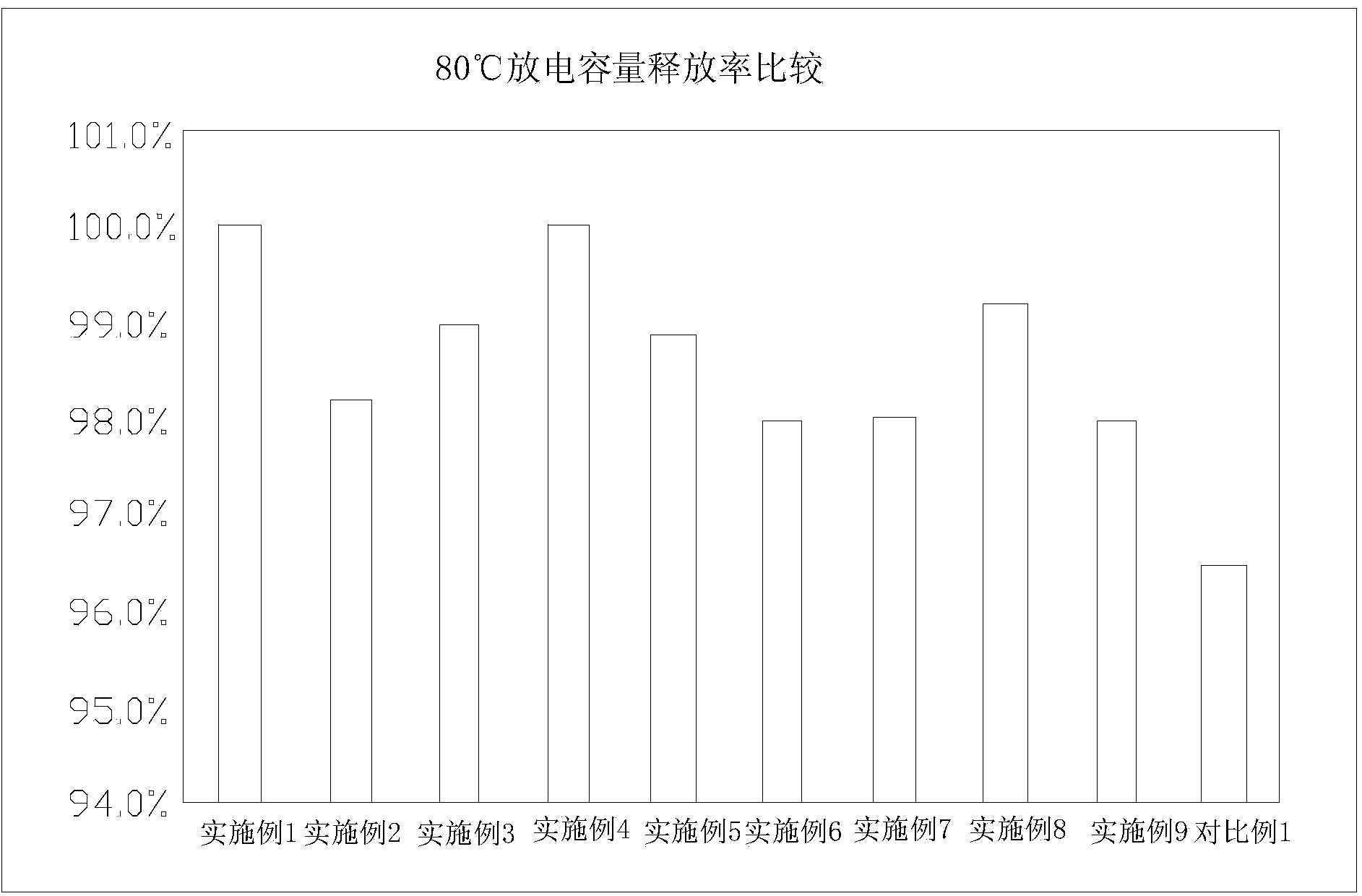
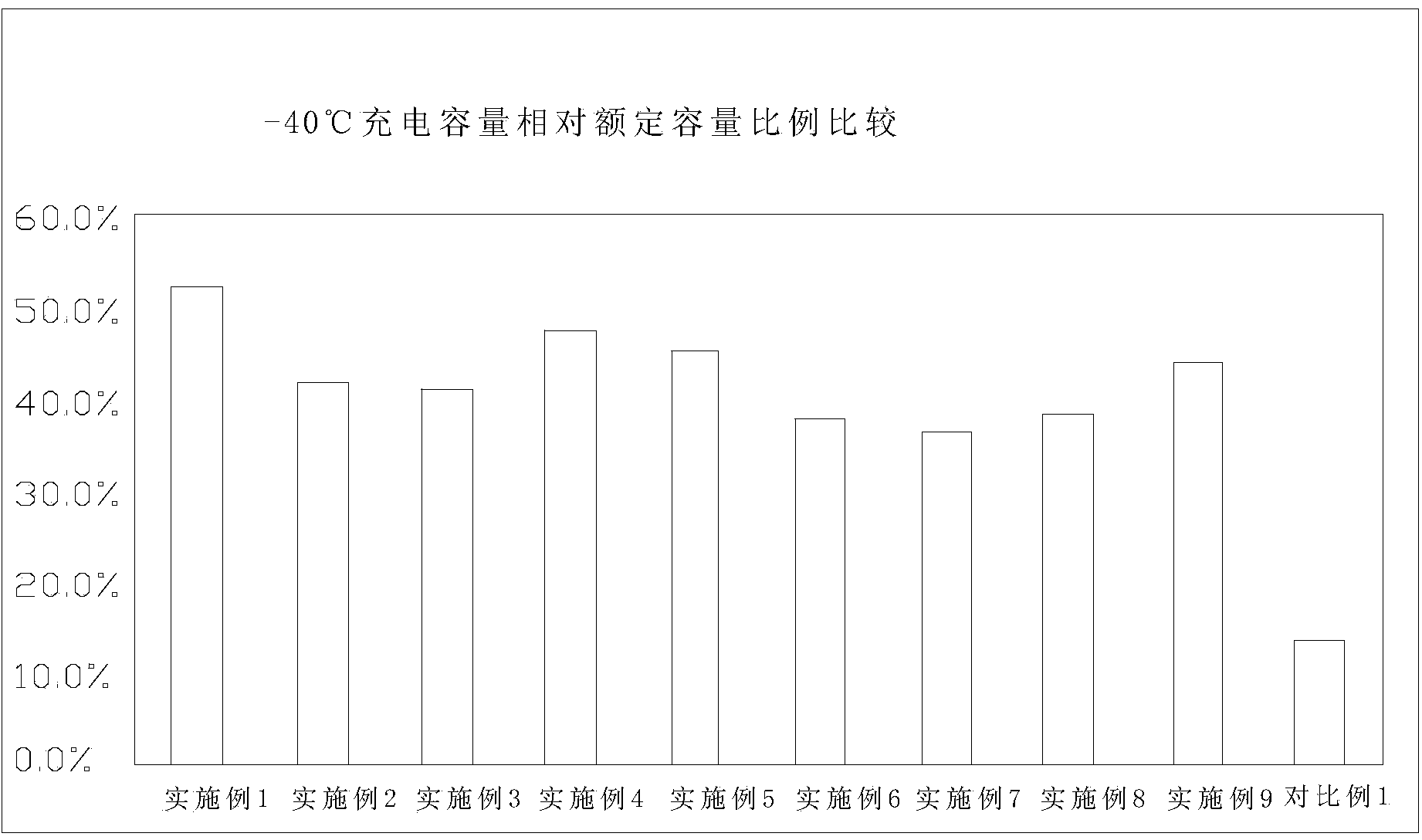
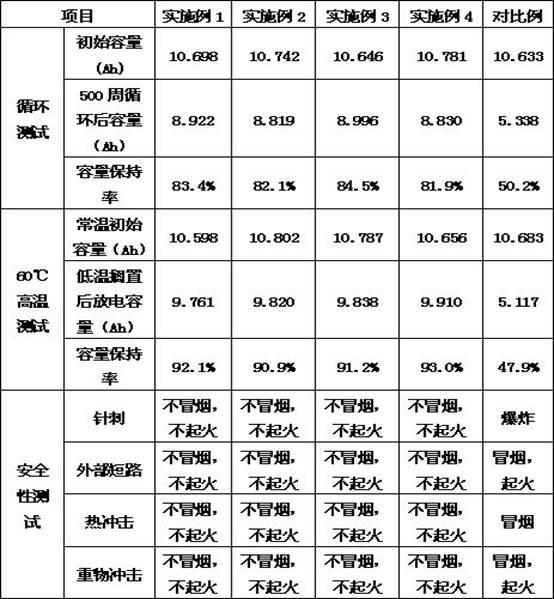
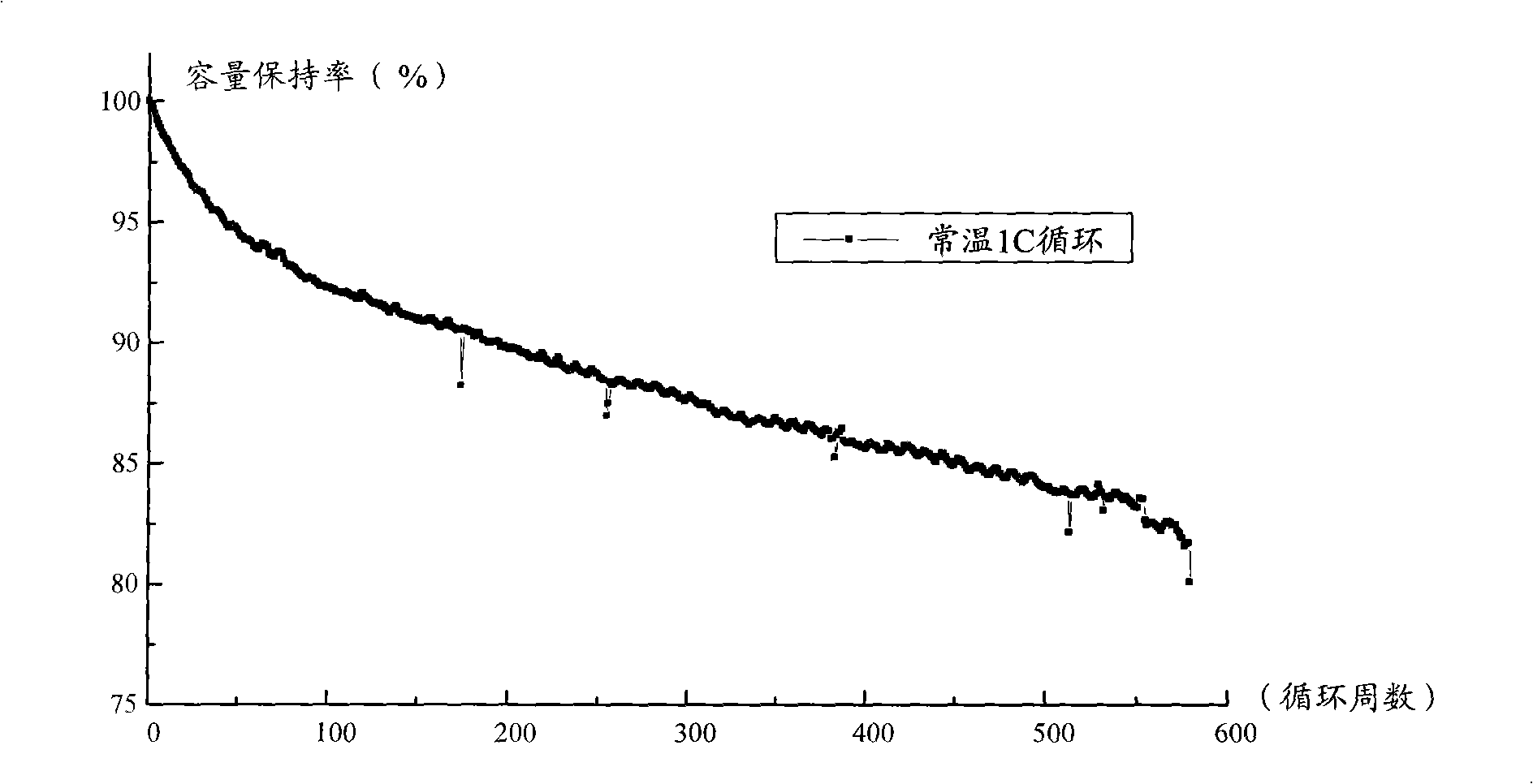
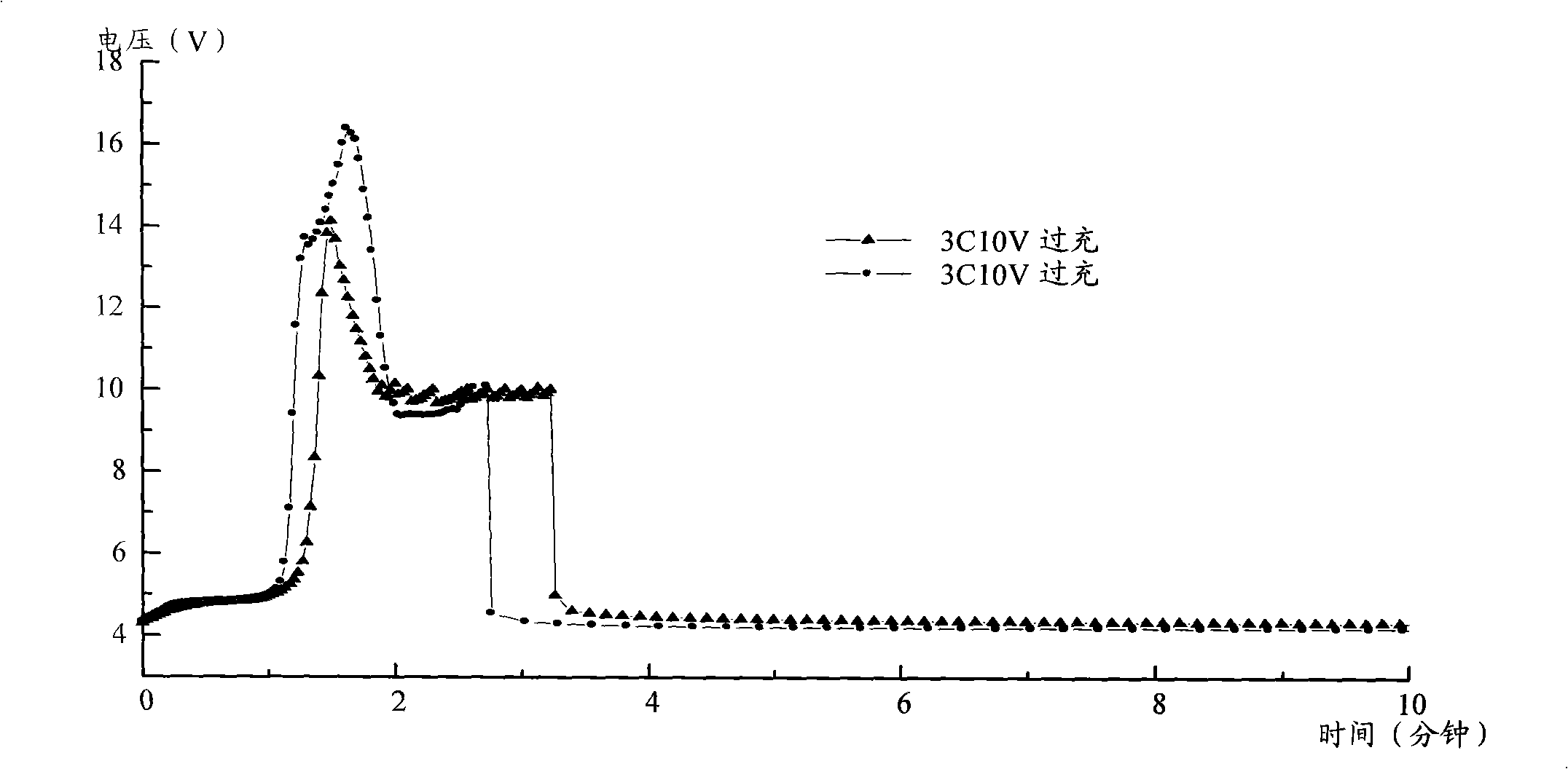
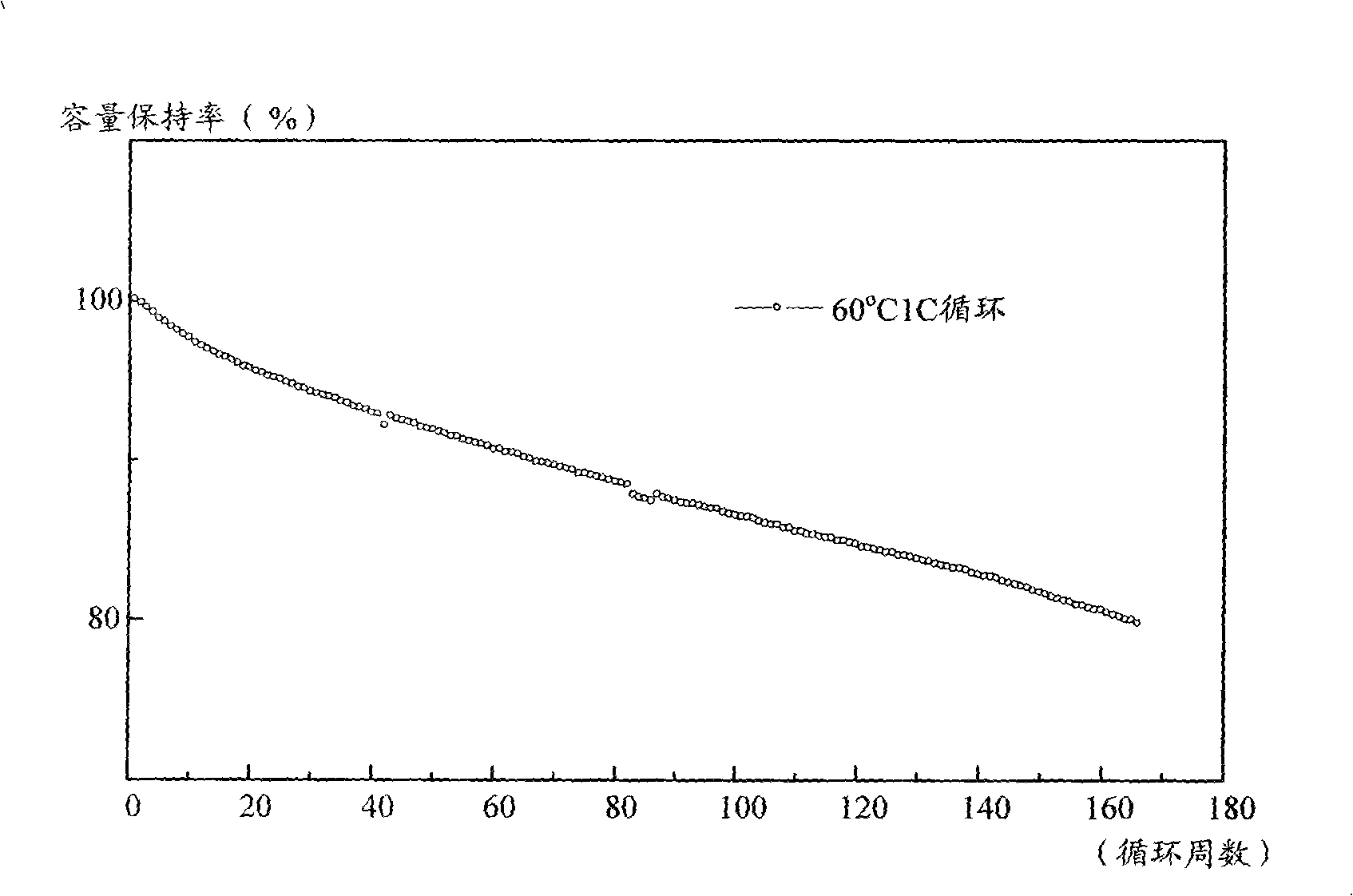
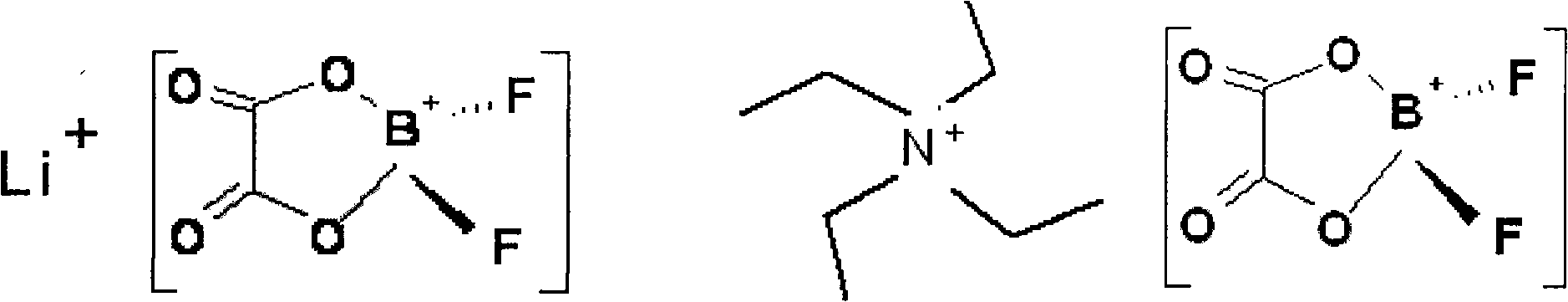
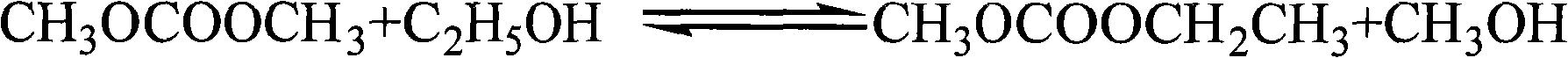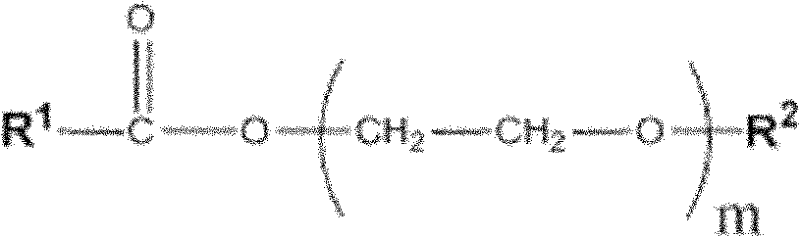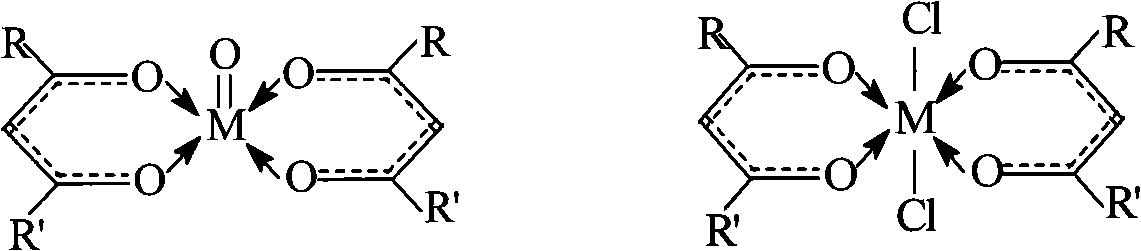Patents
Literature
244 results about "Ethyl carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH 2 O) 2 CO. ... Other components like diethyl carbonate, ethyl methyl carbonate, dimethyl carbonate and methyl acetate can be added to those electrolytes in order to decrease the viscosity and melting point.
Lithium ion battery with high voltage electrolytes and additives
ActiveUS20110136019A1Final product manufactureElectrode carriers/collectorsMethyl carbonateHigh pressure
Desirable electrolyte compositions are described that are suitable for high voltage lithium ion batteries with a rated charge voltage at least about 4.45 volts. The electrolyte compositions can comprise ethylene carbonate and solvent composition selected from the group consisting of dimethyl carbonate, methyl ethyl