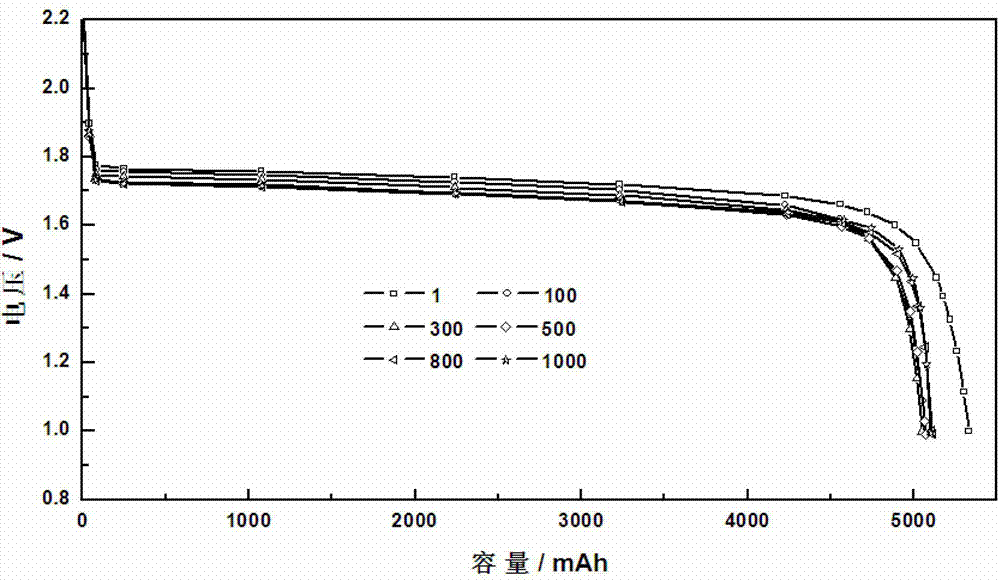
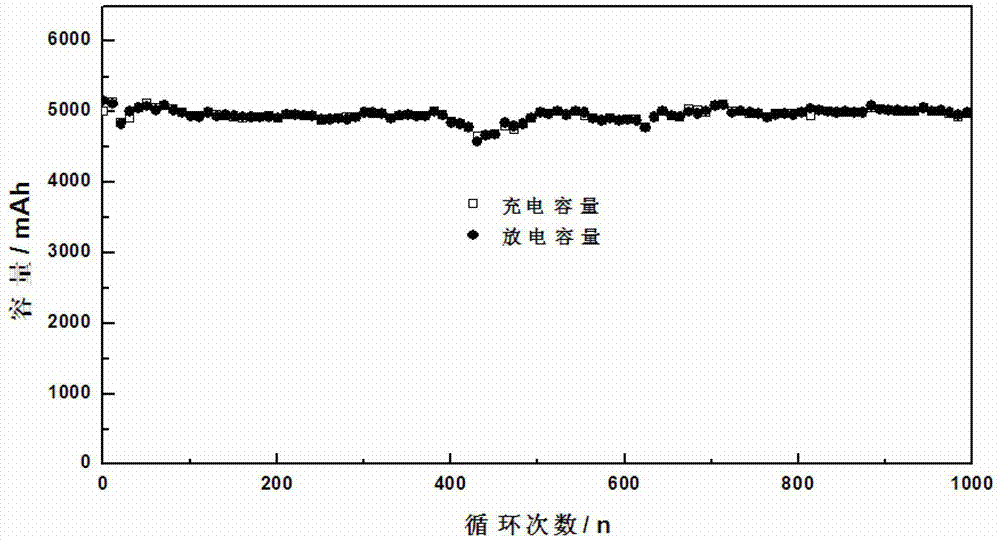
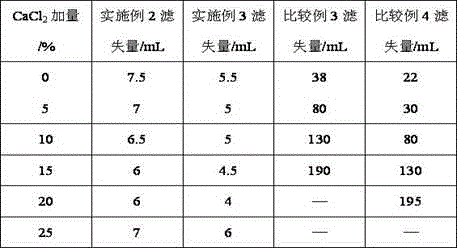
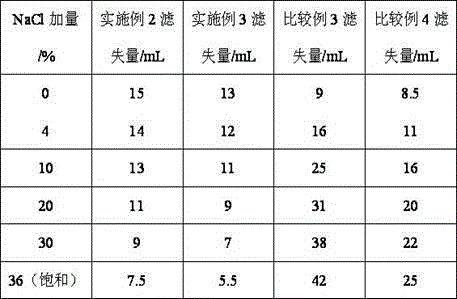
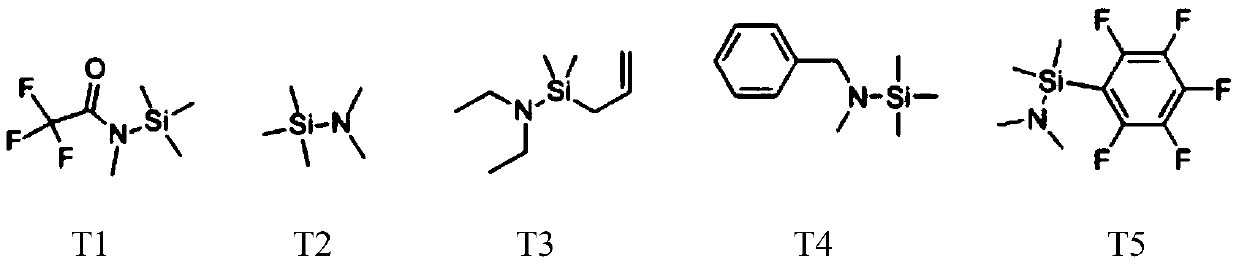
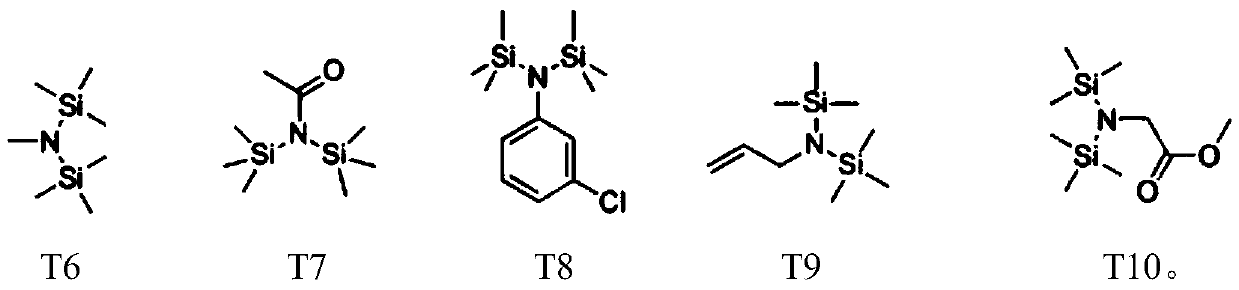
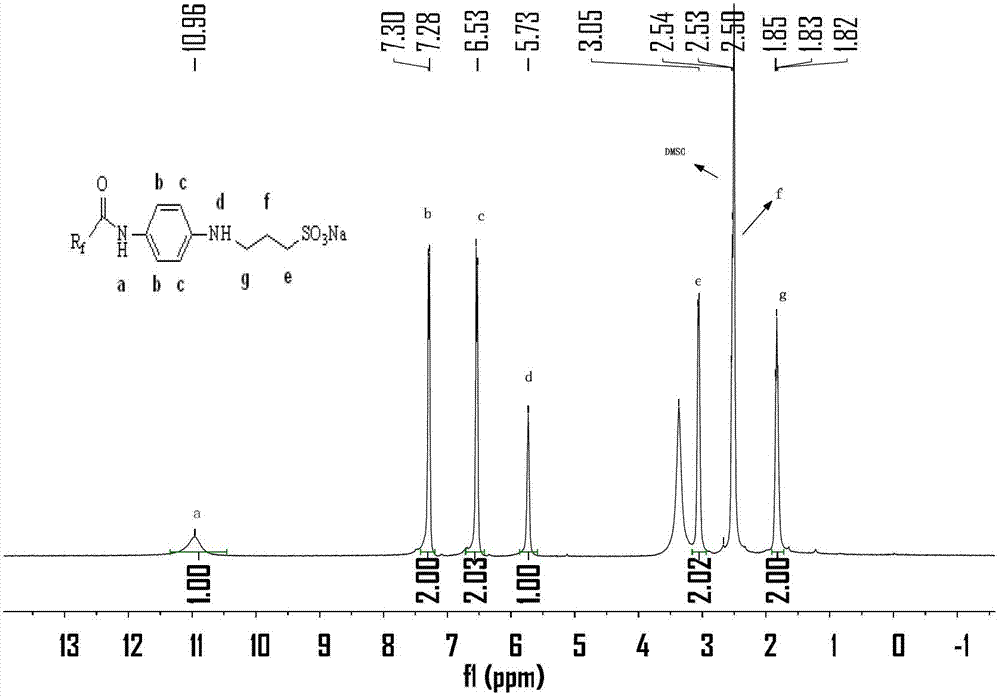
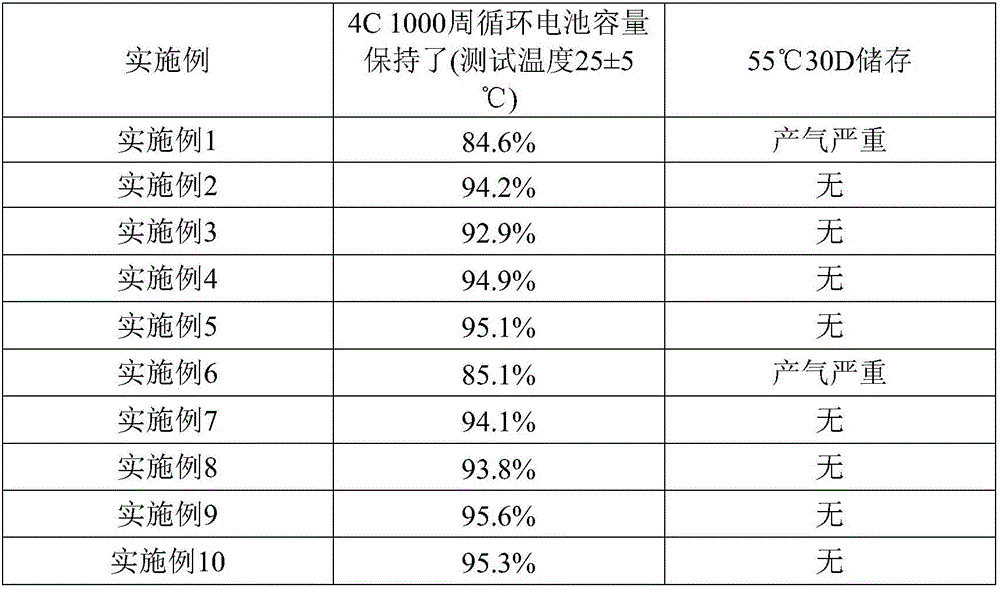
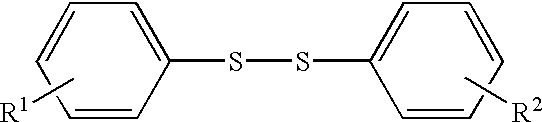Patents
Literature
68 results about "Propanesultone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A synthetic, colorless liquid or white crystalline solid that is readily soluble in water and many organic solvents such as ketones, esters and aromatic hydrocarbons. 1,3-propanesultone is used as a chemical intermediate in the production of detergents, dyes, lathering agents, bacteriostats, fungicides, insecticides, cation-exchange resins, corrosion inhibitors and vulcanization accelerators. When heated to decomposition, it emits toxic fumes of sulfur oxides. Humans are potentially exposed to residues of 1,3-propane sultone when using products manufactured from this compound. The primary routes of potential human exposure to 1,3-propane sultone are ingestion and inhalation. Contact with this chemical can cause mild irritation of the eyes and skin. It is reasonably anticipated to be a human carcinogen. (NCI05)
Non-aqueous electrolyte and non-aqueous electrolyte secondary cell
InactiveUS20040091786A1Reduce gas volumeExcellent characteristicsNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsPhysical chemistryDiphenyl disulfide
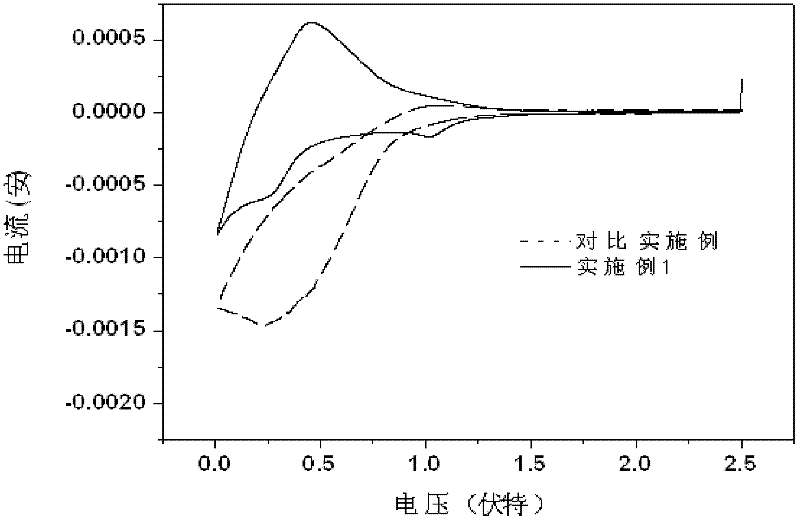
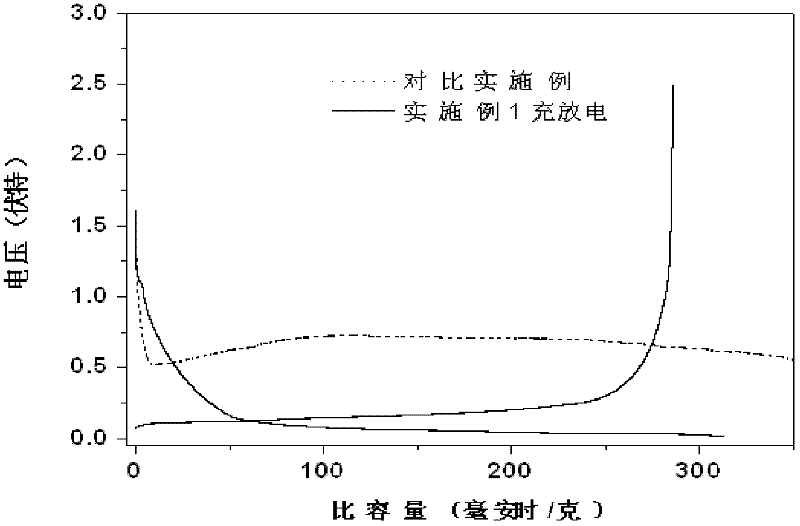
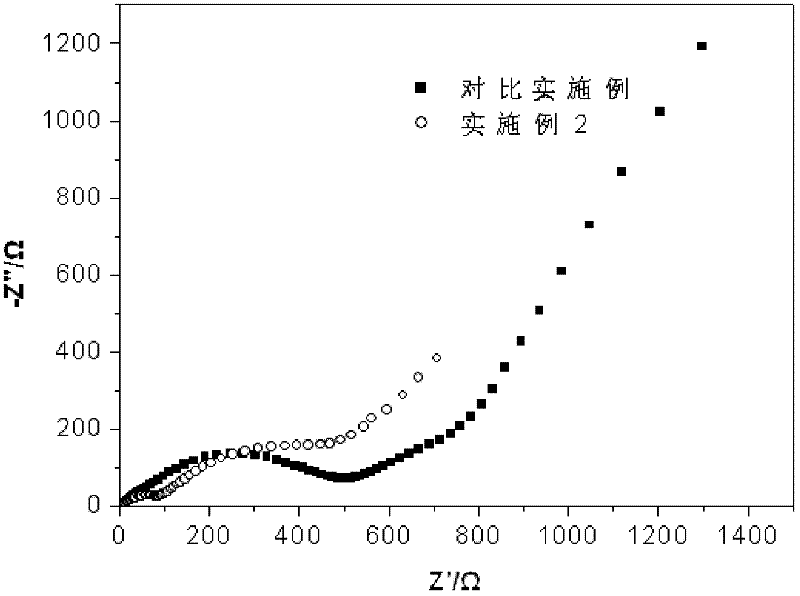
A non-aqueous electrolyte containing propylene carbonate and 1,3-propanesultone as additives can reduce the amount of a gas evolved during storage at a high temperature of a non-aqueous electrolyte secondary cell comprising the electrolyte, a non-aqueous electrolyte containing at least one compound selected from the group consisting of vinylene carbonate, diphenyl disulfide, di-p-tolyldisulfide and bis(4-methoxyphenyl)disulfide as an additive can improve cycle characteristics of a non-aqueous electrolyte secondary cell comprising the electrolyte, and a non-aqueous electrolyte containing a combination of the above two types of additives can provide a non-aqueous electrolyte secondary cell exhibiting excellent retention of capacity and storage stability.
