Lithium battery electrolyte and preparation method thereof
An electrolyte and lithium battery technology, applied in the field of lithium battery electrolyte and its preparation, can solve problems such as unstable performance and low conductivity, and achieve the effects of improving compatibility, increasing reduction potential, and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
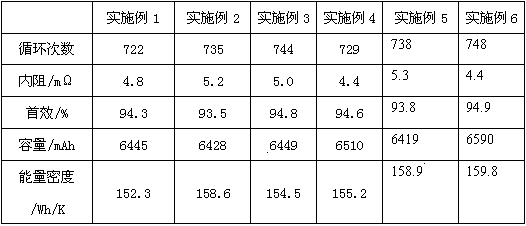
Embodiment 1
[0024] A lithium battery electrolyte, including organic solvents, additives, tris(trimethylsilane) borate, and lithium hexafluorophosphate, wherein tris(trimethylsilane) borate accounts for 0.5% of the total mass of the lithium battery electrolyte, and lithium hexafluorophosphate Accounting for 6% of the total mass of lithium battery electrolyte;
[0025] The additives include stabilizers, SEI film-forming additives, and flame retardants, and the mass ratio of the stabilizers, flame retardants, and SEI film-forming additives is 60:4:1;
[0026] The stabilizer is a mixture of ethoxypentafluorocyclotriphosphazene, hexafluoroisopropanol methyl ether, and fluoroethylene carbonate at a mass ratio of 1:1:1;
[0027] The SEI film-forming additive is composed of vinylene carbonate and vinyl sulfite in a mass ratio of 1:3;
[0028] The flame retardant is composed of triphenyl phosphate and triethyl phosphate in a mass ratio of 1:4.
[0029] The organic solvent includes dimethyl carbo...
Embodiment 2
[0033] A lithium battery electrolyte, including organic solvents, additives, tris(trimethylsilane) borate, and lithium hexafluorophosphate, wherein tris(trimethylsilane) borate accounts for 1% of the total mass of the lithium battery electrolyte, and lithium hexafluorophosphate Accounting for 8% of the total mass of lithium battery electrolyte;
[0034] The additives include stabilizers, SEI film-forming additives, and flame retardants, and the mass ratio of the stabilizers, flame retardants, and SEI film-forming additives is 50:5:1;
[0035] The stabilizer is composed of ethoxypentafluorocyclotriphosphazene, hexafluoroisopropanol methyl ether, and fluoroethylene carbonate in a mass ratio of 1:1:2;
[0036] The SEI film-forming additive is vinylene carbonate;
[0037] The flame retardant is composed of triphenyl phosphate and triethyl phosphate in a mass ratio of 2:1.
[0038] The organic solvent includes dimethyl carbonate and ethyl propionate, and the volume ratio of the t...
Embodiment 3
[0042] A lithium battery electrolyte, including organic solvents, additives, tris(trimethylsilane) borate, and lithium hexafluorophosphate, wherein tris(trimethylsilane) borate accounts for 0.2% of the total mass of the lithium battery electrolyte, and lithium hexafluorophosphate Accounting for 10% of the total mass of lithium battery electrolyte;
[0043] The additive includes a stabilizer, an SEI film-forming additive, and a flame retardant, and the mass ratio of the stabilizer, flame retardant, and SEI film-forming additive is 70:3:5;
[0044] The stabilizer is composed of ethoxypentafluorocyclotriphosphazene, hexafluoroisopropanol methyl ether, and fluoroethylene carbonate in a mass ratio of 1:2:1;
[0045] The SEI film-forming additive is composed of ethyl propylene carbonate and butenyl sulfite in a mass ratio of 1:1;
[0046] The flame retardant is composed of triphenyl phosphate and triethyl phosphate in a mass ratio of 5:1.
[0047] The organic solvent includes dimeth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Internal resistance | aaaaa | aaaaa |
| Capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com