carbonate, γ-butyrolactone, γ-valerolactone or a combination thereof. The electrolyte can further comprise a stabilization additive. The electrolytes can be effectively used with lithium rich positive electrode active materials.
Owner:IONBLOX INC
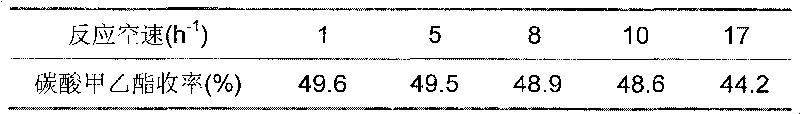
Process for preparing methyl ethyl carbonate by ester exchanging reaction
InactiveCN1900047AHigh yieldQuick responseMetal/metal-oxides/metal-hydroxide catalystsPreparation from organic carbonatesTransesterificationReaction temperature
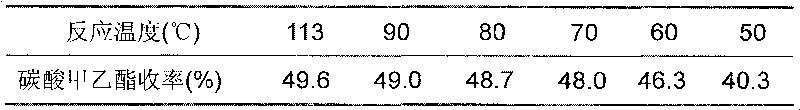
The transesterification process for preparing methyl ethyl carbonate features that the materials dimehtyl carbonate and ethanol in the molar ratio of 1-4 to 1 produce transesterification reaction at normal pressure and 50-110 deg.c in the presence of binary heterogeneous solid alkali catalyst for 1-8 hr to prepare methyl ethyl carbonate. The solid alkali catalyst has consumption of 5-25 % of ethanol weight and is prepared through soaking process or sol-gel process, and used catalyst may be separated easy from the reaction product for reuse. The transesterification reaction is completed in a reaction-rectification apparatus and has methyl ethyl carbonate yield up to 90 %.