Owner:PANASONIC CORP +1
Lithium-ion battery electrolyte, lithium-ion battery and electronic instrument
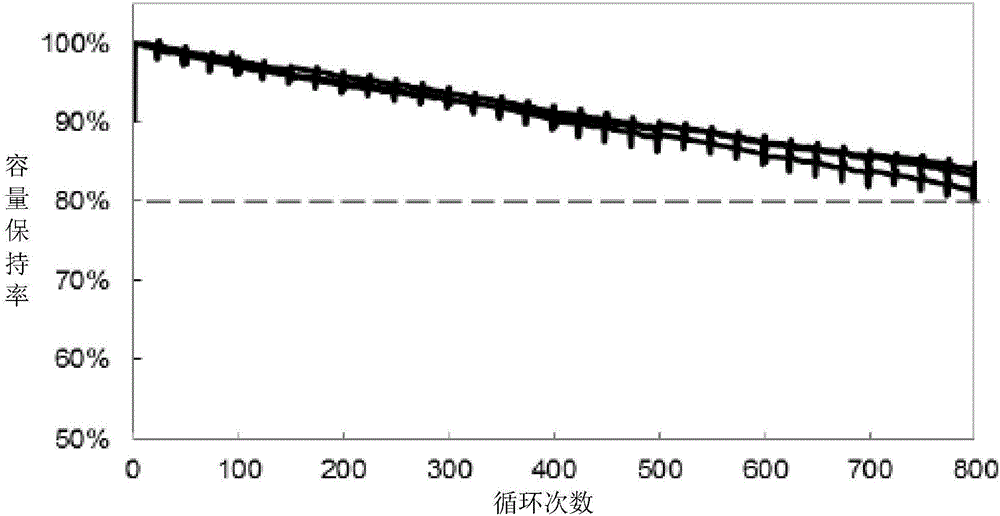
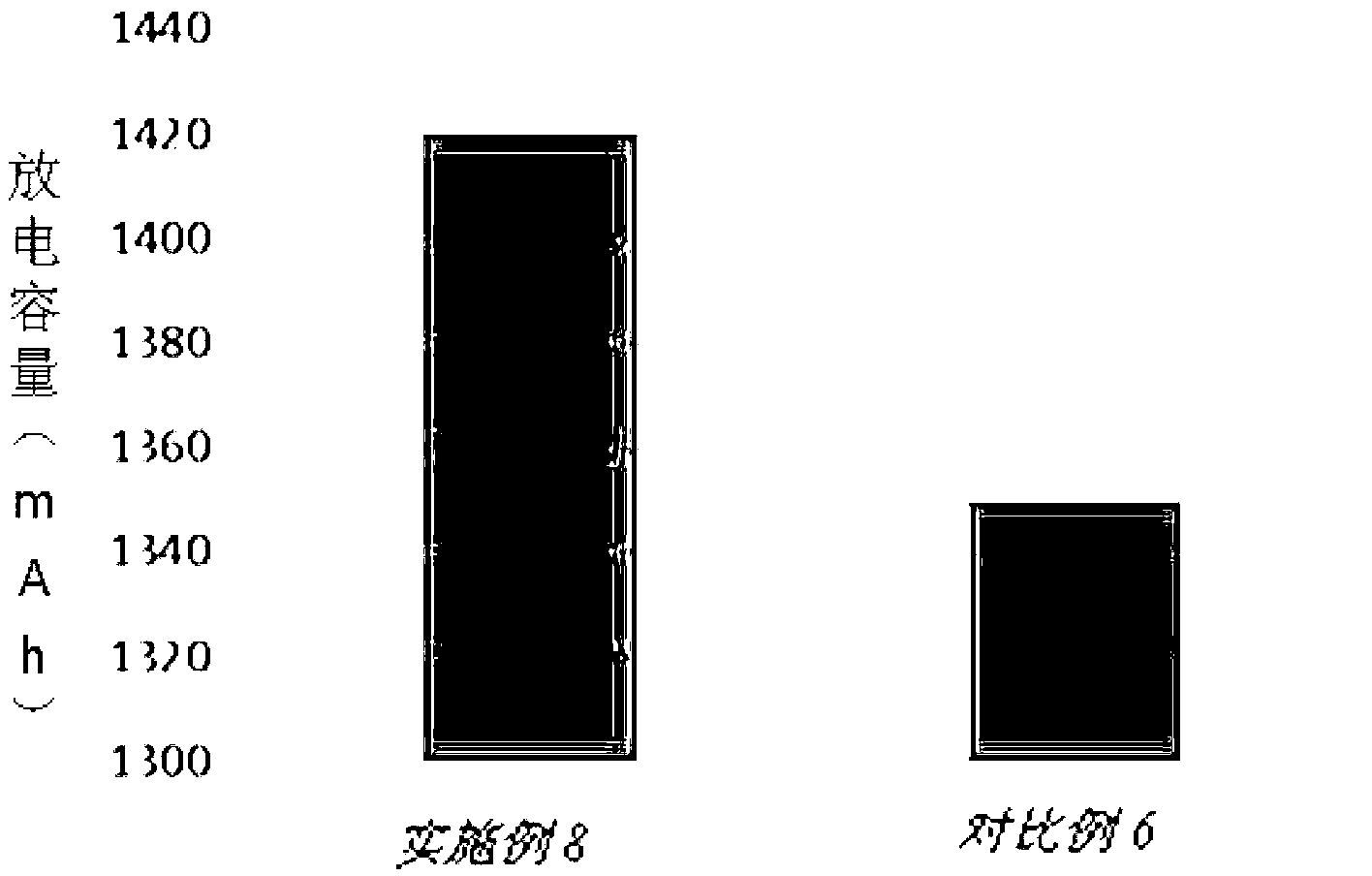
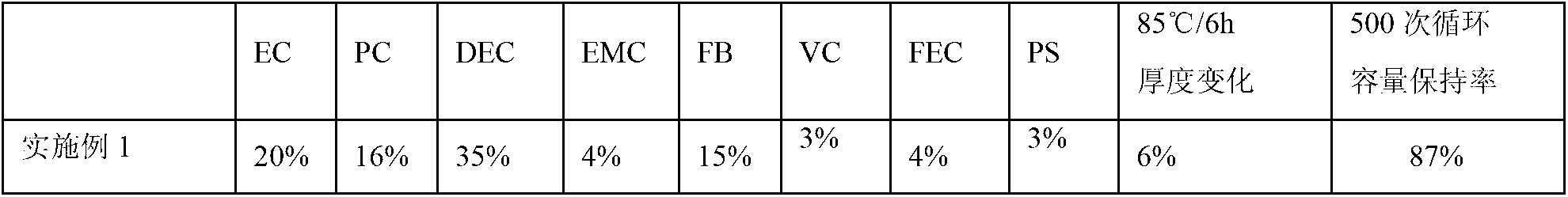
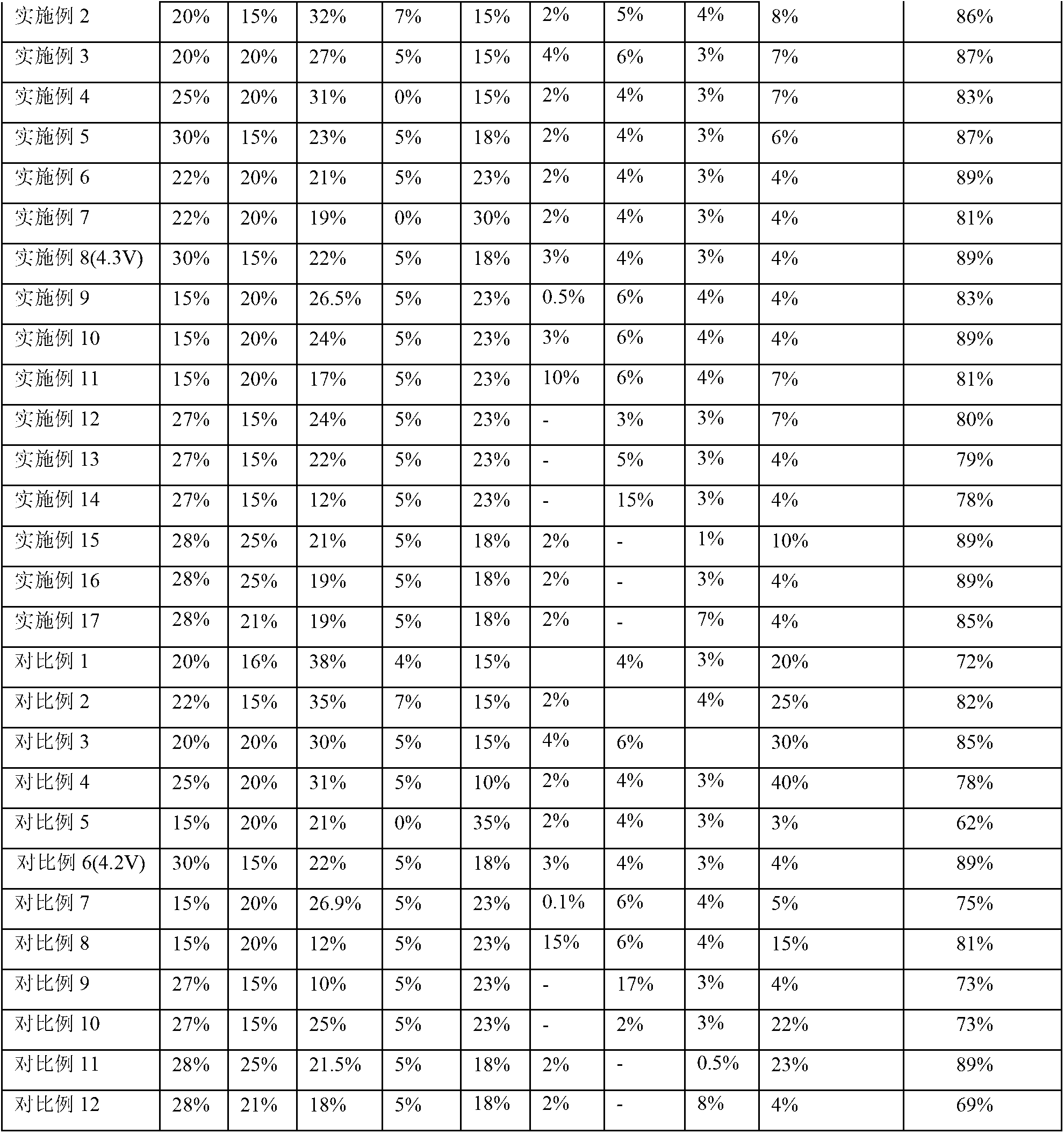
The invention provides a lithium-ion battery electrolyte, comprising a solvent and an additive, wherein the solvent comprises propyl propionate and two or three selected from a group consisting of vinyl carbonate, diethyl carbonate and propylene carbonate, and the additive comprises vinyl sulfate, fluoroethylene carbonate, adiponitrile, glycol bis(propionitrile) ether, 1,3-propanesultone and fluorobenzene. A lithium-ion battery prepared from the lithium-ion battery electrolyte has stable usability under the condition of 4.40 V or 4.45 V, has energy density of 750 Wh / L or above, cycle life of 800 times or above and a capacity retention ratio of 80% or above, so the service life of a novel high-voltage high-energy-density polymer lithium-ion battery is substantially improved.
Owner:LENOVO (BEIJING) CO LTD
Non-aqueous secondary battery having enhanced discharge capacity retention
InactiveUS6866966B2High discharge capacity retentionLead-acid accumulatorsOrganic electrolyte cells1,4-ButanediolHalogen
A discharge capacity retention of a non-aqueous secondary battery is enhanced by incorporating into its non-aqueous electrolytic solution a small amount of a substituted diphenyldisulfide derivative in which each of the diphenyl groups has a substituent such as alkoxy, alkenyloxy, alkynyloxy, cycloalkyloxy, aryloxy, acyloxy, alkanesulfonyloxy, arylsulfonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy, halogen, CF3, CCl3, or CBr3. Preferably, a small amount of methyl 2-propylcarbonate, 2-propynyl methanesulfonate, 1,3-propanesultone, divinylsulfone, 1,4-butanediol dimethanesulfonate or cyclohexylbenzene is further incorporated.
Owner:UBE IND LTD
Synthesis method for amphoteric ion modified starch for well drilling fluid
ActiveCN104926995AReduce usageAct as a solventSulfonic acids salts preparationDrilling compositionSynthesis methodsSolvent
