;
[0047] (6) After stirring the above mixture D for 1...
Embodiment 2
[0051] (1) Dissolve chloroplatinic acid hexahydrate in deionized water so that the concentration of the Pt element is 19.2 mM, and sonicate for 5 minutes until the soluble Pt salt is completely dissolved to obtain a yellow transparent solution A;
[0052] (2) Dissolve bismuth nitrate in deionized water as a solvent, so that the mass volume ratio of bismuth element to solvent b is 0.025g:30mL, and ultrasonically dissolve the soluble bismuth salt completely for 5 minutes to obtain a colorless and transparent solution B;
[0053] (3) 0.5g of Al 2 o 3 Disperse into 30mL solution B, ultrasonic 5min to make Al 2 o 3 Uniformly disperse to obtain suspension C;
[0054] (4) Under stirring conditions, add 1.66mL of A solution to C to obtain a mixture D, in which Pt and Al 2 o 3 The mass ratio is 1:100;
[0055] (5) Dissolve 30 mg of sodium borohydride in 10 mL of ice water, and sonicate for 5 minutes to form a colorless and transparent solution E;
[0056] (6) After stirring the ...
Embodiment 3
[0060] (1) Dissolve chloroplatinic acid hexahydrate in deionized water so that the concentration of the Pt element is 19.2 mM, and sonicate for 5 minutes until the soluble Pt salt is completely dissolved to obtain a yellow transparent solution A;
[0061] (2) Dissolve bismuth nitrate in deionized water as a solvent, so that the mass volume ratio of bismuth element to solvent b is 0.025g:30mL, and ultrasonically dissolve the soluble bismuth salt completely for 5 minutes to obtain a colorless and transparent solution B;
[0062] (3) 0.5g of Al 2 o 3 Disperse into 30mL solution B, ultrasonic 5min to make Al 2 o 3 Uniformly disperse to obtain suspension C;
[0063] (4) Under stirring conditions, add 1.66mL of A solution to C to obtain a mixture D, in which Pt and Al 2 o 3 The mass ratio is 1:100;
[0064] (5) Dissolve 30 mg of sodium borohydride in 10 mL of ice water, and sonicate for 5 minutes to form a colorless and transparent solution E;
[0065] (6) After stirring the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com