Owner:ZHEJIANG UNIV
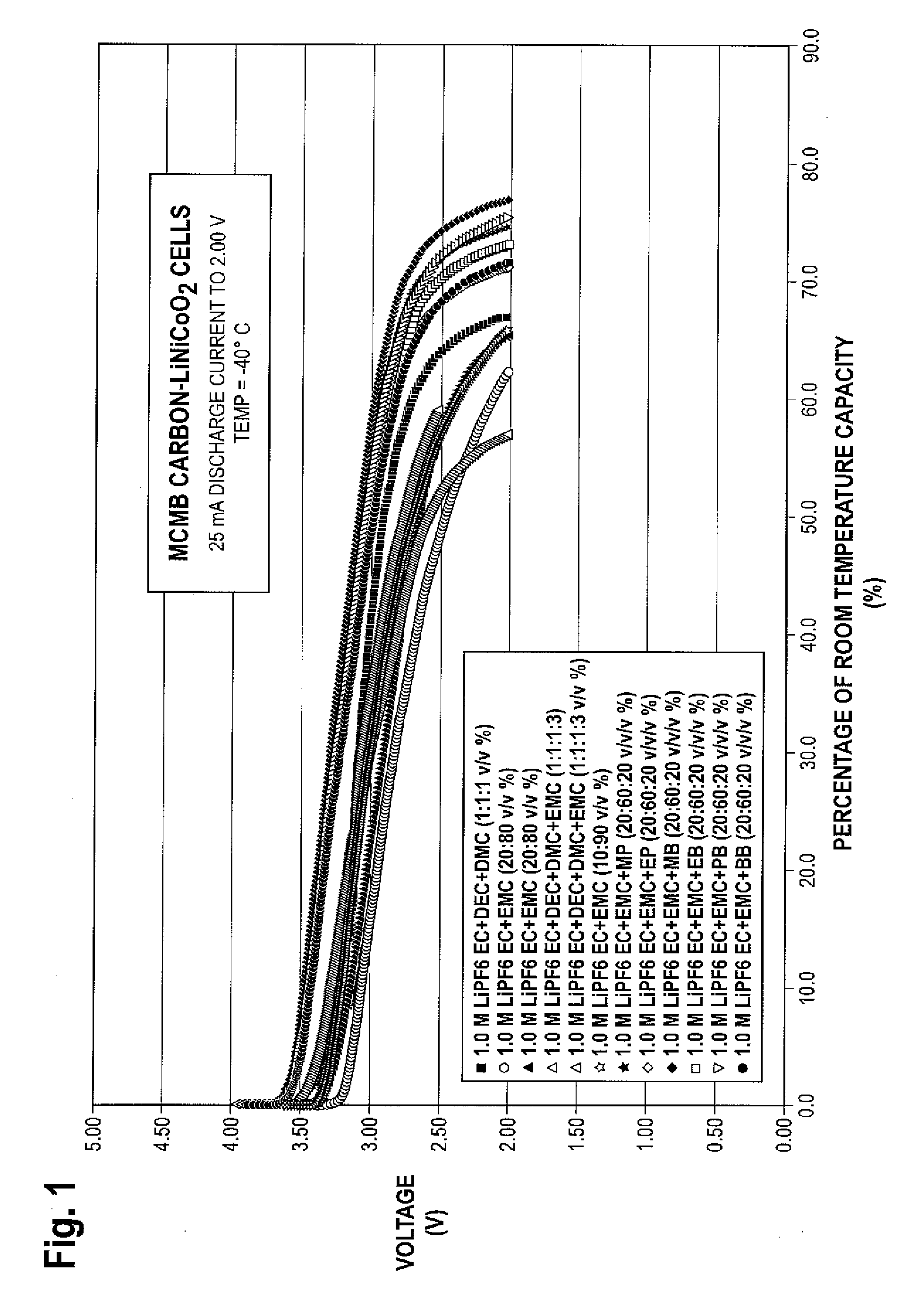
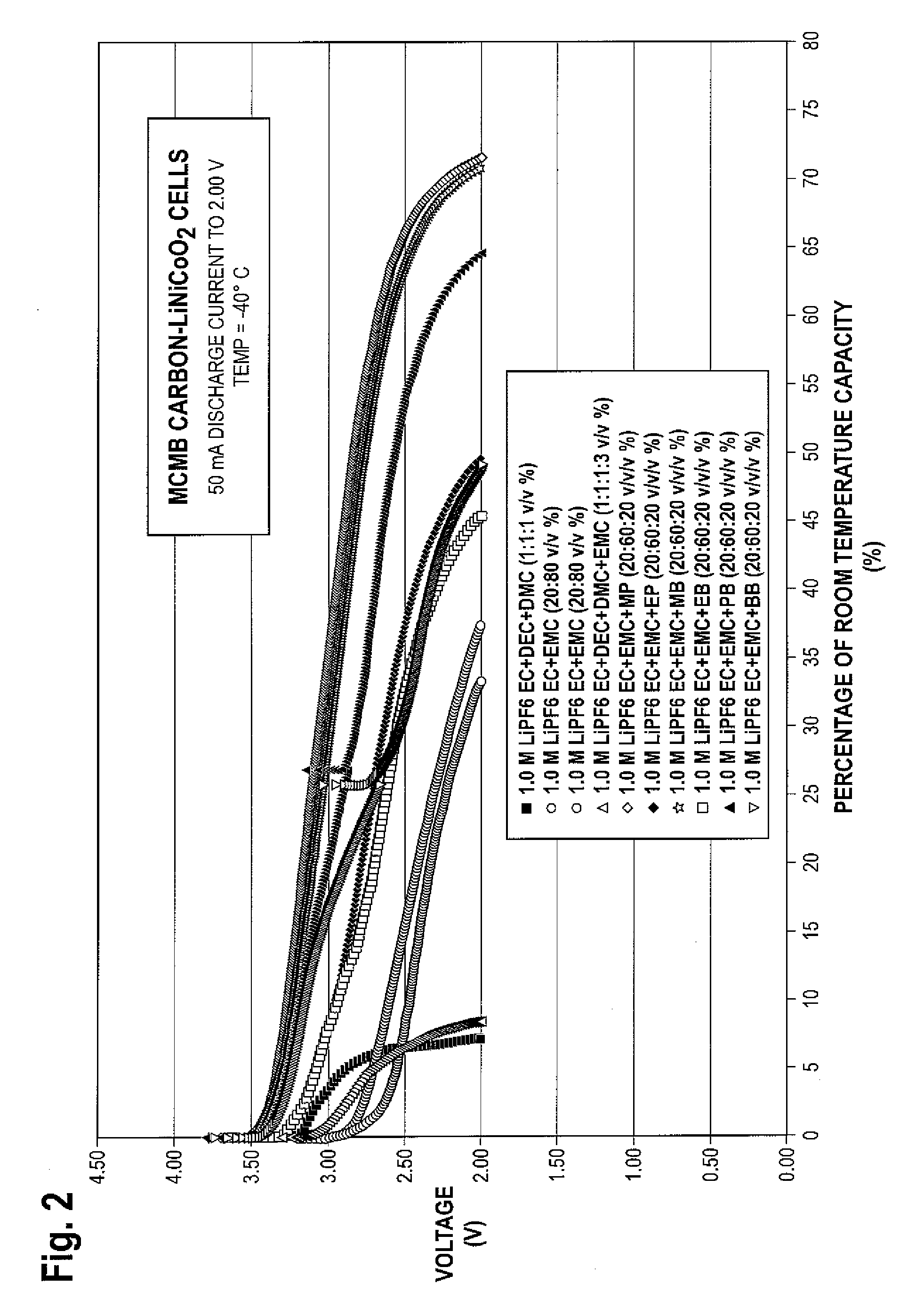
Lithium ion electrolytes and lithium ion cells with good low temperature performance
ActiveUS20090253046A1Improve low temperature performanceImprove stabilityOrganic electrolyte cellsNegative electrodesCelsius DegreeMethyl carbonate
There is provided in one embodiment of the invention an electrolyte for use in a lithium ion electrochemical cell. The electrolyte comprises a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), an ester cosolvent, and a lithium salt. The ester cosolvent comprises methyl propionate (MP), ethyl propionate (EP), methyl butyrate (MB), ethyl butyrate (EB), propyl butyrate (PB), or butyl butyrate (BB). The electrochemical cell operates in a temperature range of from about −60 degrees Celsius to about 60 degrees Celsius. In another embodiment there is provided a lithium ion electrochemical cell using the electrolyte of the invention.
Owner:CALIFORNIA INST OF TECH
Method for preparing methyl ethyl carbonate
InactiveCN101289395AHigh yieldPreparation from organic carbonatesTransesterificationMethyl carbonate
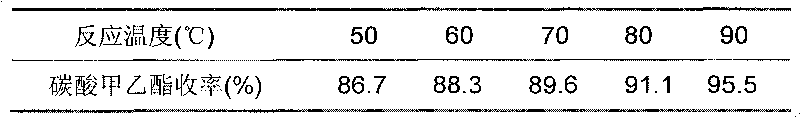
The invention relates to a preparation method of methyl ethyl carbonate, which takes methyl carbonate and ethanol as raw material and carries on transesterification with the existence of the catalyst with the reaction temperature of 50 DEG C to 60 DEG C. The temperature of the reaction materials rises from room temperature with a heating rate of five to ten DEG C per hour. The method also includes a water removing treatment to the ethanol so that the water content of the ethanol is less than or equal to 0.1 percent of the gross weight of the ethanol. By adopting the preparation method of the invention, the purity of the methyl ethyl carbonate is as high as 99.8 percent and the yield is higher than 70 percent. The method has the advantages of mild reaction condition, simple separation and purification of reaction products and low cost.
Owner:BYD CO LTD
Synthesis method of methyl ethyl carbonate
InactiveCN101704751AStable in natureHigh yieldPreparation from organic carbonatesSynthesis methodsFixed bed
The synthesis method of methyl ethyl carbonate belongs to the technical field of organic compounds synthesized by ester exchange. Dimethyl carbonate and diethyl carbonate, or dimethyl carbonate and ethanol are used as raw materials to perform ester exchange in the presence of catalyst. The reaction mode comprises two kinds of fixed bed continuous reaction and kettle-type reaction. The catalyst issolid alkali catalyst in which the carrier is active carbon, carbon molecular sieve or mesoporous carbon and the active component is Na2O, K2O, MgO, CaO, SrO or BaO. In the process of fixed bed continuous synthesis, the catalyst is stable without inactivation after long-term use, thereby retaining the high yield of the methyl ethyl carbonate, simplifying the production process, and achieving environmental friendliness and energy conservation. In the kettle-type reaction, the catalytic efficiency is high, the catalyst can be recovered by simple filtering and re-sintering, and the target product can be obtained in short time with high yield.