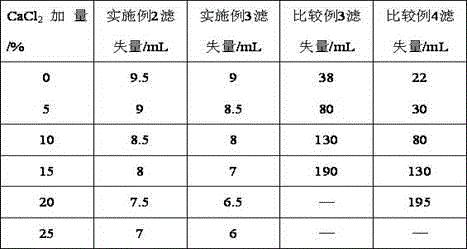
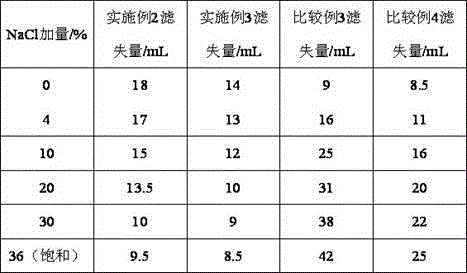
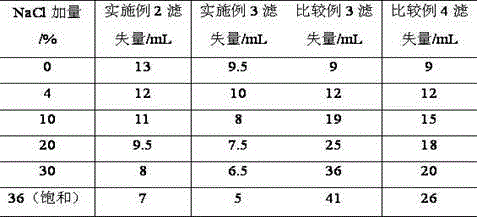
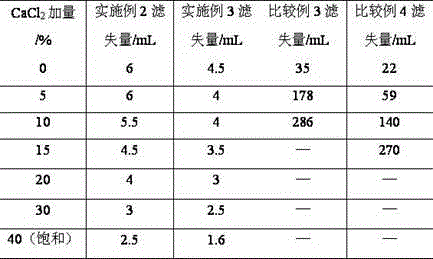
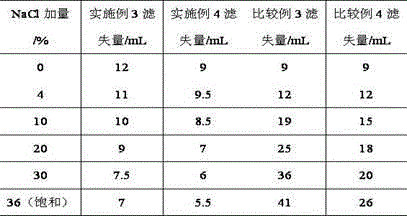
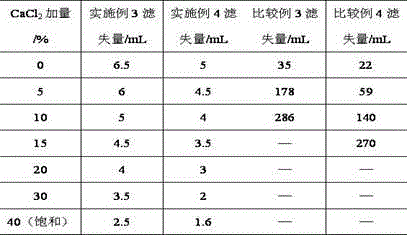
The invention provides a synthesis method for amphoteric ion modified starch for a well drilling fluid, wherein the synthesis method comprises the steps: weighing dimethylaminoethyl methacrylate and 1,3-propanesultone with the mass ratio of 2:1 to 9:1, adding 1,3-propanesultone to dimethylaminoethyl methacrylate, after reaction, filtering, extracting, and drying to obtain DMAPS; adding water to starch, stirring to obtain gelatinized starch, introducing N2, carrying out a reaction, and then adding an initiator; and dissolving acrylamide and DMAPS with a solvent, adding into the gelatinized starch after treatment, carrying out a reaction for 4-6 h at the temperature of 60-80 DEG C, to obtain a colloidal solid, cleaning with acetone to obtain a white precipitate, and finally drying and crushing to obtain the amphoteric ion modified starch. The modified starch obtained by the method can resist NaCl saturation and resist high content of CaCl2, has the filtrate loss reduction amount of a polymer decreased along with the increase of the salt content, and besides has the advantages of excellent shale inhibition performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
Electrolyte solution for negative lithium titanate battery, lithium ion battery and preparation method thereof
ActiveCN103117414AWide variety of sourcesEasy to operateFinal product manufactureElectrolyte accumulators manufactureSolventCarbonate
The invention discloses an electrolyte solution for a negative lithium titanate battery, a lithium ion battery and a preparation method thereof. The electrolyte solution is characterized in that lithium hexafluorophosphate is adopted as electrolyte, ethylene carbonate, methyl ethyl carbonate, diethyl carbonate and propylene carbonate are adopted as solvents, and one or more of fluoroethylene carbonate, double oxalate ithium borate, 1,3-propanesultone or vinylene carbonate are adopted as a film-formation additive.
Owner:CALB CO LTD +1
Preparation method for amphoteric ion modified starch for well drilling fluid
The invention provides a preparation method for amphoteric ion modified starch for a well drilling fluid, wherein the preparation method comprises the steps: weighing N,N-dimethylallylamine and 1,3-propanesultone with the molar ratio of 2:1 to 8:1, carrying out a reaction for 0.5-4 h at the temperature of 10-60 DEG C, filtering, extracting, and drying to obtain N,N-dimethylallyl propanesulfonate; adding water to starch, stirring to obtain gelatinized starch, introducing N2, carrying out a reaction, and then adding an initiator; and dissolving acrylamide and N,N-dimethylallyl propanesulfonate with a solvent, adding into the gelatinized starch after treatment, carrying out a reaction for 4-6 h at the temperature of 60-80 DEG C, to obtain a colloidal solid, cleaning with acetone to obtain a white precipitate, and finally drying and crushing to obtain the amphoteric ion modified starch. The modified starch obtained by the method can resist NaCl saturation and resist high content of CaCl2, has the filtrate loss reduction amount of a polymer decreased along with the increase of the salt content, and besides has the advantages of excellent shale inhibition performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
High-voltage lithium ion battery and electrolyte thereof
ActiveCN103078138AImprove high temperature storage performanceImprove securitySecondary cellsHigh temperature storageSolvent
The invention provides a high-voltage lithium ion battery and electrolyte thereof. The electrolyte of the high-voltage lithium ion battery comprises non-aqueous organic solvent and lithium salt which is dissolved in the non-aqueous organic solvent. To calculate by the total mass of the solvent and additives of the high-voltage lithium ion battery, the non-aqueous organic solvent comprises the following components mass percentage: 35-53 percent of cyclic carbonate, 17-39 percent of chain carbonate and 15-30 percent of fluorobenzene (FB). The electrolyte of the high-voltage lithium ion battery additionally comprises at least one of the following additives by mass percentages: 0.5-10 percent of vinylene carbonate (VC), 3-15 percent of fluoroethylene carbonate (FEC) and 1-7 percent of 1, 3-propanesultone (PS). The high-voltage lithium ion battery comprises a positive plate, a negative plate, a diaphragm which is arranged between the positive plate and the negative plate close to each other, and electrolyte which is the electrolyte of the high-voltage lithium ion battery. Therefore, gas which is produced by the battery during high-temperature storage under high voltage can be reduced, the high-temperature storage performance and the safety performance of the battery are improved and the circulating performance of the battery under the high voltage is improved.
Owner:NINGDE AMPEREX TECH
Synthesis method for amphoteric ion copolymer for well drilling fluid
ActiveCN104926986AReduce usageAct as a solventSulfonic acids salts preparationDrilling compositionSynthesis methodsWell drilling
The invention provides a synthesis method for an amphoteric ion copolymer for a well drilling fluid, wherein the synthesis method comprises the steps: weighing dimethylaminoethyl methacrylate and 1,3-propanesultone with the mass ratio of 2:1 to 9:1, adding 1,3-propanesultone to dimethylaminoethyl methacrylate, after reaction, filtering, extracting, and drying to obtain DMAPS; adding the obtained DMAPS and AM into a solvent, dissolving, then transferring into a reactor, next introducing N2, carrying out a deoxidization reaction, at the same time, heating up to 50-70 DEG C, then adding an initiator, carrying out a reaction to obtain a colloidal solid, and precipitating with acetone to obtain a white precipitate; and drying the obtained precipitate, and then crushing to finally obtain the amphoteric ion copolymer. The copolymer obtained by the method can sustain a temperature as high as 150 DEG C, resists NaCl saturation, resists CaCl2 saturation, has the filtrate loss reduction amount of a polymer decreased along with the increase of the salt content, and besides has the advantages of excellent shale inhibition performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
Non-aqueous electrolytic solution and lithium secondary battery using the same
ActiveUS7261975B2Improve battery performanceOrganic chemistryNon-aqueous electrolyte accumulatorsElectrolytic agentElectrical battery
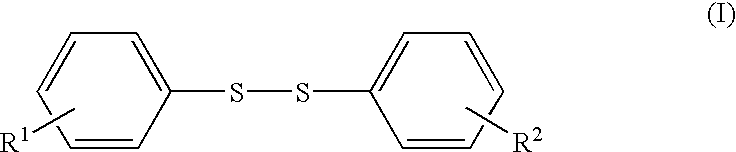
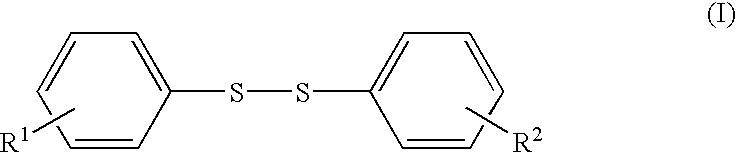
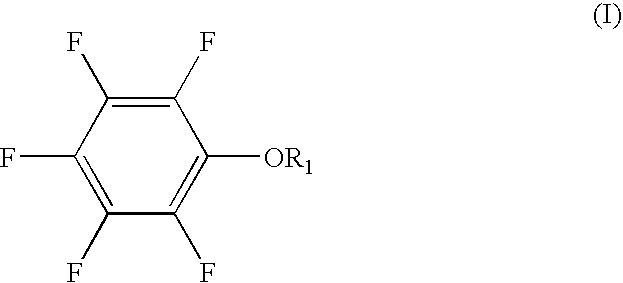
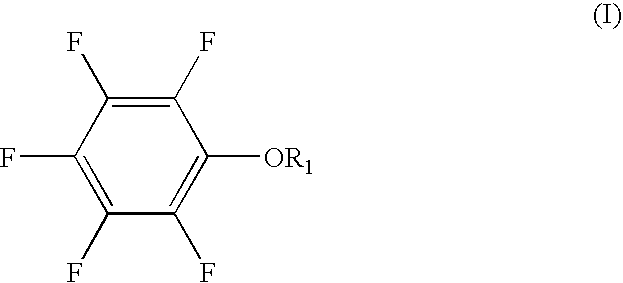
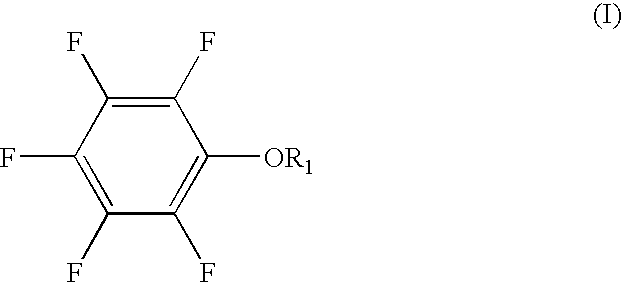
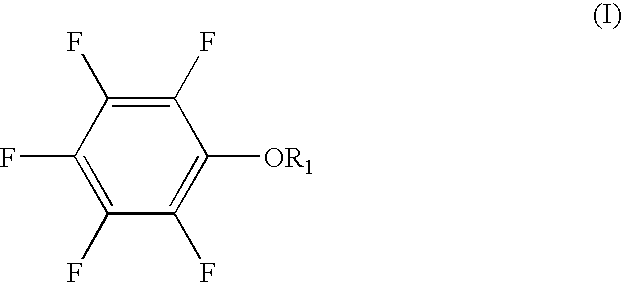
The present invention provides a lithium secondary battery which is improved particularly in cycle characteristics. Disclosed is a lithium secondary battery which uses a non-aqueous electrolytic solution obtained by dissolving electrolyte salt in a non-aqueous solvent. The non-aqueous electrolytic solution further contains a pentafluorophenyloxy compound represented by the formula (I), and vinylene carbonate and / or 1,3-propanesultone.In the formula (I), R1 is a substituent selected from the group consisting of an alkylcarbonyl group having 2 to 12 carbon atoms, an alkoxycarbonyl group having 2 to 12 carbon atoms, an aryloxycarbonyl group having 7 to 18 carbon atoms, and an alkanesulfonyl group having 1 to 12 carbon atoms. At least one hydrogen atom of the substituent can be substituted with a halogen atom or an aryl group having 6 to 18 carbon atoms.
Owner:MU IONIC SOLUTIONS CORP
Sulfur-containing electrolyte with film forming function for lithium ion battery as well as preparation method and application thereof
InactiveCN102231441ASuppression of co-embeddingImprove initial discharge capacitySecondary cellsSodium-ion batteryCharge discharge
The invention provides a sulfur-containing electrolyte with a film forming function for a lithium ion battery as well as a preparation method and application thereof. The sulfur-containing electrolyte with a film forming function is prepared by adding a functional additive to a common electrolyte, wherein the added function additive accounts for 1-5% of the common electrolyte; the common electrolyte contains a cyclic carbonate solvent, a linear carbonate solvent and conductive lithium salts; and the functional additive is one or two of 1,3-propanesultone or propenyl-1, 3-sultone. The functional additive has a higher reduction potential, can form a compact and stable SEI film in the initial charge process, thus restraining cyclic carbonate from co-intercalating towards a graphite electrode effectively, enlarging the application range of cyclic carbonate, improving the initial charge capacity, the cycle life and the high-low temperature property of the battery effectively. The sulfur-containing electrolyte with a film forming function can be used for preparing a lithium ion battery and the prepared lithium ion battery has good charge-discharge performance.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Non-aqueous electrolyte and lithium ion battery containing same
The present invention provides a non-aqueous electrolyte and a lithium ion battery containing the same, and belongs to the technical field of electrolyte research. The non-aqueous electrolyte comprises a non-aqueous organic solvent, a conductive lithium salt and an additive, the additive comprises based on the total weight of the electrolyte: 0.1-5% of an anode film formation additive, 0.1-10% ofa cathode film formation additive and 0.1-5% of a gas expansion inhibition additive, wherein the anode film formation additive comprises at least one of vinyl-vinylene carbonate, 4-Fluoro-1,3-dioxolan-2-one, Ethylene Sulfate, 1,3-propanesultone and vinylene carbonate; and the cathode film formation additive is at least one of nitriles, phosphate compounds and a boracic-like compound. The side reaction of the electrolyte and the electrode material in the storage and cyclic process of the battery is reduced, the battery gas expansion is inhibited so as to the storage and cycle lives of the battery are obviously improved.