Owner:JILIN UNIV
A high-capacity secure 26650 lithium ion battery and its making method
InactiveCN101262077AHigh feasibilityFinal product manufactureCell electrodesMethyl carbonatePhosphate
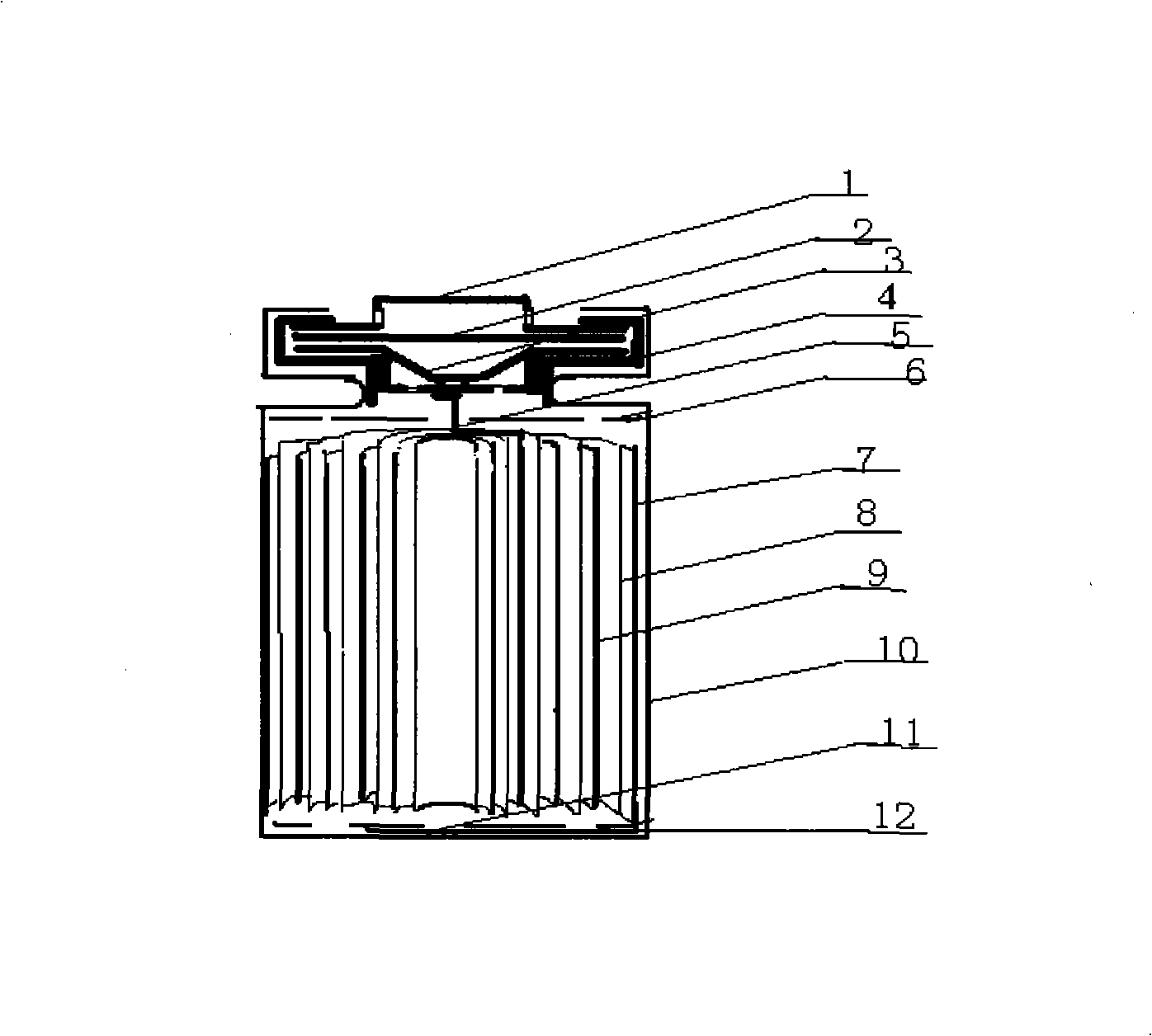
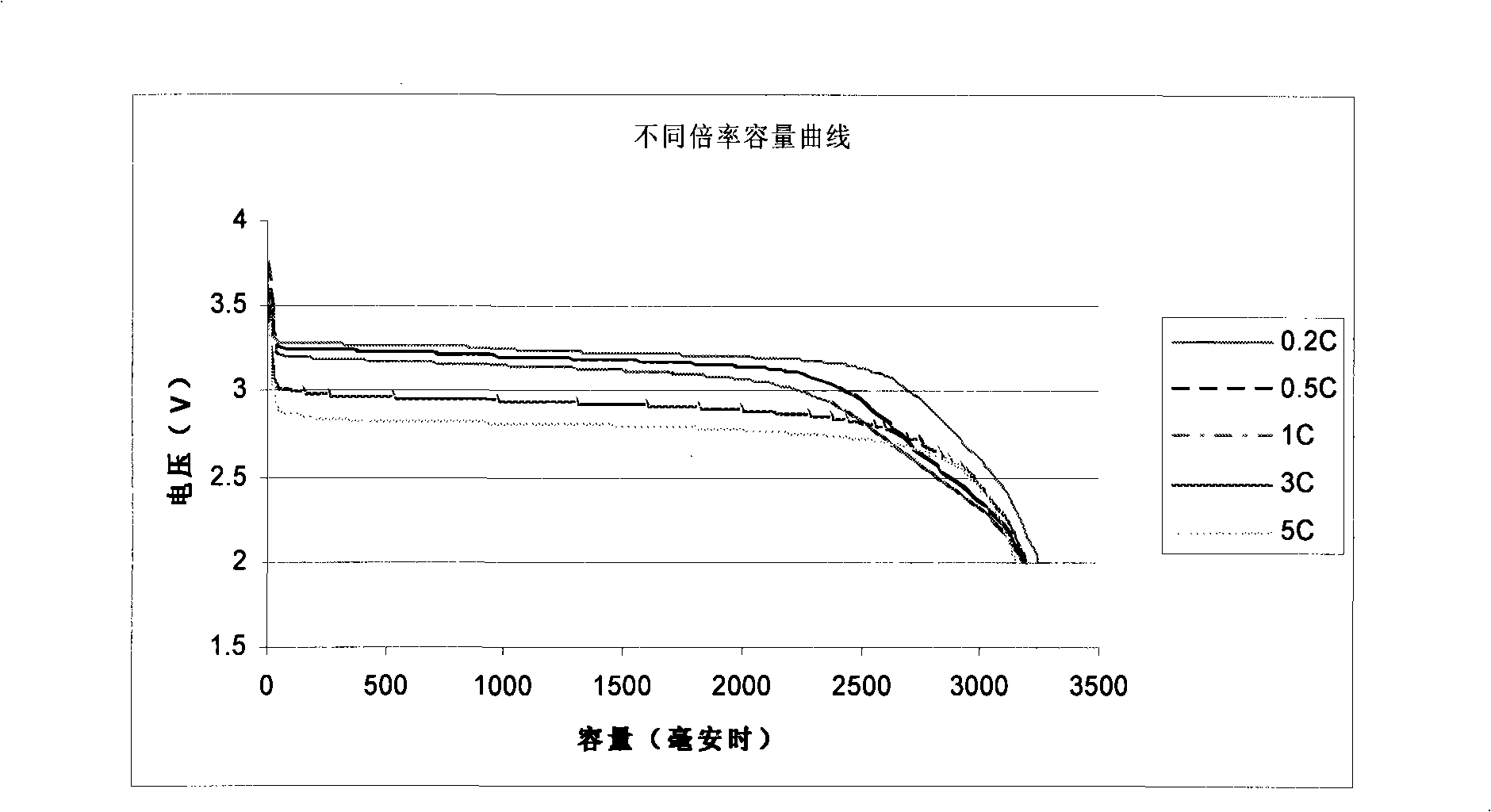
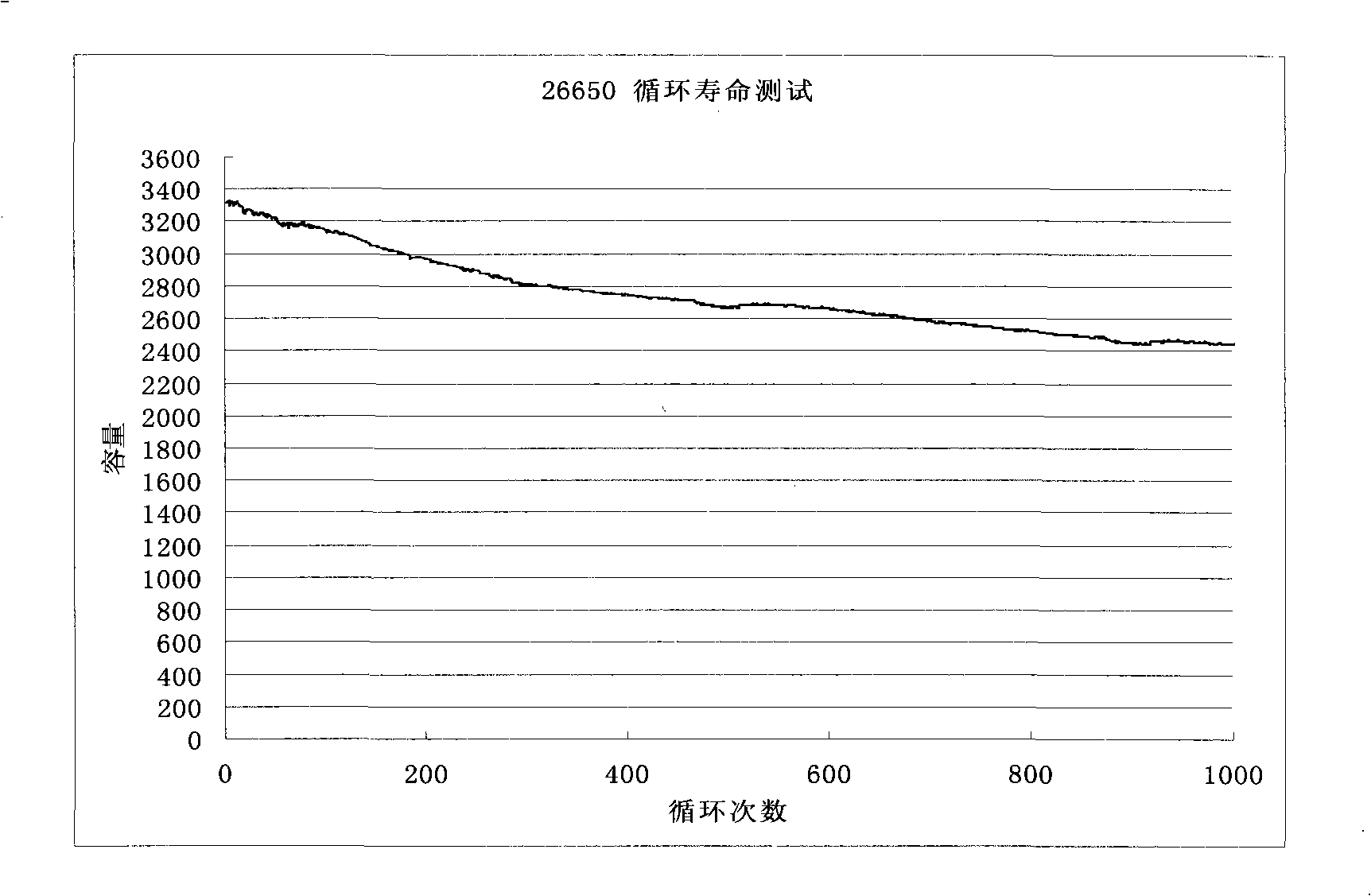
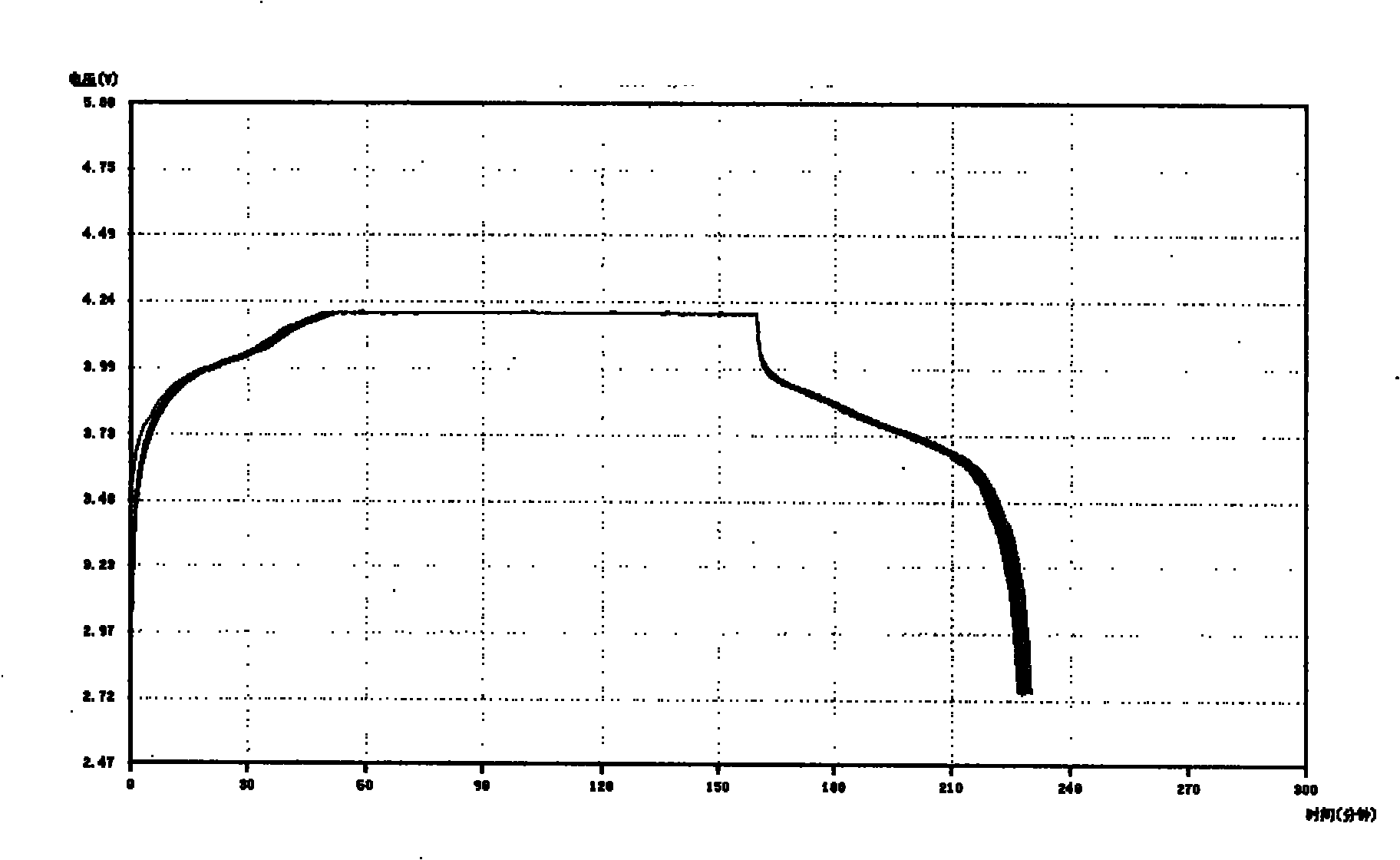
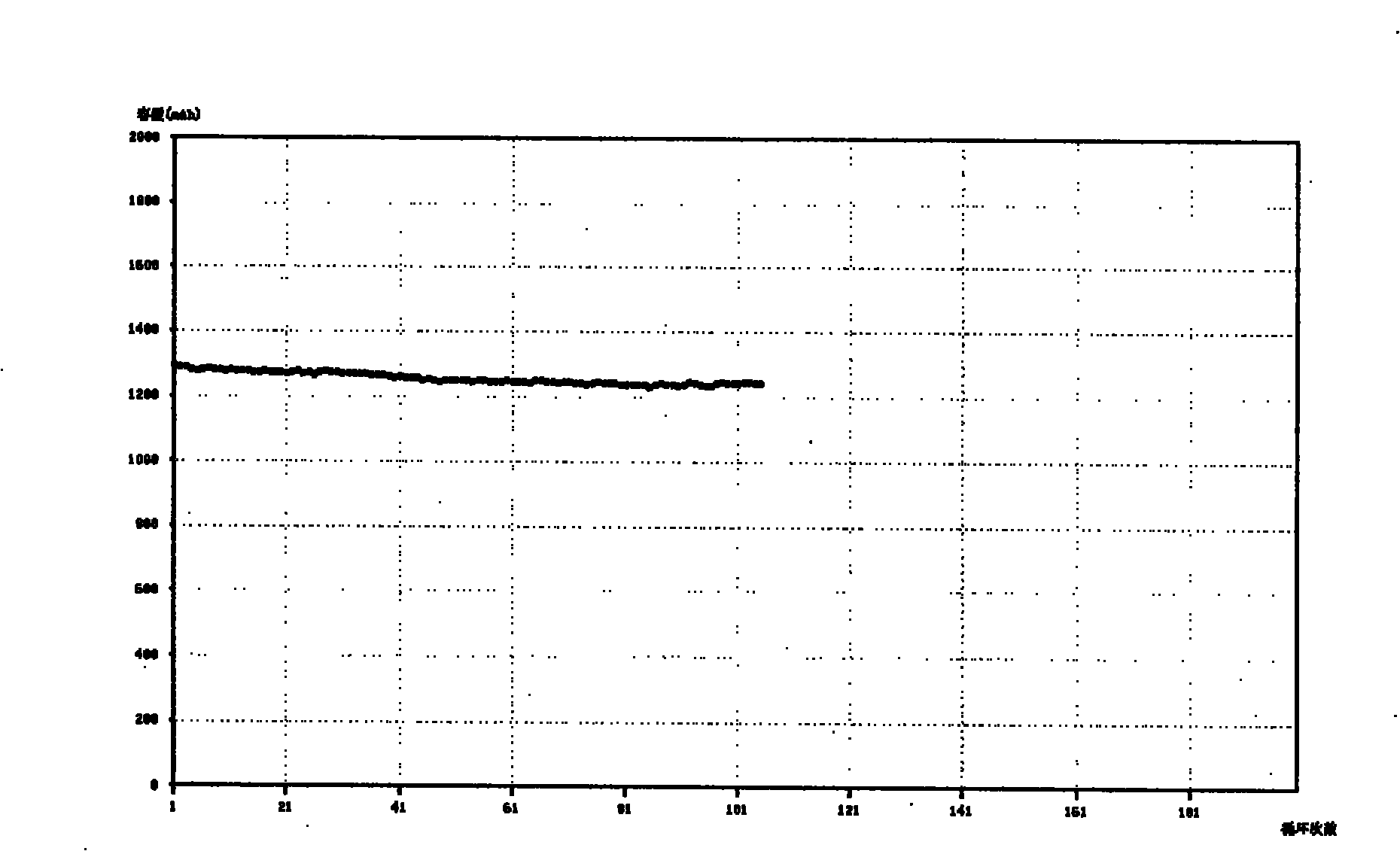
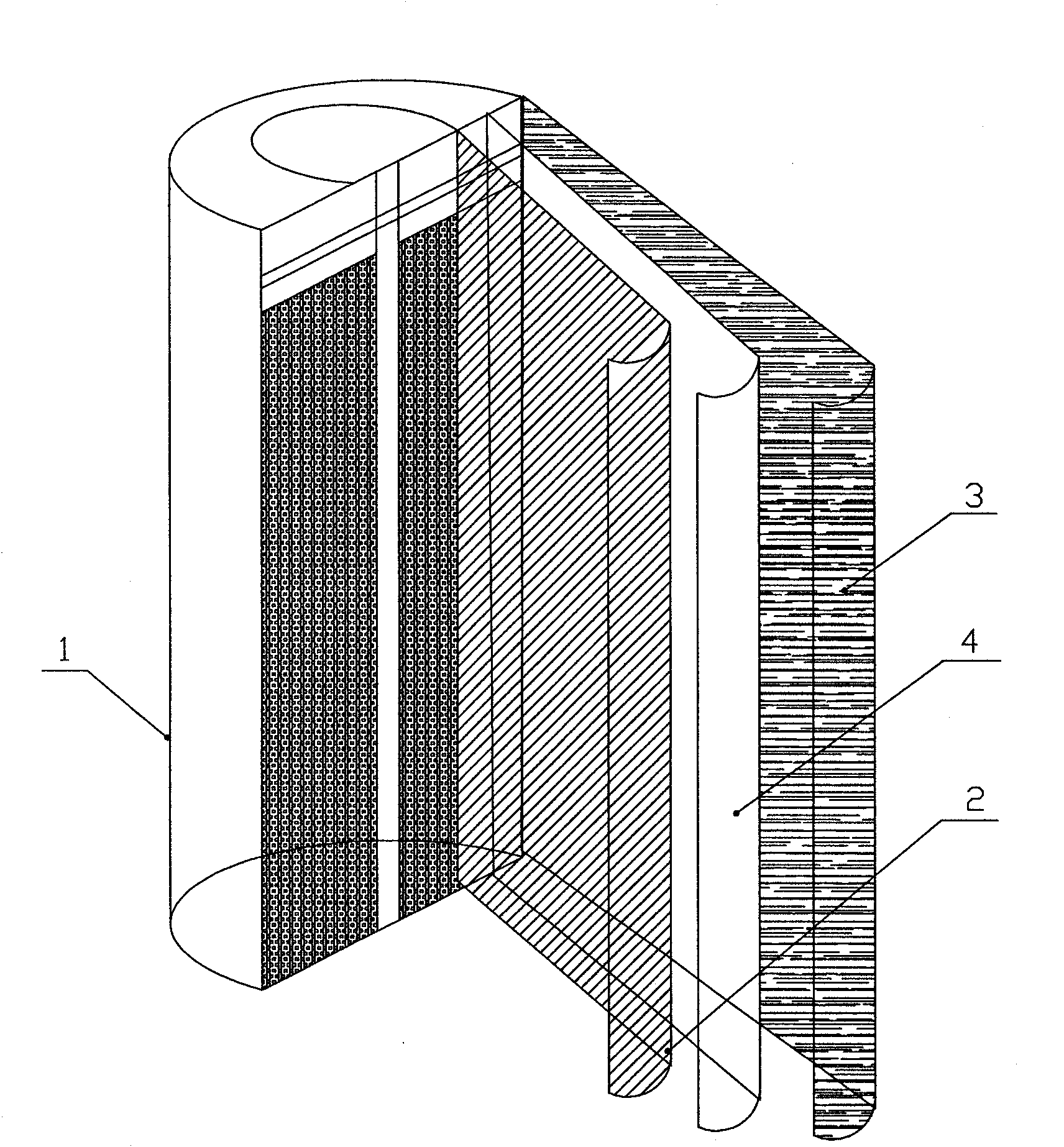
The invention relates to the technical field of a secondary power (a chargeable battery) which can be reused, in particular to a 26650 type lithium ion battery which is safe and has high capacity and a preparation method thereof. Lithium ferrous phosphate is used as cathode material; graphite materials are adopted as anode materials; mixture of EC (ethylene carbonate), DMC (Di Methyl Carbonate), EMC (methyl ethyl carbonate) (based on volume ratio of 1: 1: 1) is used as electrolyte solution, and LiPF6 (lithium hexafluorophosphate ) with molar ration 1.0 to 1.4 mol / L is adopted as electrolyte; multi-porous PP (polypropylene) and PE (polyethylene) are used as separating membrane, and a metal tank is used as a shell, thus the 26650 type battery is made with the diameter of 26mm and the height of 65mm; the capacity of the battery can reach 3200mAh, the internal resistance is within 30mOmega, and the cycle life thereof is more than 1000 times. The 26650 type lithium ion battery mainly solves the limitation problem of the existing lithium ion battery in the respect of safe and large size, and develops towards large size, has longer service life and higher safety and belongs to an environment protection chemical power with wider application.
Owner:上海德朗能电池有限公司
Lithium-ion secondary battery and manufacturing method thereof
InactiveCN101887990AImprove securityMeet environmental protection requirementsCell electrodesFinal product manufactureMethyl carbonateManganate
The invention discloses a lithium-ion secondary battery and a manufacturing method thereof. The lithium-ion secondary battery comprises an anode material, a cathode material, a diaphragm and electrolyte, wherein, the anode material and the cathode material are coated in a metal casing, the diaphragm is arranged between the anode material and the cathode material, and the electrolyte is filled in the diaphragm; the anode material is prepared by mixing a Ni-Co-Mn ternary material with a lithium manganate material base on a proportion of 1-9:9-1 by weight parts; the cathode material is made of graphite; the electrolyte is prepared by mixing ethylene carbonate, dimethyl carbonate with methyl ethyl carbonate based on the volume ratio of 1:1:1; and the diaphragm is porous polypropylene, polyethylene or a modified polymer of the porous polypropylene and the polyethylene. The invention has the advantages of excellent safety performance, least production cost and larger specific capacity, and through safety tests such as overcharge, over-discharge, short circuit, needling, extrusion, heavy impact, hot box and the like, the safety performance meets the standard testing requirements of UL1642, IEC60949-3 and GB / T18287-2004, and products meet the requirements and reach RoHS instruction requirement.
Owner:濮阳市星驰电源制造有限公司
Preparation method of polymer lithium ion battery
ActiveCN101685878AStable structureMeet capacityCell electrodesFinal product manufactureHigh temperature storagePolymer science