Owner:ZHUHAI COSMX BATTERY CO LTD
Lithium-ion battery electrolyte
ActiveCN103413971AImprove high temperature performanceGood capacity retentionSecondary cellsOperabilityStructural formula
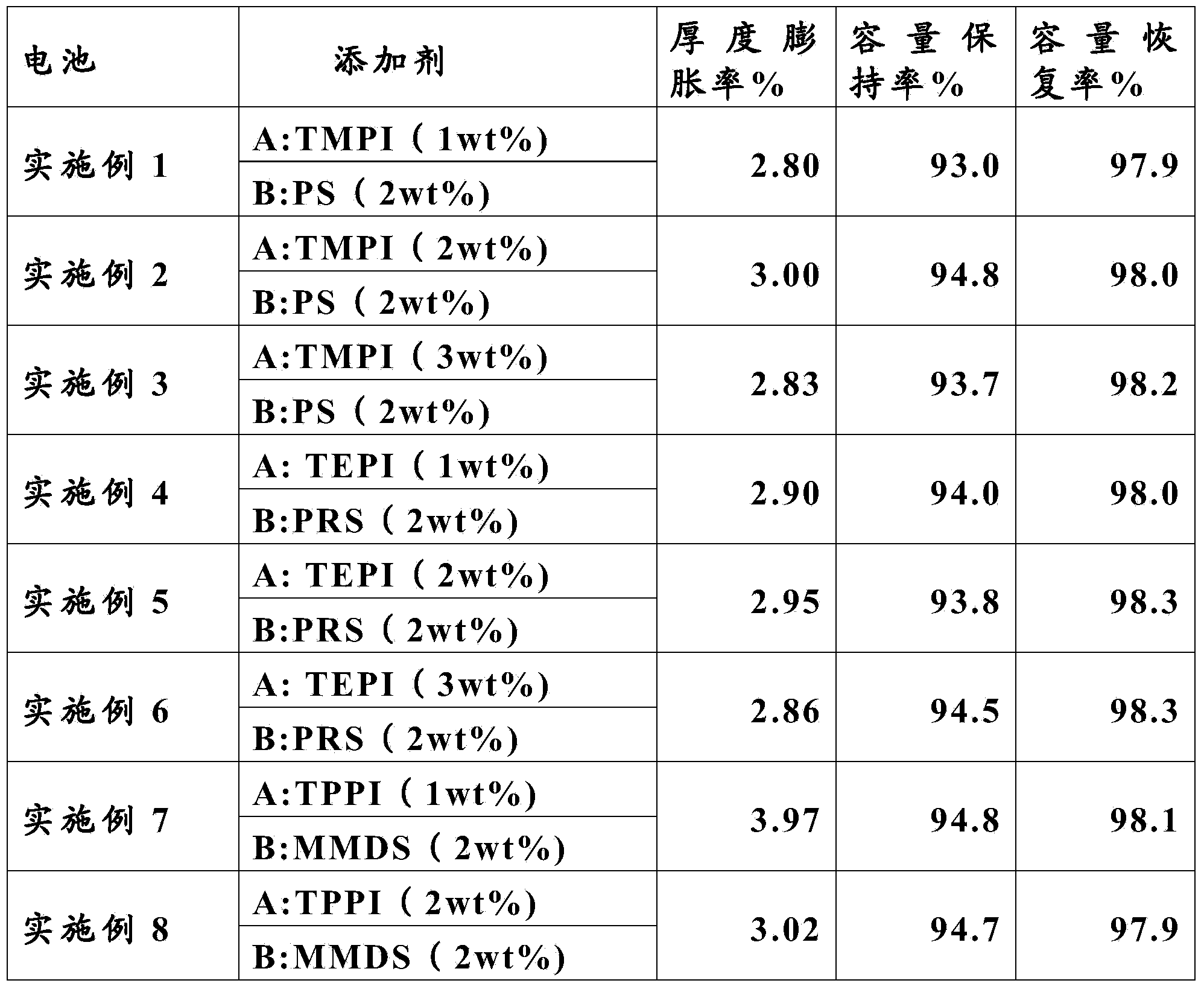
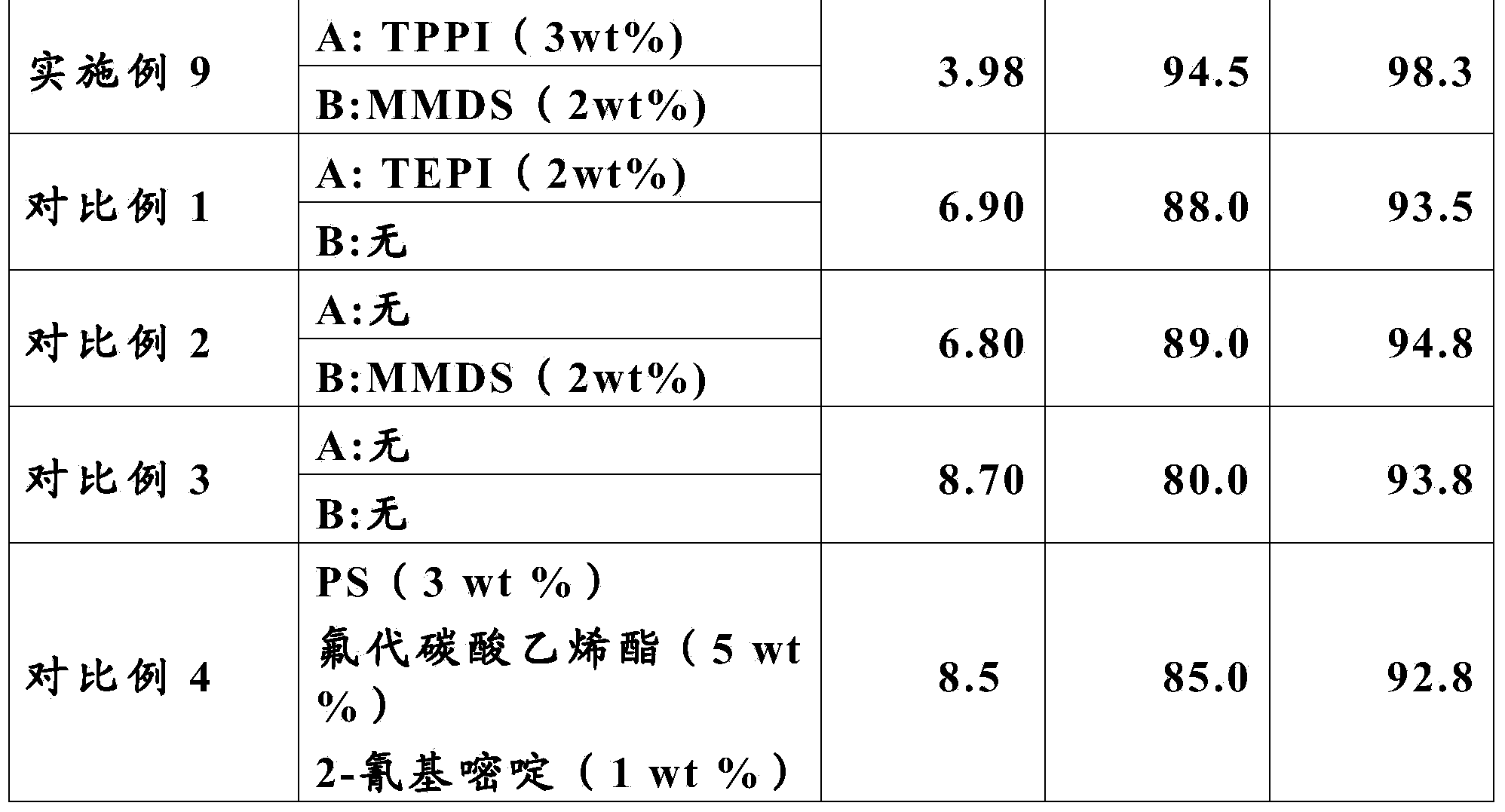
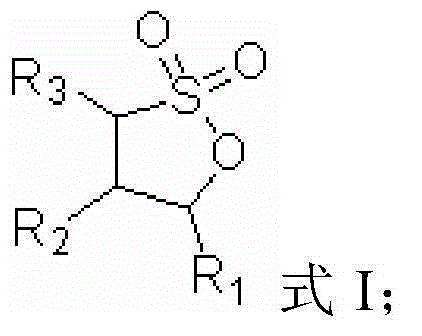
The invention relates to a lithium-ion battery electrolyte. The lithium-ion battery electrolyte consists of a lithium salt, a solvent and additives, wherein the additives consist of an additive A and an additive B, the additive A accounts for 0.01-10wt% of the electrolyte, the additive B accounts for 0.01-8wt% of the electrolyte. The structural formula of the additive A is shown in the specification, wherein R1, R2, R3 are independent C1-C3 alkyl, and R4 is an alkyl with a C1-C5 linear chain or branched chain; the additive B is selected from one or more of 1,3-propanesultone, propenyl-1,3-sulfonic lactone and methylene methanedisulfonate. The lithium-ion battery electrolyte provided by the invention has good circulation at a high temperature of 85 DEG C, ensures that a battery also has good capacity retention ratio when being stored at a high temperature, fully meets the application requirement of a lithium-ion battery in certain new fields, and is strong in operability and low in cost.
Owner:轻工业化学电源研究所 +1
Lithium ion battery and electrolyte thereof
InactiveCN103682440AEasy to storeImprove high temperature storage characteristicsSecondary cellsOrganic electrolytesOrganic solventDecomposition
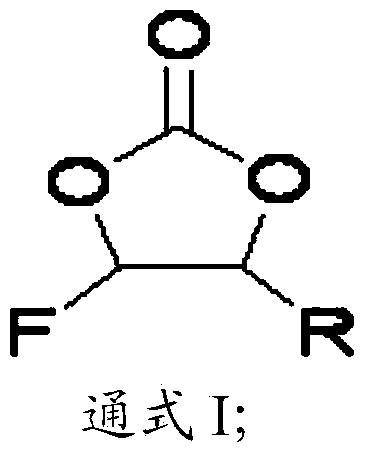
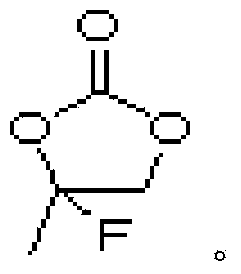
The invention discloses a lithium ion battery and an electrolyte thereof. The electrolyte comprises a nonaqueous organic solvent, a lithium salt dissolved in the nonaqueous organic solvent, and an additive dispersed in the nonaqueous organic solvent, and the additive is composed of 1,3-propanesultone, 4-fluorine-5-alkyl substituted-1,3-dioxolame-2-one represented by a general formula I, wherein R in the formula I is an alkyl group containing 1-5 carbon atoms; the lithium ion battery adopts the electrolyte as an electrolyte. Because the compound represented by the general formula 1 is promoted to form a solid electrolyte interfacial film by virtue of 1,3-propanesultone, so as to effectively restrain the decomposition reaction of the electrolyte, so that the high-temperature memory property of the lithium ion battery can be improved obviously.
Owner:NINGDE AMPEREX TECH +1
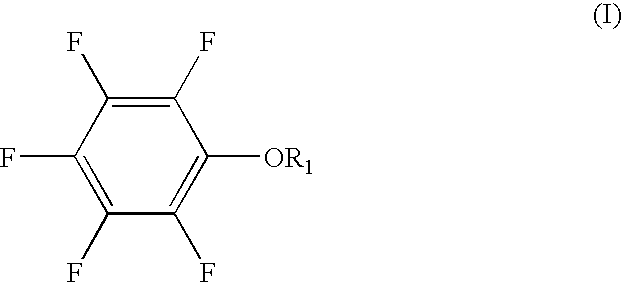
Lithium ion secondary battery and electrolyte thereof
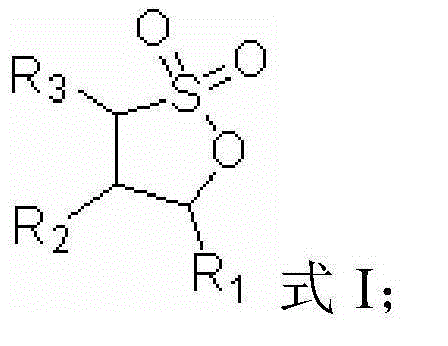
ActiveCN105006593AImprove low temperature discharge performanceImprove high temperature cycle performanceSecondary cellsOrganic solventPhysical chemistry
The present invention provides a lithium ion secondary battery and an electrolyte thereof. The lithium ion secondary battery electrolyte comprises a non-aqueous organic solvent, a lithium salt dissolved in the non-aqueous organic solvent, and an additive dissolved in the non-aqueous organic solvent, wherein the additive is vinylene carbonate (VC), a lithium-containing compound of sulfonimide LiN(CxF2x+1SO2)fluoro(CyF2y+1SO2) (wherein x and y are positive integers), and fluoro 1,3-propanesultone having a structure represented by a formula I, R1, R2 and R3 are respectively and independently selected from fluorine atom, the mass of the vinylene carbonate (VC) is 0.1-2% of the mass of the non-aqueous organic solvent, the concentration of the lithium-containing compound of sulfonimide LiN(CxF2x+1SO2)fluoro(CyF2y+1SO2) is 0.02-0.2 M, and the mass of the fluoro 1,3-propanesultone is 0.5-5% of the mass of the non-aqueous organic solvent. The lithium ion secondary battery comprises the electrolyte. With the Lithium ion secondary battery electrolyte of the present invention, the low temperature discharge performance and the high temperature cycle performance of the lithium ion secondary battery can be improved. The formula I is defined in the specification.
Owner:CONTEMPORARY AMPEREX TECH CO
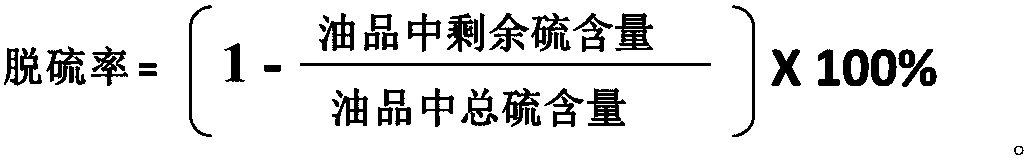
Method for catalyzing fuel oil desulfurization by coupling of sulfonic heteropolyacid salt with eutectic solvent
InactiveCN108179021AEasy to synthesizeHigh recovery rateRefining with oxygen compoundsHydrocarbon oils treatment productsRoom temperatureFuel oil
The invention belongs to the technical field of desulfurization, and relates to fuel oil desulfurization, in particular to a method for catalyzing fuel oil desulfurization by coupling of a sulfonic heteropolyacid salt with a eutectic solvent. According to the method, a sulfonic heteropolyacid salt catalyst and a choline eutectic solvent are added to fuel oil and uniformly stirred, then an oxidantaqueous hydrogen peroxide solution is added, and continuous stirring and sufficient reaction are performed, wherein the sulfonic heteropolyacid salt is prepared from 1,3-propanesultone and triethylamine by mixing, and the eutectic solvent is prepared from a quaternary ammonium salt and a hydrogen bond donor by mixing. The method adopts mild reaction conditions, can be exerted at room temperature under normal pressure and is simple to operate. After the reaction, the eutectic solvent and an oil product form two phases, the oil product can be separated by simple pouring, and the method is harmless to human and environment. The prepared catalyst and eutectic solvent can be recycled directly and also be used after regeneration. The highest oil product recovery rate can reach 100%, and the method is green.
Owner:JIANGSU UNIV
Polyether sulfonate anionic surfactant, and preparation method and application thereof
ActiveCN106519212ASimple processImprove applicabilityTransportation and packagingSulfonic acids salts preparationSulfonateSolvent free
The invention discloses a polyether sulfonate anionic surfactant, and a preparation method and an application thereof. The preparation method comprises the following steps: 1, placing dehydrated polyether, a catalyst and alkali metal hydroxide in a reaction kettle, and carrying out a reaction at 30-60 DEG C for 0.2-0.8 h to obtain polyether alkali metal salt; and 2, controlling the temperature in the kettle, continuously adding molten 1,3-propanesultone into the kettle, and carrying out a reaction at 30-60 DEG C for 0.8-1.5 h to obtain the polyether sulfonate anionic surfactant. The preparation method for synthesizing the anionic surfactant through ring opening of 1,3-propanesultone under a solvent-free condition under the catalysis of the catalyst has the advantages of simple process, environmental protection, high conversion rate, and realization of stable performances of the above product.
Owner:WUHAN OXIRAN SPECIALTY CHEM CO
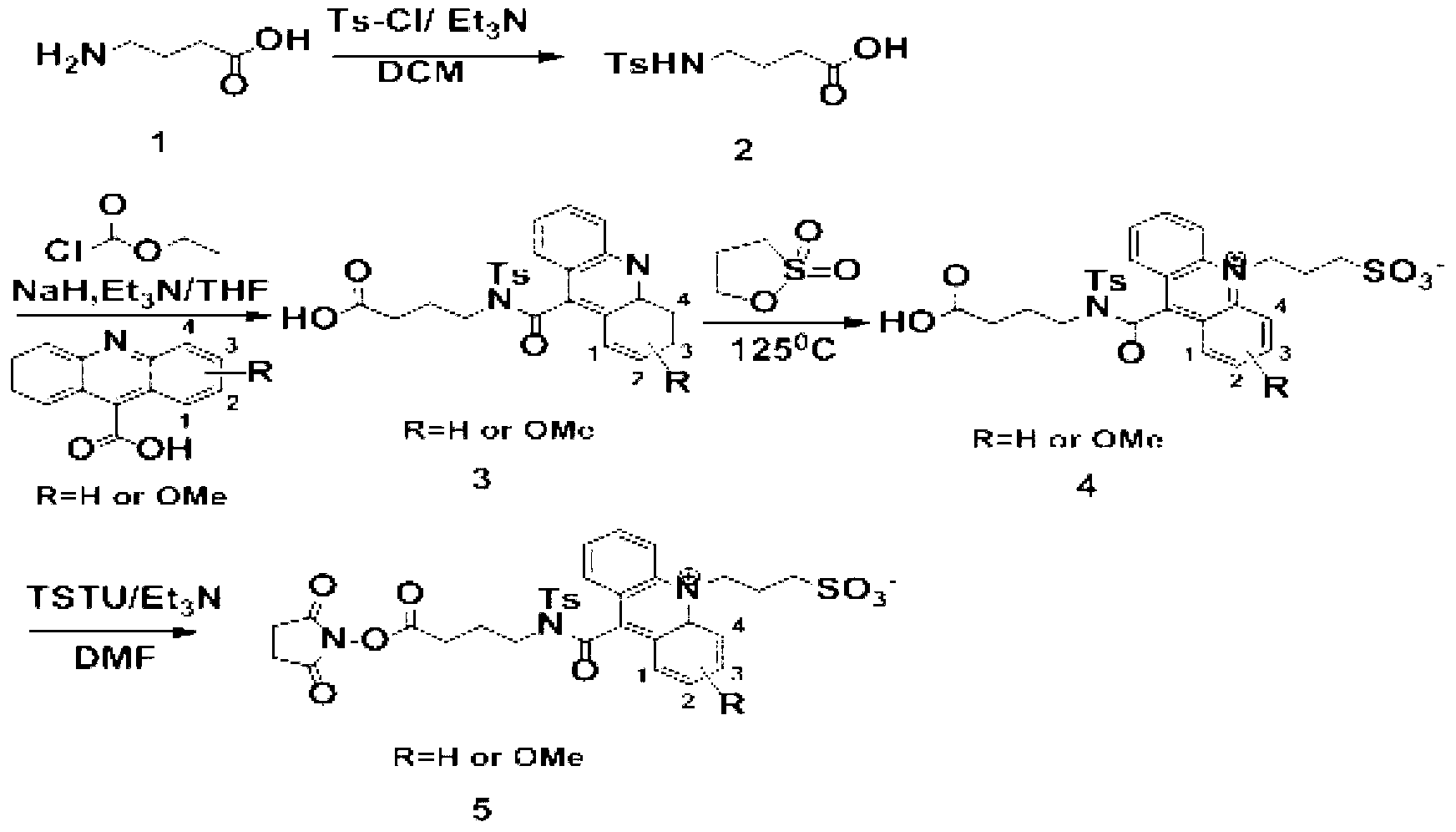
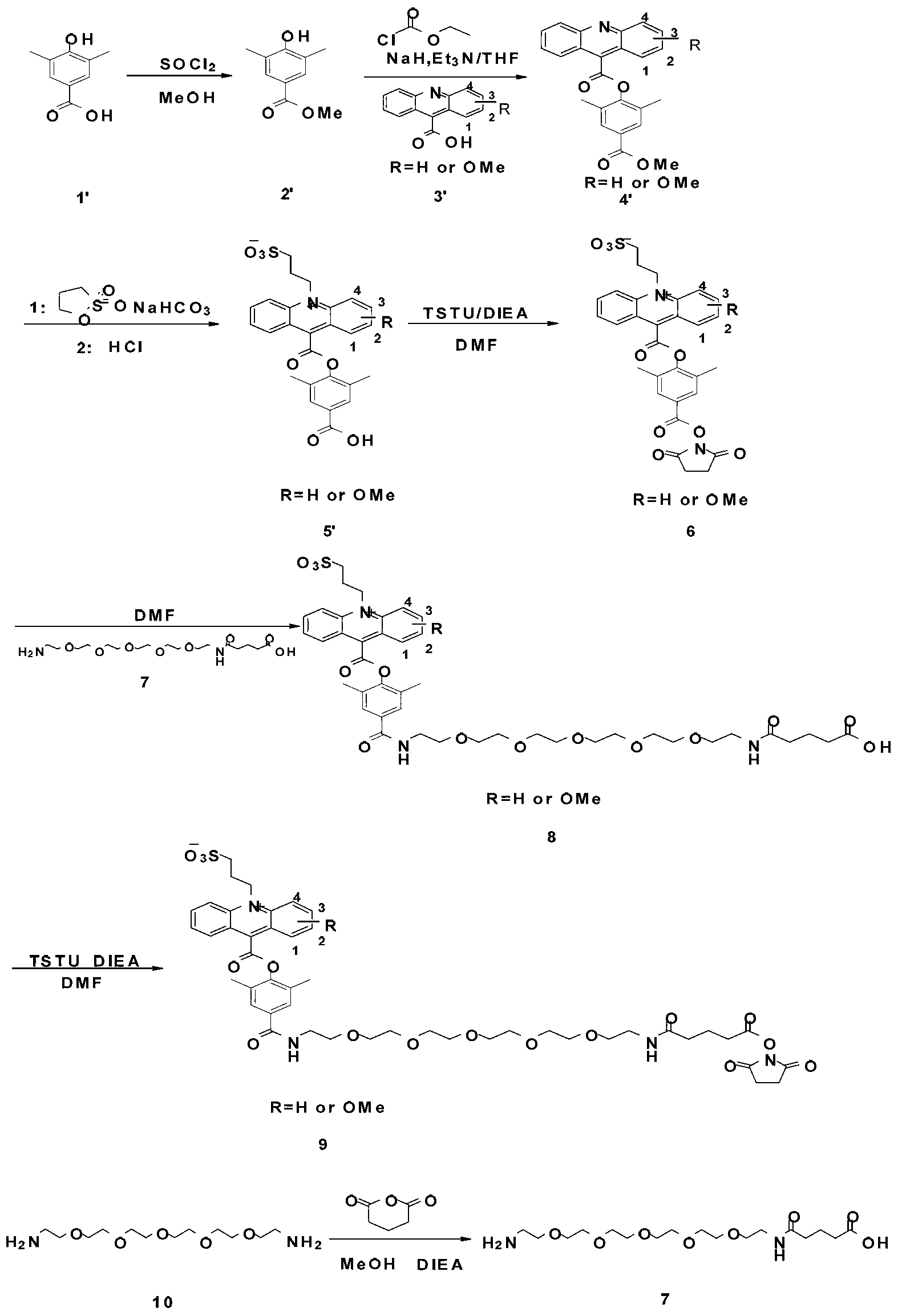
Preparation method for a series of acridine chemiluminescence agents
The invention provides a preparation method for a series of acridine chemiluminescence agents. The preparation method comprises the following steps: 1, dissolving 4-aminobutyric acid in a first solvent, stirring, cooling, sequentially adding triethylamine and p-toluenesulfonyl chloride, and carrying out pressure reduction solvent removing to obtain a product (a compound 2); 2, placing 9-acridinecarboxylic acid in a round bottom flask, adding a second solvent to dissolve the 9-acridinecarboxylic acid, stirring, cooling, sequentially adding a third solvent, carrying out a reaction, filtering the obtained solid, collecting the filtrate to obtain a filtrate A, dissolving the compound 2 with the second solvent, cooling, slowly adding an inorganic alkali in batches, carrying out a reaction, slowly adding the filtrate A to the reaction system in a dropwise manner, heating the reaction system to a room temperature, and carrying out the reaction overnight; and 3, placing a compound 3 in a flask, adding 1,3-propanesultone, stirring, carrying out a heating reaction, cooling to a room temperature, washing, filtering, and collecting the solid to obtain a compound 4 crude product. With the method, characteristics of simple process, green environmental protection, and low cost are provided.