The invention belongs to a preparation method of a polymer lithium ion battery in the technical field of battery product development, which is characterized in that the polymer lithium ion battery comprises an anode system, a cathode system and gel polymer electrolyte, wherein, the anode system adopts lithium manganate and lithium nickelate cobaltate manganate in a weight ratio of 1:1-4, the cathode system adopts natural graphite and / or artificial graphite in a weight ratio of 0-100:100-0, the gel polymer electrolyte comprises a monomer / an initiator / organic electrolyte, the monomer is methyl acrylate / tetraethenol diacrylate, the initiator is a radical initiator, the organic electrolyte is ethylene carbonate / methyl ethyl carbonate / diethyl carbonate. The product prepared by the invention canmeet the requirements of the battery on capacity and overcharge protection, is excellent in high temperature storage and cyclicity and is widely applied to such electronic equipment as MP3, MP4, portable DVD and the like.
Owner:ZHENGZHOU BAK BATTERY CO LTD
Lithium iron phosphate battery with lithium ion battery electrolyte suitable for ultralow-temperature charging and discharging
ActiveCN103367803AFacilitate quick migrationImprove ionic conductivitySecondary cellsMethyl carbonateEthyl butyrate
The invention relates to a lithium iron phosphate battery with lithium ion battery electrolyte suitable for ultralow-temperature charging and discharging. The lithium ion battery electrolyte comprises lithium salt, a multi-element organic solvent and additives, wherein the additives comprise a low-melting-point additive, a film forming additive and a high-temperature additive; the multi-element organic solvent contains at least three of ethylene carbonate, diethyl carbonate, dimethyl carbonate, methyl ethyl carbonate, propylene carbonate and butylene carbonate; the low-melting-point additive contains at least one of 4-methyl-1,3-dioxolane, methyl acetate, methyl propionate, methyl butyrate, ethyl butyrate, propyl butyrate and butyl acetate; the high-temperature additive is at least one of methyl ester, di-n-propyl carbonate and 1,3-propane sultone. The lithium iron phosphate battery with the electrolyte can charge and discharge at an ultralow temperature and in a high-temperature environment, and is stable in performance and long in recycling life.
Owner:HANGZHOU LIAO TECH
Non-aqueous electrolyte for lithium manganate power battery
ActiveCN102610859APrevent high temperature swellingAvoid capacity lossSecondary cellsElectrolytic agentMethyl carbonate
The invention discloses non-aqueous electrolyte for a lithium manganate power battery. The non-aqueous electrolyte comprises 70-90% of carbonic ester compound, 3-20% of various functional additives and 11-17% of lithium hexafluorophate, wherein the carbonic ester compound is one of or mixture of ethylene carbonate (EC), propylene carbonate (PC), butane carbonate (BC), dimethyl carbonate (DMC), diethyl carbonate (DEC), dipropyl carbonate (DPC), methyl ethyl carbonate (EMC), methyl propyl carbonate (MPC) and methyl butyl carbonate (BMC); and the additives comprise 0.5-10% of a film forming additive, 0.5-10% of a high-temperature additive, 0.5-10% of an anti-overcharge additive, 0.5-10% of a flame retardant additive and 0.001-2% of a stability additive. In the non-aqueous electrolyte for the lithium manganate power battery of the invention, the performance of a solid phase interfacial film in the battery is improved, the compatibility of the electrolyte with negative electrode material is enhanced, and the cycle performance as well as the safety performance of the battery is greatly improved.