Owner:SHENZHEN MAXCHEMTECH
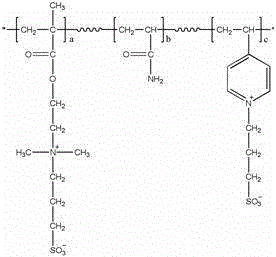
Aporate amphion switching biology isolate medium as well as its preparing method and usage
InactiveCN101157059AIrreversible adsorption is smallUnique craftCation exchanger materialsOrganic anion exchangersMicrosphereIon exchange
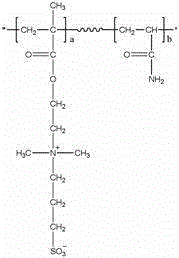
The invention relates to a non-porous amphoteric ion exchange biological separation medium. The structural formula is shown in figure, and the non-porous amphoteric ion exchange biological separation medium has a preparation method that 40 to 60 ML 1.0 mol / L of diluted HCl is added into 4.0 to 6.0 g non-porous monodisperse P (GMA / EDMA) resin, which is reacted for 2 to 3 hours in the constant temperature at 30 to 40 DEG C after being supersonicly dispersed for 10 to 15 minutes, filtered, and repeatedly cleaned with distilled water to be neutral; the water solution of 30 to 50 ML dimethylamine is further added into the resultant, which is reacted for 20 to 24 hours in the constant temperature at 60 to 70 DEG C; the resultant is repeatedly cleaned with a great amount of water and acetone, and performed the vacuum drying, thereby obtaining ammoniation microspheres; the microspheres after ammoniation are laid in acetonitrile of 120 to 150 ML, 3 to 5 g 1,3-Propanesultone is added, the return flow agitation is performed for 20 to 24 hours at 80 to 90 DEG C after the ultrasonic dispersion, and the resultant is cleaned with a great amount of acetone and ethyl alcohol, and performed the vacuum drying, thereby obtaining the non-porous amphoteric ion exchange biological separation medium. The invention takes 3.0 micron non-porous monodisperse porous poly-glycidylmethacrylate-co-ethylene dimethacrylate resin as a matrix, the adoption of a new chemical modified method prepares novel protein which can simultaneously separate the acidity and the alkality, the protein has the advantages of easy regeneration, strong acid and strong base resistance, high mechanical strength, rapid separation velocity, and high protein quality and activity recovery ratio, and the protein can be widely used for rapid separation and purification of gene engineering products and the protein.
Owner:NINGXIA UNIVERSITY
Lithium secondary cell and its nonaqueous electrolyte
InactiveUS7781106B2Non-aqueous electrolyte accumulatorsOrganic electrolyte cellsElectric capacityGraphite
Owner:UBE IND LTD
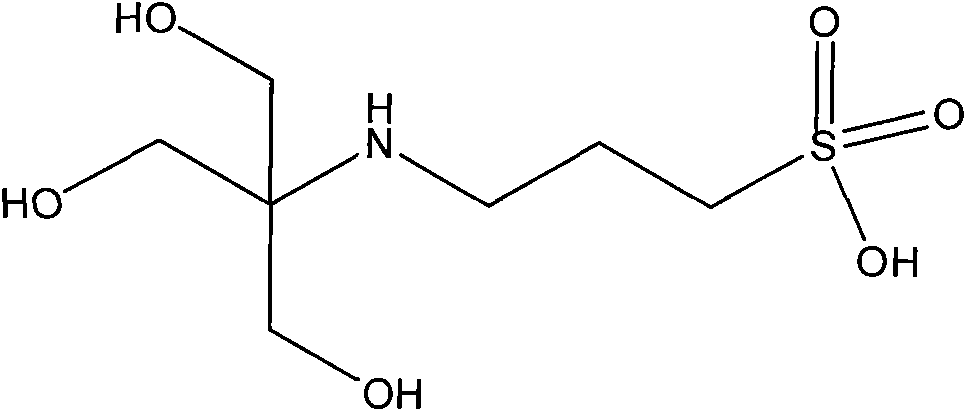
N-tris (hydroxymethyl) methyl-3-aminopropanesulfonic acid compound and preparation method thereof
InactiveCN101845003AHigh purityHigh yieldSulfonic acid preparation3-aminopropanesulfonic acidSolvent
The invention relates to an N-tris (hydroxymethyl) methyl-3-aminopropanesulfonic acid compound and a preparation method thereof. The N-tris (hydroxymethyl) methyl-3-aminopropanesulfonic acid compound is prepared by the following steps of putting tris (hydroxymethyl) aminomethane and 1,3-propanesultone with the same molar ratio into a 500mL four-hole flask with a stirring paddle, a thermometer and a condensation pipe, adding 250-300mL of C1-C8 alcohol as a solvent, stirring and dissolving the mixture with a proper speed, heating a reaction system to boiling, and treating the alcohol by condensing reflux; reacting for 1-8h; putting a reaction product into a refrigerator, treating the product by cooling to 0 DEG C and filtering, wherein a filter cake is an obtained crude product; obtaining a white powder final product by washing the crude product by absolute ethyl alcohol for 2-3 times, and obtaining the N-tris (hydroxymethyl) methyl-3-aminopropanesulfonic acid compound by putting the final product into an oven and drying the product for 20-28h under the temperature of 70-120 DEG C. The invention has the advantages of little synthesis steps and simple operation and has the product yield of 75 percent and the product purity no less than 99 percent.
Owner:EAST CHINA UNIV OF SCI & TECH
Non-aqueous electrolyte and non-aqueous electrolyte secondary battery
InactiveUS7132199B2Excellent characteristicsRaise the ratioNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsPhysical chemistryPropylene carbonate
A non-aqueous electrolyte containing propylene carbonate and 1,3-propanesultone as additives can reduce the amount of a gas evolved during storage at a high temperature of a non-aqueous electrolyte secondary cell comprising the electrolyte, a non-aqueous electrolyte containing at least one compound selected from the group consisting of vinylene carbonate, diphenyl disulfide, di-p-tolydisulfide and bis(4-methoxyphenyl)disulfide as comprising the electrolyte, and a non-aqueous electrolyte containing a combination of the above-two types of additives can provide a non-aqueous electrolyte secondary cell exhibiting excellent retention of capacity and storage stability.
Owner:PANASONIC CORP +1
Novel sulfonic acid functionalized rhenium ionic liquid and preparation method and application thereof
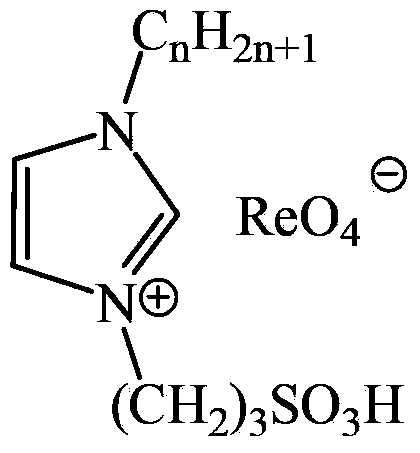
ActiveCN103450088AHigh catalytic activityImprove stabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsRheniumPerformance index
The invention relates to novel sulfonic acid functionalized rhenium ionic liquid and a preparation method and application of the sulfonic acid functionalized rhenium ionic liquid. Alkyl imidazole reacts with 1,3-propane sulfonic acid lactone to generate ionic liquid intermediates A, then the ionic liquid intermediates A are acidized to obtain intermediates B, and the acidized ionic liquid intermediates B react with silver perrhenate to obtain the product of the sulfonic acid functionalized rhenium ionic liquid. The ionic liquid is used for FCC gasoline oxidation sweetening, an oil phase is extracted after the reaction, the content of sulphur is measured to be lower than 10ppm, other performance indexes of oils are not obviously changed, and a Euro V standard is basically met.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Preparation method of electronic grade 1, 3-propanesultone
InactiveCN104177326AQuality improvementIndicator qualifiedOrganic chemistryMolecular sieveDistillation
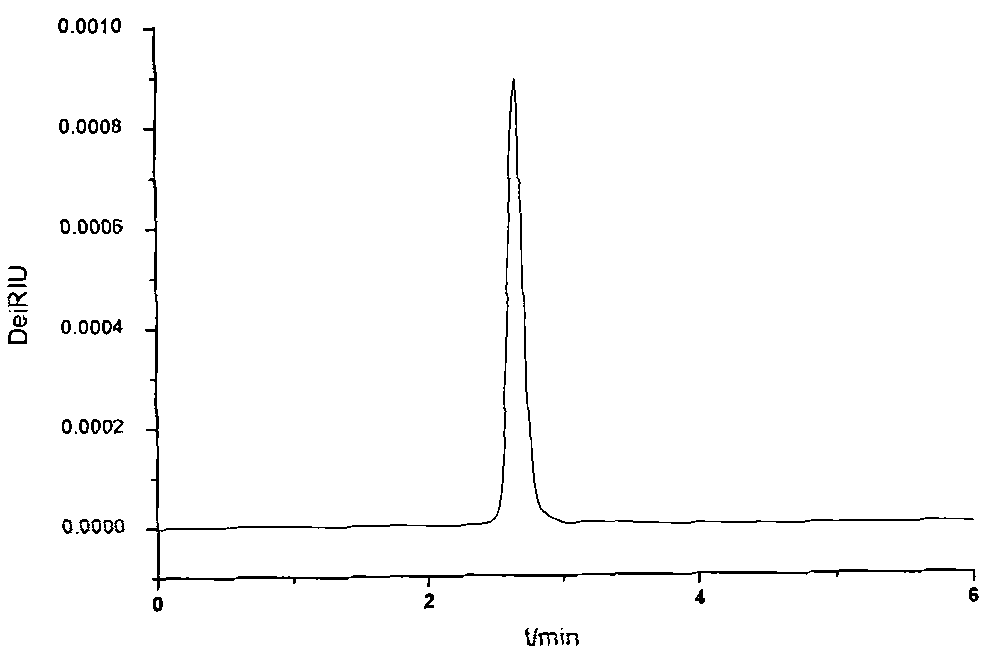
The invention discloses a preparation method of electronic grade 1, 3-propanesultone. The preparation method comprises the following steps: a) firstly, putting 1, 3-propanesultone into a reaction kettle provided with a molecular sieve, and standing for 1-10 hours at 30-40 DEG C to obtain 1, 3-propanesultone with the acid value of 0-50ppm and the water mass content of 0-100ppm; b) after filtering, carrying out reduced-pressure distillation, wherein the distillation temperature is 110-130 DEG C and the distillation pressure is (-0.5)-(-0.1)MP; and c) finally, continuously passing through a glass column of the molecular sieve to obtain the 1, 3-propanesultone with the acid value of 0-50ppm, the water content of 0-100ppm and the content of sodium ions of 0-20ppm. The preparation method disclosed by the invention is simple and convenient to operate and low in cost, and the indexes of the prepared electronic grade 1, 3-propanesultone are qualified. The prepared electronic grade 1, 3-propanesultone is stable in quality.