Owner:广东金光高科股份有限公司
Electrolyte for power lithium ion battery
InactiveCN102544582AImprove wettabilityStable electrochemical propertiesSecondary cellsPolyolefinSolvent
The invention discloses high-wettability and high-pourability electrolyte for a power lithium ion battery. The electrolyte comprises a wetting additive, a lithium salt, a non-aqueous organic solvent and other additives, wherein the wetting additive is a nonionic surfactant, and the wetting additive accounts for 0.001 to 5 percent of the mass of the electrolyte; the non-aqueous solvent is one or a mixture of more of ethylene carbonate, propylene carbonate, methyl ethyl carbonate, dimethyl carbonate, diethyl carbonate and gamma-butyrolactone, and the non-aqueous solvent accounts for 50 to 90 percent of the mass of the electrolyte; the lithium salt at the concentration of 0.6 to 1.5 mol / L is at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(oxalato)borate and lithium trifluoromethanesulfonate; and the other additives accounts for 0.5 to 8 percent of the mass of the electrolyte. The electrolyte has high wettability for a polyolefin diaphragm and electrode active materials, and has long cycle life.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS
Method for synthesizing vinylene carbonate
ActiveCN101407508AAvoid the problem of crystallization clogging pipesEasy to separate by filtrationOrganic chemistryFiltrationUltraviolet lights
The invention discloses a method for synthesizing vinylene carbonate, and the method mainly comprises the following steps: (1) chlorine gas is introduced into the raw material of ethylene carbonate in ultraviolet light condition, thus synthesizing chlor-ethylene carbonate; (2) taking mixture of ester, ether and hydrocarbon as the organic solvent, the chlor-ethylene carbonate which is obtained in the step (1) produces elimination reaction with triethylamine, thus eliminating hydrogen chloride and generating the vinylene carbonate; and (3) the mixed product obtained in the step (2) is distilled and purified after filtration. By adopting the mixed solvent of ester, ether and hydrocarbon, the reaction product is easily isolated from triethylamine hydrochloride, thus increasing the yield and the production efficiency; and BHT can be used as a polymerization inhibitor in the reaction process, thus controlling the polymerization of the vinylene carbonate effectively and increasing the yield.
Owner:江苏瀚康新材料有限公司
Electrolyte solution for negative lithium titanate battery, lithium ion battery and preparation method thereof
ActiveCN103117414AWide variety of sourcesEasy to operateFinal product manufactureElectrolyte accumulators manufactureSolventCarbonate
The invention discloses an electrolyte solution for a negative lithium titanate battery, a lithium ion battery and a preparation method thereof. The electrolyte solution is characterized in that lithium hexafluorophosphate is adopted as electrolyte, ethylene carbonate, methyl ethyl carbonate, diethyl carbonate and propylene carbonate are adopted as solvents, and one or more of fluoroethylene carbonate, double oxalate ithium borate, 1,3-propanesultone or vinylene carbonate are adopted as a film-formation additive.
Owner:CALB CO LTD +1
Method for interesterification synthesis of diphenyl carbonate by dimethyl carbonate
ActiveCN102050740AHigh catalytic activityHigh activityPhysical/chemical process catalystsPreparation from organic carbonatesCarbon nanotubeHigh activity
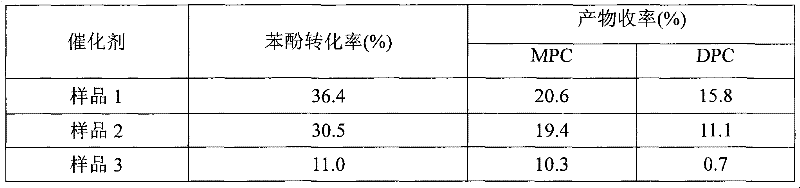
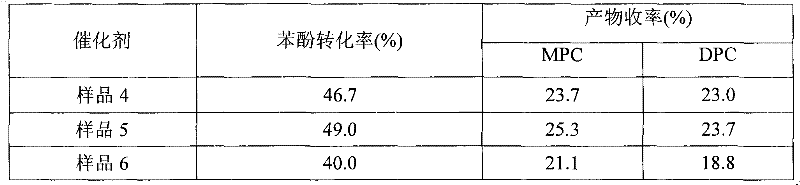
The invention relates to a method for interesterification synthesis of diphenyl carbonate by dimethyl carbonate. In the method, phenol and the dimethyl carbonate are used as raw materials, and carbon nano tube-loaded titanium dioxide is used as a catalyst; the carbon nano tube-loaded titanium dioxide used as the catalyst can be prepared by a sol gel method, a immersion method or a hydrothermal method; and the catalyst takes carbon nano tube (CNT) as a carrier and titanium dioxide as an active component. The catalyst has the high activity, good selectivity and high stability on reaction for preparing the diphenyl carbonate by using the dimethyl carbonate and the phenol, so that the total yield of the dimethyl carbonate and alkyphenyl carbonic ester reaches 49.0%, no byproducts are generated, the catalyst can be repeatedly used for four times, and the conversion rate of the phenol still keeps above 45%. The carbon nano tube-loaded titanium dioxide catalyst is a multi-phase catalyst, is easy to separate, can be recycled and has potential industry application value.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Electrolytic solution for improving lithium manganate lithium ion battery performance
InactiveCN101350430AReduce solubilityGood high temperature storage performanceSecondary cellsCyclic processManganese
The invention relates to the field of lithium batteries, disclosing an electrolyte which can be used to improve the performance of LiMn2O4 Li-ion battery, composed of ethylene carbonate, methyl ethyl carbonate, diethyl carbonate, propylene carbonate, 1,3-propane sultone, lithium hexafluorophosphate and lithium bis(oxalate)borate. During battery formation, the film forming additive forms a stable high-temperature resisting passivating film (SEI film) on the surface of the electrode, and the film is capable of restraining the reaction between the battery anode (mainly composed of LiMn2O4) and the electrolyte, reducing Mn-ion dissolution and alleviating the capacity attenuation of the battery during the cyclic process.
Owner:SHENZHEN HAIYING TECH
Degradable polymer/natural mineal filler composite material and preparation method thereof
The invention belongs to a composite material of degradable poly-methyl ethyl carbonic acid resin / natural mineral filling and the preparing method. It adopts the natural mineral as the filling, andthe carbonic acid resin as the basic body, smelts and commixes the filling and the basic body on the existent / nonexistent condition of the coupling agent to make the composite material. The weight percent content of the filling is 5-80%, the better filling 10-40%, and the basic-body material is heat-degraded to obtain the methyl ring carbonic-acid ethyl ester which can be used as organic agent.
Owner:SUN YAT SEN UNIV
An electrolyte for super capacitance cell
InactiveCN101079511AFulfil requirementsImprove conductivityCapacitor electrolytes/absorbentsSecondary cellsCapacitanceOrganic solvent
The invention discloses an electrolyte to prepare hyper capacitance battery, which comprises the following parts: lithium salt, non-water organic solvent and additive, wherein the non-water organic solvent is at least two parts of dimethyl carbonate, diethyl carbonate, propylene carbonate ester, ethyl carbonate ester, sulfite vinyl ester, sulfite propone ester, butane ester carbonate, Y-butyrolactone, methyl vinyl carbonate, methyl propyl ester carbonate, acetic ester and acetonitrile. The invention is compatible with capacitance and battery, which possesses high-power density, large current discharge and good circulating life.
Owner:CENT SOUTH UNIV
Difunctional electrolyte and preparation method thereof
InactiveCN101587777AMeet energy storage requirementsGood compatibilityHybrid capacitor electrolytesElectrolytic capacitorsEthyl acetateLithium-ion battery
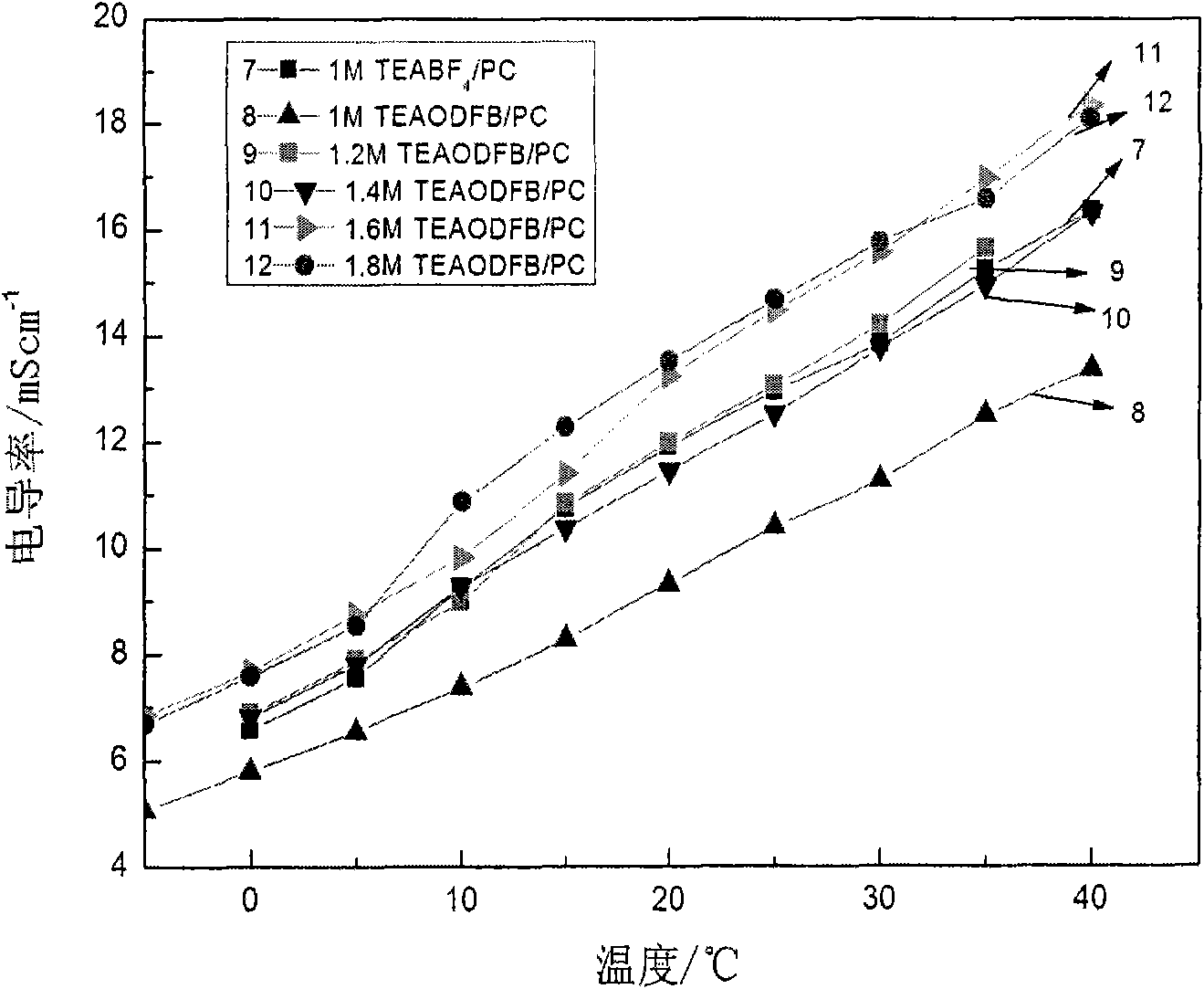
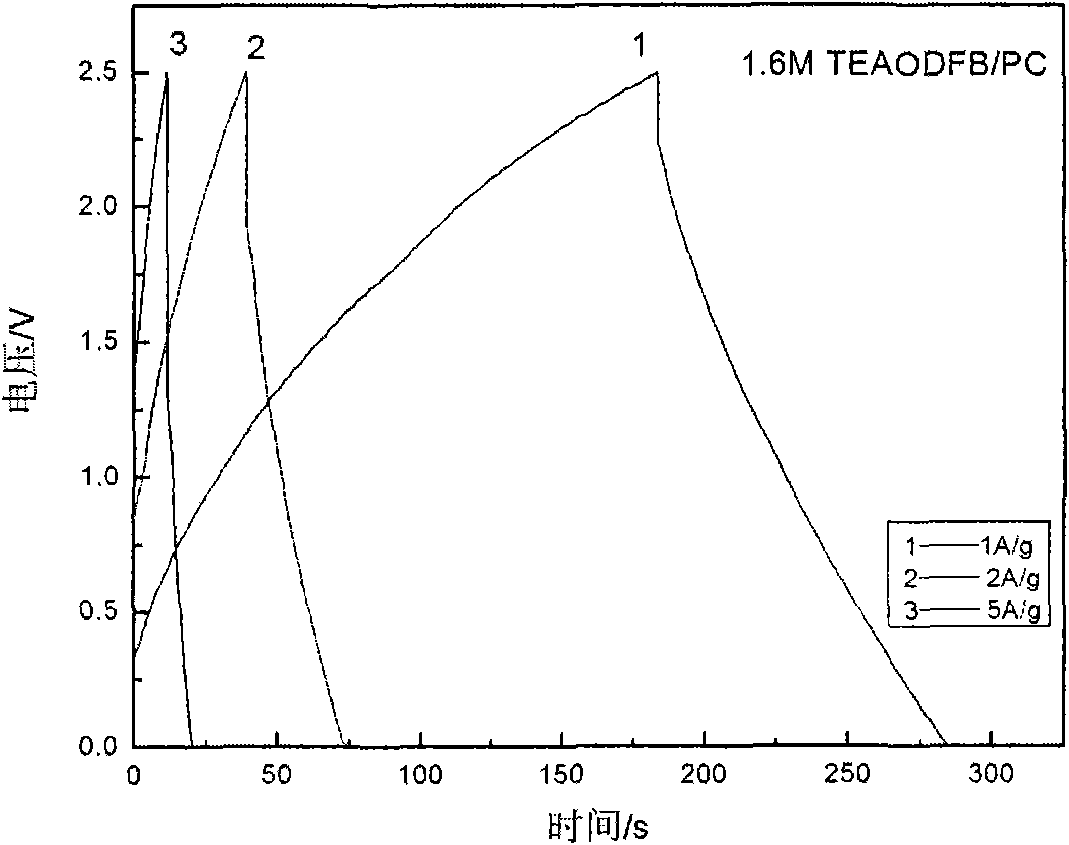
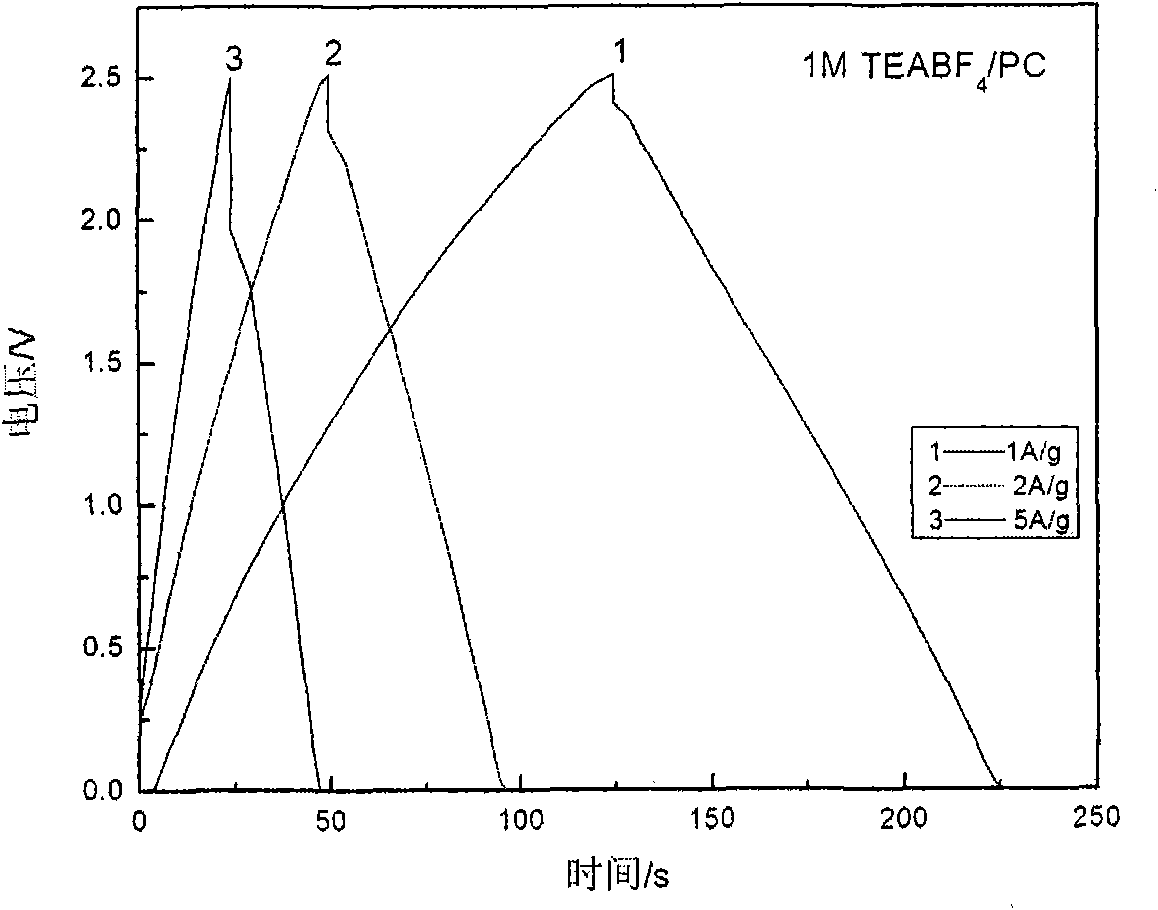
The invention relates to a difunctional electrolyte and preparation method thereof. The electrolyte is prepared by dissolving LiODFB, TEAODFB and additives to PC radical nonaqueous organic solvent. The PC radical nonaqueous organic solvent is one or multiple-solvent system at least containing propene carbonate, and ethylene carbonate, dimethyl carbonate, diethyl carbonate, methyl ethyl carbonate, propyl methyl carbonate, ethyl, methyl acetate, ethyl acetate, ethylene sulfite, propylene sulfite, acetonitrile, gamma-D lactone. The electrolyte of the invention has difunctional characteristic, which not only satisfies the request for the lithium ion battery by Li[+] chemical energy saving, but also satisfies the request for a super capacitor double electrode layer energy saving, at the same time being the difunctional electrolyte suitable for the super capacitor battery. The electrolyte is beneficial to promote the contribution for the energy and environment problem solution and provides a developing way for the electrolyte development.
Owner:CENT SOUTH UNIV +1
Method for preparing methyl ethyl carbonate
InactiveCN1394847ANo pollution in the processHigh yieldCarbonic/haloformic acid esters purification/separationMethyl carbonateReaction temperature
The preparation method of methylethyl carbonate uses dimethyl carbonate and diethyl carbonate as raw material, in the presence of catalyst makes them implement ester exchange reaction, and is characterized by that the dosage (mass) ratio of raw material dimethyl carbonate and diethyl carbonate is 1:2-2:1, its catalyst is load type metal oxide loaded on alumina, its metal oxide content (wt%) is 2%-30%, and the rest is Al2O3. In the ester exchange reaction the addition quantity of metal oxide is 0.1%-10% of total quantity of raw material, its reaction temp. is 50-200 deg.C, reaction pressure is normal pressure and its reaction time is 2-48 hr.
Owner:ZHEJIANG UNIV
Process and apparatus for preparing diethyl carbonate
The present invention discloses a technological method of producing diethyl carbonate with a reaction distillation bulkhead column, and a device thereof. The reaction distillation bulkhead column is characterized in that a bulkhead is arranged in the vertical direction in a conventional distillation column, and the whole column is divided into four zones: a public distillation zone, a reaction zone, a side line zone and a public stripping zone. Methyl carbonate and ethyl alcohol are used as raw materials, diethyl carbonate and methanol are generated in the reaction zone through transesterification reaction with catalyst, the byproduct methanol with a concentration more than 95 percent is generated at the column top, the product diethyl carbonate with a concentration more than 99.5 percent can be prepared after the catalyst and the diethyl carbonate in the column reactor are separated, the separated catalyst, and the methyl ethyl carbonate, ethyl alcohol, diethyl carbonate and methanol, which are collected from the side line, can be recycled. The advantages of the bulkhead column and the reaction distillation are fully developed in the present invention, the transesterification reaction and the product separation can be realized in one column, and the equipment investment and the energy consumption can be effectively reduced.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Adjustable fuel power booster component composition
InactiveUS20070204506A1Reduce gas emissionsBiofuelsLiquid carbonaceous fuelsMethyl carbonateInternal combustion engine
An adjustable fuel power booster component composition having three components: (A) an ignition accelerator which is preferably normal propyl nitrate and / or diterbutyl peroxide; (B) propylene glycol monoalkyl ether and / or butylene glycol monoalkyl ether; and (C) methyl carbonate and / or ethyl carbonate and / or propyl carbonate and / or butyl carbonate, which may be used mixed in any proportion with methylal (dimethoxymethane) or ethylal (diethoxymethane). The adjustable fuel power booster component composition of the present invention can be used by itself or in mixture with gasoline, diesel or burning oils in combustion engines without the need for modification thereof. The adjustable fuel power booster component composition enables low energy content alcohol based fuels to substitute conventional fuels, such as gasoline or diesel, in conventional non-modified internal combustion engines, thereby generating lower amounts of toxic gas emission all the while proving more power.
Owner:BRENES MARIO ARAYA
Electrolyte of the lithium ion battery for ultra-low temperature discharge and its lithium ion battery
InactiveCN101017918AImprove performanceExcellent cycle indexOrganic electrolyte cellsSecondary cellsDimethoxyethaneEthyl carbonate
The related Li-ion cell fit to discharge at ultra-low temperature comprises an anode, a cathode, a membrane, and the electrolyte. Wherein, the electrolyte comprises: lithium hexafluorophosphate and lithium tetrafluoroborate with weight ratio as 1:5-10:1, and the solvent (including ethylene carbonate, dimethyl carbonate, methyl- ethyl carbonate, and dimethoxyethane). Wherein, the dimethoxyethane is 0.5-10wt%. Compared with prior art, this product can work well at -40deg.
Owner:HANGZHOU SKYRICH POWER CO LTD
Lithium secondary cell electrolyte
InactiveCN1339846AImprove cycle lifeImprove charge and discharge efficiencySecondary cellsOrganic conductorsOrganic solventPhysical chemistry
The present invention belongs to the field of lithium iron cell producing technology and the nonaqueous electrolyte liquid consists of organic carbonate as solvent in 85-90 wt% and electrolyte salt in 10-15 wt%, the solvent consists of one of vinyl carbonate and alloy carbonate and one or more of dimethyl carbonate, diethyl carbonate and methyl ethyl carbonate; and the electrolyte salt is one of LiPF6, LiAsF6, LiClO4 and LiBF4. Into the electrolyte liquid, sulfur containing compound in 0.01-10 wt% may be also added to raise the charge and discharge efficienty and circulation life of the cell.The electrolyte liquid of the present invention is well compatible with positive and negative electrode material in secondary lithium ion cell.
Owner:TIANJIN CHEM RES & DESIGN INST
Composite lithium ion battery electrolyte and lithium ion battery comprising same
ActiveCN109361017AImprove cycle performanceInhibit side effectsSecondary cellsOrganic electrolytesMethyl carbonateLithium-ion battery
The invention discloses composite lithium ion battery electrolyte and a lithium ion battery comprising the same. The composite lithium ion battery electrolyte comprises an organic solvent, lithium salt and an additive. The organic solvent is at least two of propylene carbonate (PC), ethyl methyl carbonate (EMC), dimethyl carbonate (DMC), dipropyl carbonate (DPC), allyl ethyl carbonate (AEC), allylmethyl carbonate (AMC) and the like; and the additive is selected from at least two of unsaturated carbonate, sulfur-containing organic matters, lithium borate dioxalate, lithium difluorophosphate and fluoro-imide salt. For the respective physical and chemical characteristics of the organic solvent and the additive, the types of the additive are screened and combined, by adjustment on an electrolyte additive, the ratio by which respective advantages can be played and respective shortcomings can also be suppressed is found out, the high-voltage capacity of the battery is improved, and the cycle life of the battery is prolonged.
Owner:산산어드밴스드머테리얼스(취저우)컴퍼니리미티드
Lithium iron phosphate power battery with improved low temperature charge performance
InactiveCN108306018AImprove low temperature performanceNo negative impact on energy densityElectrode carriers/collectorsSecondary cellsDifluorophosphateElectrical battery
The invention discloses a lithium iron phosphate power battery with an improved low temperature charge performance, belongs to the technical field of lithium iron phosphate power batteries, and aims to solve the problem that the negative pole can easily reach a cut-off voltage, and the charge capacity is reduced therefore. The lithium iron phosphate power battery is characterized by comprising a positive plate, a negative plate, and electrolyte. The lithium salt is lithium hexafluorophosphate (11.5-15.0%). The organic solvent is a four component mixed solvent, which is composed of propylene carbonate, methyl carbonate, ethyl carbonate, and diethyl carbonate according to a ratio of 10-40: 1-10: 20-50: 10-30. The additive is one or more of vinylene carbonate, fluoroethylene carbonate, propargyl sulfonate, lithium difluorophosphate, lithium difluoro(oxalato)borate, and lithium tetrafluoro(oxalato)phosphate. The interface impedance of the negative pole can be reduced, the low temperature properties of the lithium iron phosphate power battery are obviously improved, and the low temperature charge performance of the lithium iron phosphate power battery is improved.