Owner:武汉松石科技股份有限公司
Non-aqueous electrolytic solution and lithium secondary battery using the same
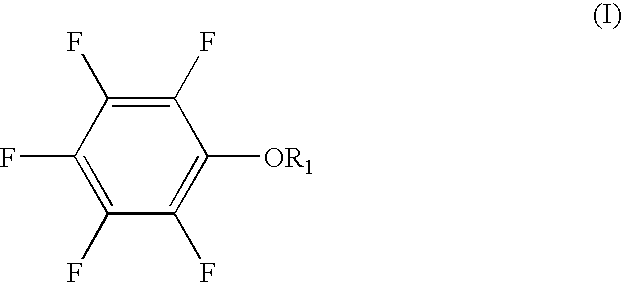
ActiveUS20070054185A1Improve battery performanceExcellent characteristicsOrganic chemistryNon-aqueous electrolyte accumulatorsTO-18Aryl
The present invention provides a lithium secondary battery which is improved particularly in cycle characteristics. Disclosed is a lithium secondary battery which uses a non-aqueous electrolytic solution obtained by dissolving electrolyte salt in a non-aqueous solvent. The non-aqueous electrolytic solution further contains a pentafluorophenyloxy compound represented by the formula (I), and vinylene carbonate and / or 1,3-propanesultone. In the formula (I), R1 is a substituent selected from the group consisting of an alkylcarbonyl group having 2 to 12 carbon atoms, an alkoxycarbonyl group having 2 to 12 carbon atoms, an aryloxycarbonyl group having 7 to 18 carbon atoms, and an alkanesulfonyl group having 1 to 12 carbon atoms. At least one hydrogen atom of the substituent can be substituted with a halogen atom or an aryl group having 6 to 18 carbon atoms.
Owner:MU IONIC SOLUTIONS CORP
Method for purifying 1,3-propanesultone
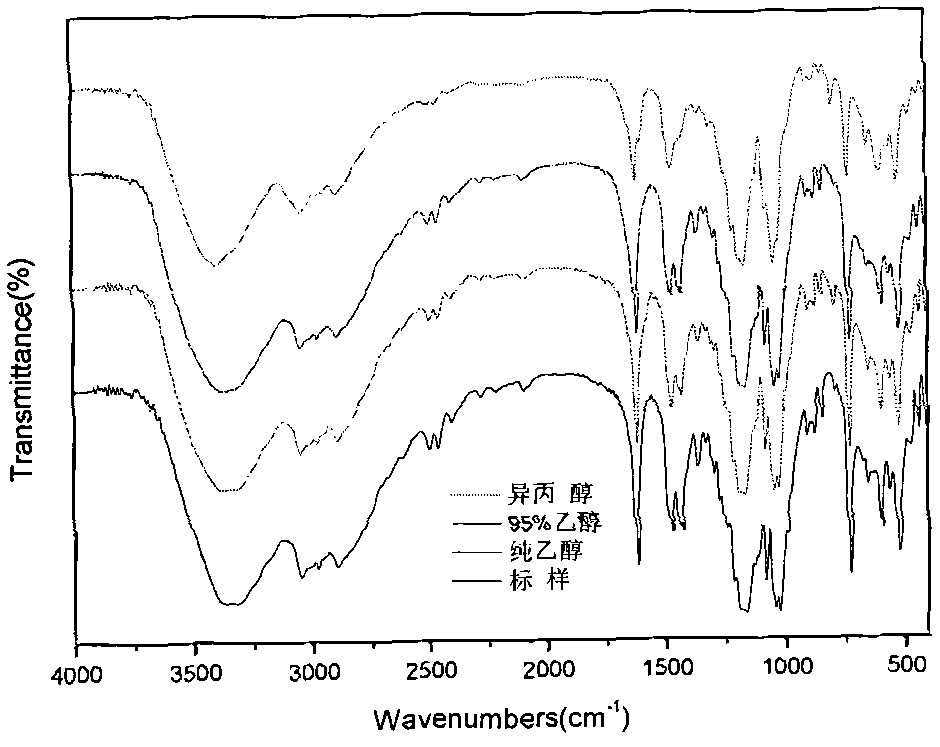
The invention provides a method for purifying 1,3-propanesultone. The method comprises the following steps: S1, providing crude 1,3-propanesultone; and 2, purifying the crude 1,3-propanesultone: cooling and crystallizing the crude 1,3-propanesultone to obtain crystals and a raffinate, separating out the crystals, heating the crystals, and separating the obtained solid substance to obtain the finished 1,3-propanesultone. The method for purifying the 1,3-propanesultone has the advantages of short time, high purity and wide application values.
Owner:HUIZHOU CAPCHEM CHEM CO LTD
Preparation method for amphoteric ion copolymer for well drilling fluid
The invention provides a preparation method for an amphoteric ion copolymer for a well drilling fluid, wherein the preparation method comprises the steps: weighing dimethylaminoethyl methacrylate and 1,3-propanesultone with the mass ratio of 2:1 to 9:1, adding 1,3-propanesultone to dimethylaminoethyl methacrylate, after reaction, filtering, extracting, and drying to obtain DMAPS; respectively weighing DMAPS, AM and VPPS in proportion, adding into a solvent, dissolving, then transferring into a reactor, then introducing N2, carrying out a deoxidization reaction, next adding an initiator, carrying out a reaction to obtain a colloidal solid, and precipitating with acetone to obtain a white precipitate; and drying the obtained precipitate, and then crushing to finally obtain the amphoteric ion copolymer. The copolymer obtained by the method can sustain a temperature as high as 180 DEG C, resists NaCl saturation, resists CaCl2 saturation, has the filtrate loss reduction amount of a polymer decreased along with the increase of the salt content, and besides has the advantages of excellent shale inhibition performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
A lithium battery
InactiveCN106058153AImprove high temperature resistanceImprove low temperature performanceCell electrodesFinal product manufactureAdhesiveEngineering
A lithium battery is disclosed. The lithium battery comprises a cathode sheet, an anode sheet, a separating membrane, an electrolyte and an outer package. The cathode sheet comprises a cathode current collector and a cathode material with which the cathode current collector is coated, and the anode sheet comprises an anode current collector and an anode material with which the anode current collector is coated. The cathode material comprises a cathode active compound, a cathode conductive agent and a cathode adhesive. The anode material comprises an anode active compound, an anode conductive agent, a thickening agent and an anode adhesive. The lithium battery has a high specific capacity and high stability. The energy density of a battery core assembled by the cathode having a high specific capacity and the lithium titanate anode in match can be 90 Wh / kg, and is obviously higher than the energy density of lithium titanate batteries at present. High-temperature resistance of the electrolyte is improved. A gas generating amount is reduced. Cyclic properties are good. The service lifetime is prolonged. High and low temperature properties and flame retardance of the electrolyte are improved. Wettability and pouring performance of the electrolyte are improved through adding 1,3-propanesultone.
Owner:YUNNAN ENERGY RES INST CO LTD
Method for preparing self-curing ionic liquid
InactiveCN109174179AEasy to operateProcess environmental protectionFatty acid esterificationOrganic-compounds/hydrides/coordination-complexes catalystsBiodieselSelf curing
The invention discloses a method for preparing a self-curing ionic liquid. The preparation method comprises the following steps: reacting polyethyleneimine serving as a matrix with 1,3-propane sultoneor 1,4-butyl sultone by a one-step method, and performing suction filtering, washing and drying to prepare an ionic liquid product, wherein the molar ratio of the polyethyleneimine to the 1,3-propanesultone or 1,4-butyl sultone is 1:1-1:10. The method is simple in process and low in cost, the self-curing ionic liquid which serves as a heterogeneous catalyst and is applied to biodiesel productionby ester exchange has excellent catalytic activity and repeated use stability, can be reclaimed conveniently after reaction, and has a large-scale application prospect.
Owner:FUZHOU UNIV
Sulfonate type and sulfonate inner salt type fluorocarbon surfactant as well as preparation and applications thereof
InactiveCN103240034AEasy to degradeSimple preparation processTransportation and packagingSulfonic acids salts preparationChemical industryPapermaking
The invention relates to a sulfonate type and sulfonate inner salt type fluorocarbon surfactant as well as a preparation method and applications thereof. The structure formula of the fluorocarbon surfactant is shown in the specification. The preparation method comprises the steps of: with hexafluoropropylene oxide polymer as a raw material, performing amidation on the raw material and 4-nitroaniline to obtain a product, carrying out nitroreduction on the product, and performing an open-loop alkylation reaction on the product subjected to nitroreduction and 1,3-propanesultone to obtain the sulfonate type and sulfonate inner salt type fluorocarbon surfactant. The sulfonate type and sulfonate inner salt type fluorocarbon surfactant is applied in the fields of textiles, chemical industry, papermaking, oil fields, leather and firefighting. The synthesized sulfonate type and sulfonate inner salt type fluorocarbon surfactant is more easily degraded by replacing the traditional perfluoroalkyl chain with a perfluoropolyether chain, belongs to novel environment-friendly fluorocarbon surfactants, and can be widely applied in multiple fields; and the sulfonate type and sulfonate inner salt type fluorocarbon surfactant is simple in preparation technique, and low in cost and has good application prospects.
Owner:DONGHUA UNIV +1
Electrolyte for storage battery
InactiveCN105958119AImprove performanceImprove stabilitySecondary cellsOrganic electrolytesHigh temperature storageSolvent molecule
The invention provides electrolyte for a storage battery. The electrolyte comprises lithium salt, a solvent and an additive. The additive is one of or the combination of many kinds of vinylene carbonate, 1,3-propanesulfonate, 1,3-propanesultone, glycol sulfite, dimethyl sulfite and lithium bis(oxalate)borate. The usage amount of the additive is 0.5%-5% of the total mass of the lithium salt and the organic solvent. According to the electrolyte, the additive forms a passivation layer on the surface of a graphene-based lithium titanate electrode when the battery is charged for the first time. The passivation layer coats on the surface of an electrode material stably. The solvent molecules are effectively prevented from passing through and prolapsing from the material through adoption of the formed passivation film, therefore, the flatulence phenomenon resulting from side reaction of the electrolyte and the graphene-based lithium titanate is prevented, while the Li<+> can freely intercalate and prolapse through the passivation layer. Through adoption of the method, the stability of the graphene-based lithium titanate battery in the circulation and high temperature storage processes can be greatly improved, and the performance of the battery can be greatly improved.
Owner:天津普兰能源科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com