Owner:CAMEL GRP WUHAN OPTICS VALLEY R&D CENT CO LTD
Method for preparation of methyl ethyl carbonate with co-precipitation catalyst
The invention relates to a preparation method of chemical raw materials, in particular to a method for preparation of methyl ethyl carbonate with a co-precipitation catalyst. The method adopts coprecipitation technique to carry one or more of the active components Al2O3, CaO, La2O3, Fe2O3, Mn2O3, Cs2O, MgO, BaO, SrO and K2O, wherein Y is one or more oxides of Si and Al, and Z is one or more oxides of Si, Al and Ti. The invention has the advantages that: macropore can significantly improve the mass transfer effect, and micropore can significantly increase the specific surface area of the carrier and improve the dispersity of the active center. Magnesium nitrate, aluminum chloride and lanthanum sulfate are adopted as the active components and are mixed with a precursor to obtain the 15%MgO-5%Al2O3-3%La2O3 / Al2O3-SiO2 catalyst, which can be applied to dimethyl carbonate and ethanol ester exchange fixed bed continuous reaction. The catalyst active center metal oxide prepared by precipitation method has smaller crystal grains, higher dispersity and surface area, and the prepared catalyst has catalytic effect superior to the catalyst prepared by impregnation method.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for synthesizing diphenyl carbonate through ester exchange reaction of dimethyl carbonate and phenylethyl acetate
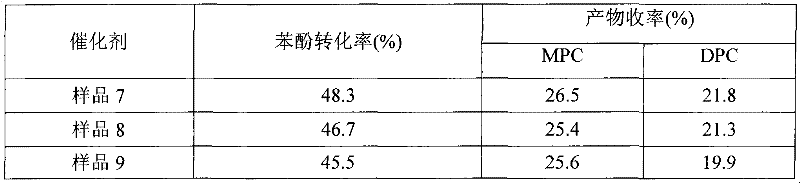
InactiveCN101628874AImprove performanceEasy to useOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from organic carbonatesDiketoneHalogen
The invention discloses a method used for synthesizing diphenyl carbonate through ester exchange reaction by using dimethyl carbonate (DMC) and phenylethyl acetate (PA) as raw materials. The method is characterized in that a catalyst system used by the method basically consists of beta-diketone compounds (seen in structural general formulas 1 and 2) belonged to IVB-class metals, and also can be homologous compound thereof. The catalyst system can exist stably in the air, the catalyst has good selectivity and catalytic activity on products comprising methyl phenyl carbonate (MPC) and the diphenyl carbonate (DPC), and the using amount of the catalyst is little. Therefore, the catalyst system can be widely applied to the production of the diphenyl carbonate. In the structural general formulas 1 and 2, M is Ti, Zr and Hf; and R and R' are substituted groups on the beta-diketone compounds, and can be alkyl, alkoxyl and halogen.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Low-temperature electrolyte for supercapacitor and preparation method thereof
InactiveCN101593625ALow melting pointImprove solubilityElectrolytic capacitorsSulfolanePropionitrile
The invention discloses low-temperature electrolyte for a supercapacitor and a preparation method thereof. The electrolyte comprises solute and non-aqueous organic solvent. The solute is ionic liquid tetraethylammonium-oxalate-difluoro-borate (TEAODFB) which is battery-grade TEAODFB obtained by purifying a product of reaction of a chlorine-containing compound, an oxalate-containing compound and a fluoroboric acid containing compound in acetonitrile or carbonic ester medium by adopting a method of reduced pressure evaporation or low-temperature recrystallization. The non-aqueous organic solvent is one or the combination of acetonitrile, propionitrile, methoxy propionitrile, ethylene carbonate, propylene carbonate, dimethyl carbonate, diethyl carbonate, methyl ethyl carbonate, gamma-butyrolactone, N,N-dimetbylformamide, tetrahydrofuran and sulfolane. The concentration of the adopted electrolyte is 0.8 to 2mol / L. The obtained low-temperature electrolyte has high specific capacity and charge / discharge cyclic life at the temperature of 30 DEG C below zero.
Owner:CENT SOUTH UNIV +1
Lithium battery electrolyte and preparation method thereof
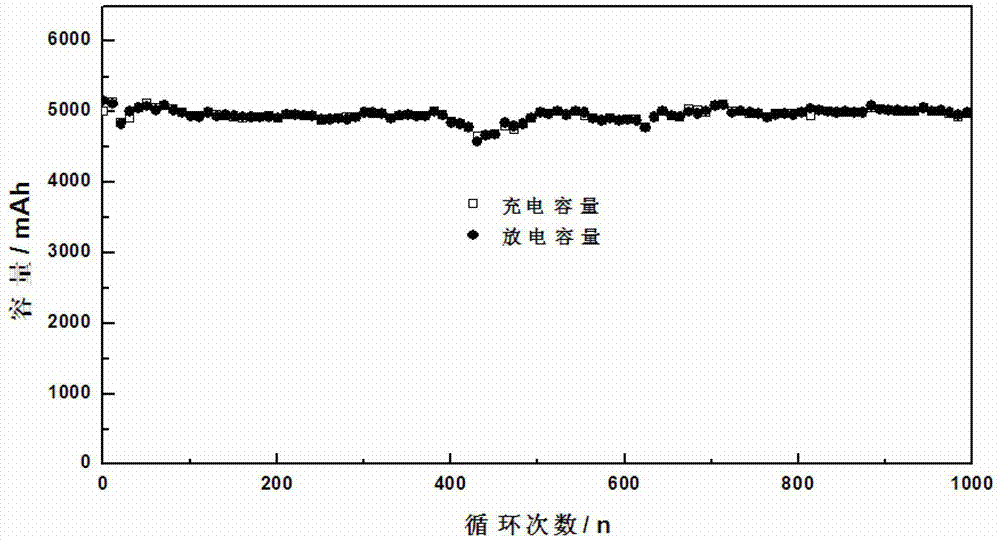
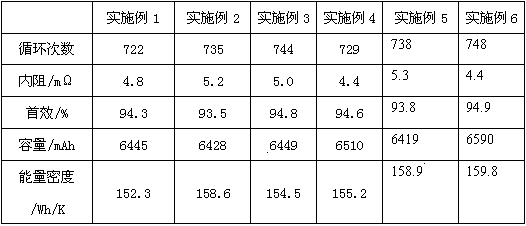
InactiveCN109585923AGood compatibilityReduce hydrogen contentFinal product manufactureLi-accumulatorsInternal resistanceOmega
The present invention relates to the technical field of lithium batteries, and in particular to a lithium battery electrolyte and a preparation method thereof. The lithium battery electrolyte comprises an organic solvent, an additive, tris(trimethylsilyl)borate and lithium hexafluorophosphate. The additive comprises a stabilizer, an SEI film forming additive and a flame retardant, wherein the massratio of the stabilizer, the SEI film forming additive and the flame retardant is 50-80:1-5:1-5; the stabilizer is compounded by an organic fluorine compound; the SEI film-forming additive is at least one of vinylene carbonate, ethylene sulfite, allyl ethyl carbonate and butylene glycol sulfite; and the flame retardant is compounded by the triphenyl phosphate and the triethyl phosphate accordingto the mass ratio of 1-5:1-5. The conductivity of the electrolyte of the lithium battery is above 12ms / cm and near 13 ms / cm, the lithium battery has good preformances, the number of cycles is not smaller than 700, the internal resistance is not larger than 5.2 m[Omega], the first effect is not smaller than 93%, the capacity reaches up to 6510 mAh, and the energy density reaches up to 158.6 Wh / K.
Owner:广东永邦新能源股份有限公司
Mixed solvent for removing acid gas
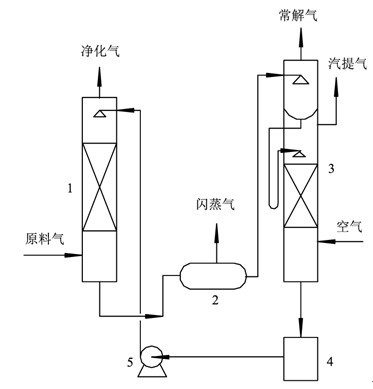
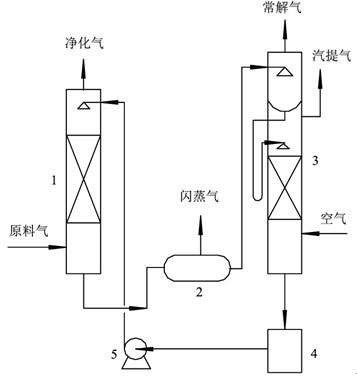
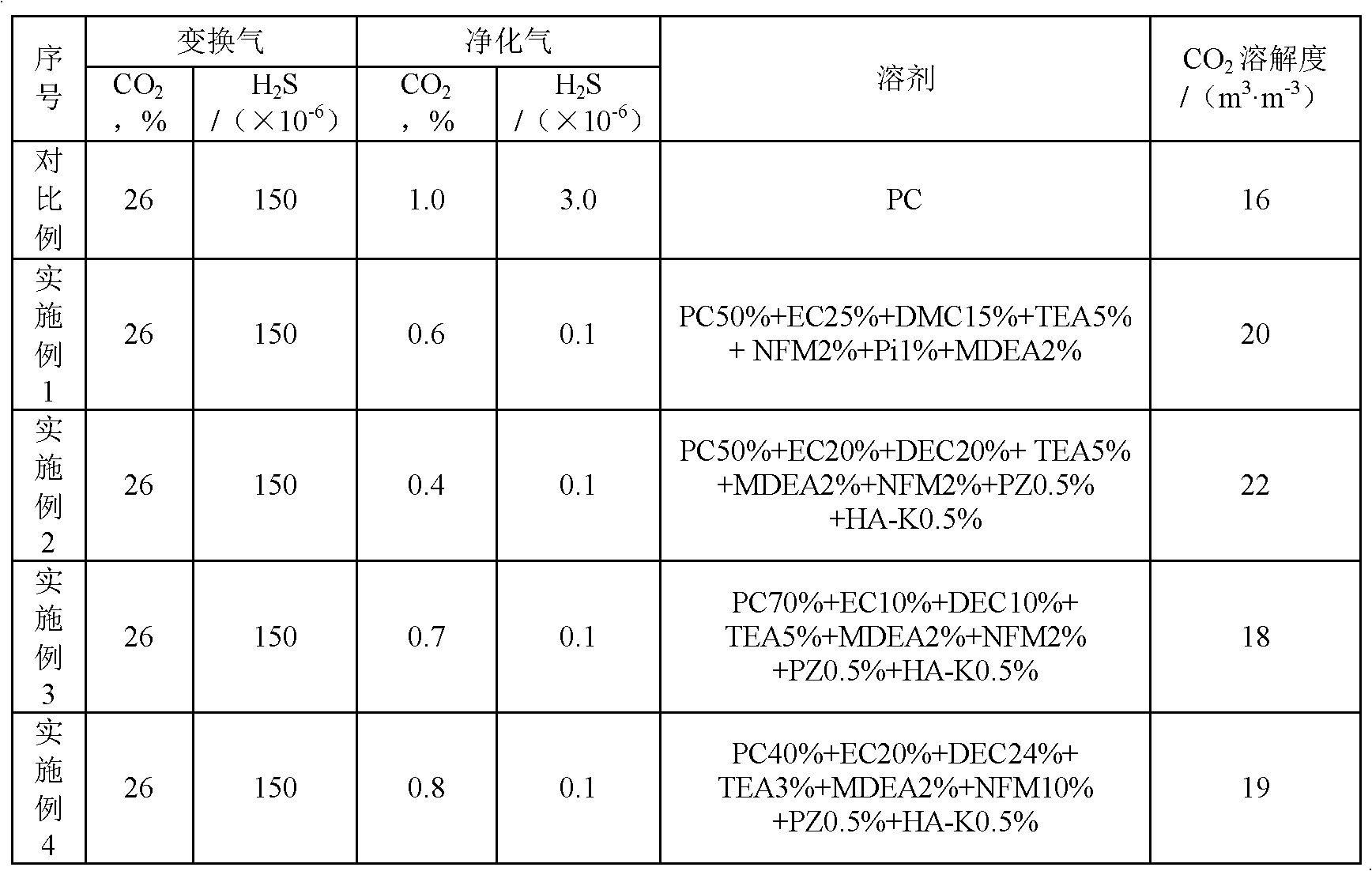
InactiveCN102657998AFlexibleOptimized formulaDispersed particle separationAbsorption capacityMethyl carbonate
The invention discloses a mixed solvent for removing acid gas, which belongs to the technical field of gas separation and is characterized by being composed of two or more than two of main absorption components, assisting absorption components and active components in mixing mode, wherein the main absorption components comprise propylene carbonate (PC), ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC) and N-methyl-2-pyrrolidone (NMP), the assisting absorption components comprise N-methyldiethanolamine (MDEA), triethanolamine (TEA), diglycolamine (DGA) and dimethylethanolamine (DMEA), and the active components comprise phosphoric acid (Pi), piperazine (PZ), urotropin (HA-K), N-Formylmorpholine formylmorpholine (NFM), 2-amino-2methyl-1-propyl alcohol (AMP) and morpholine by-products. By means of the mixed solvent for removing acid gas, on one hand, absorption capacity and purification of CO2 are improved, and on the other hand, desulfuration accuracy is greatly improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com