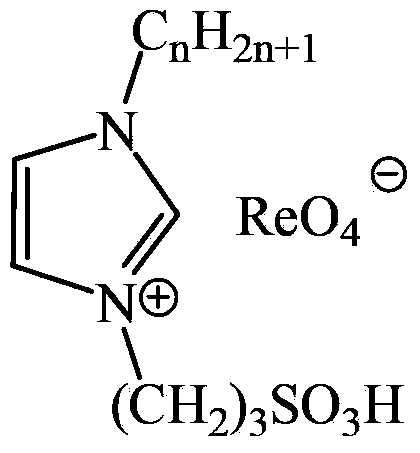
Novel sulfonic acid functionalized rhenium ionic liquid and preparation method and application thereof
A technology of sulfonic acid functionalization and ionic liquid, applied in chemical instruments and methods, refining with oxygen-containing compounds, organic chemistry, etc., can solve the problems of increasing the cost of desulfurization agent, poor effect, catalyst deactivation, etc., and achieve recovery Problems, rich variety, high stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
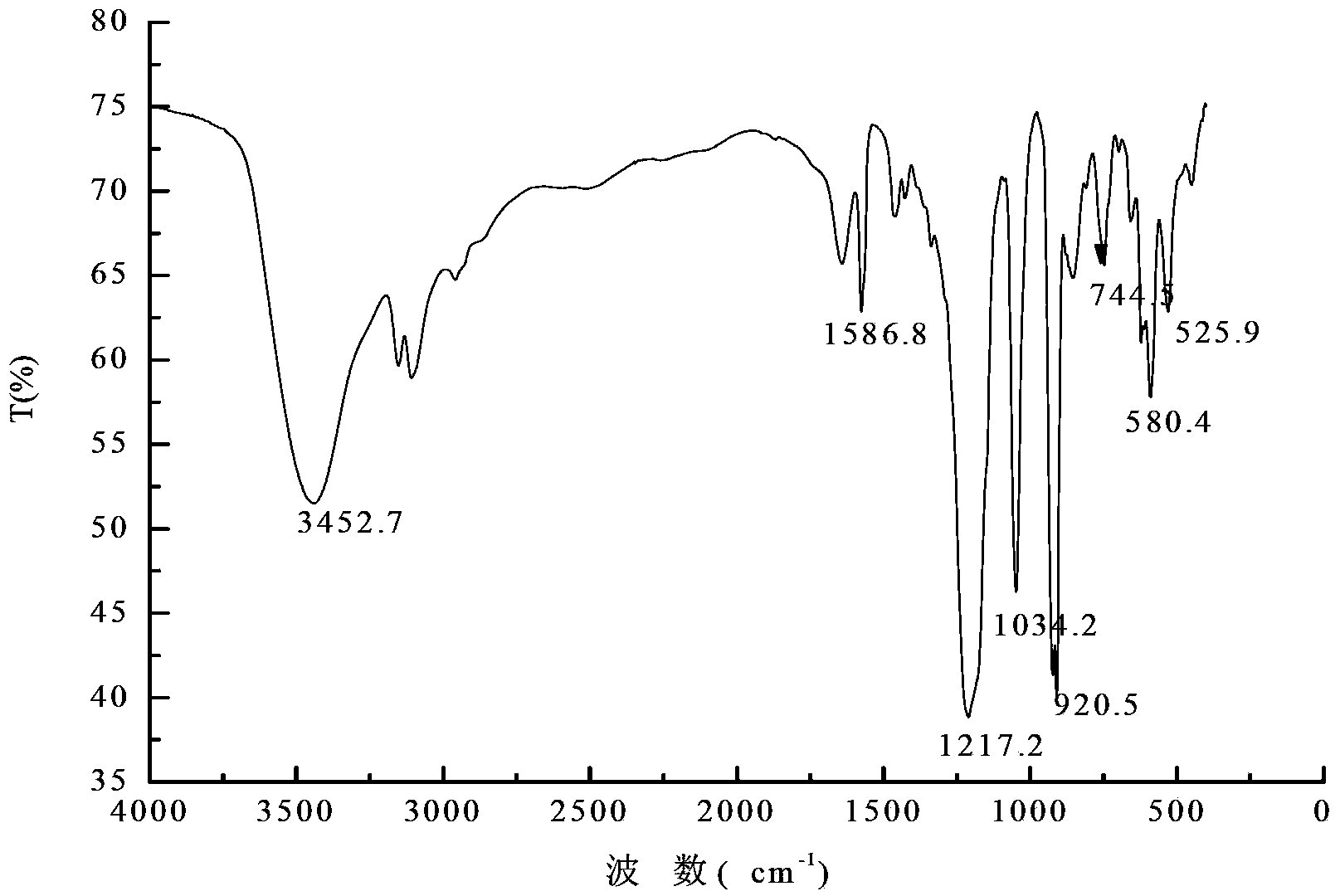
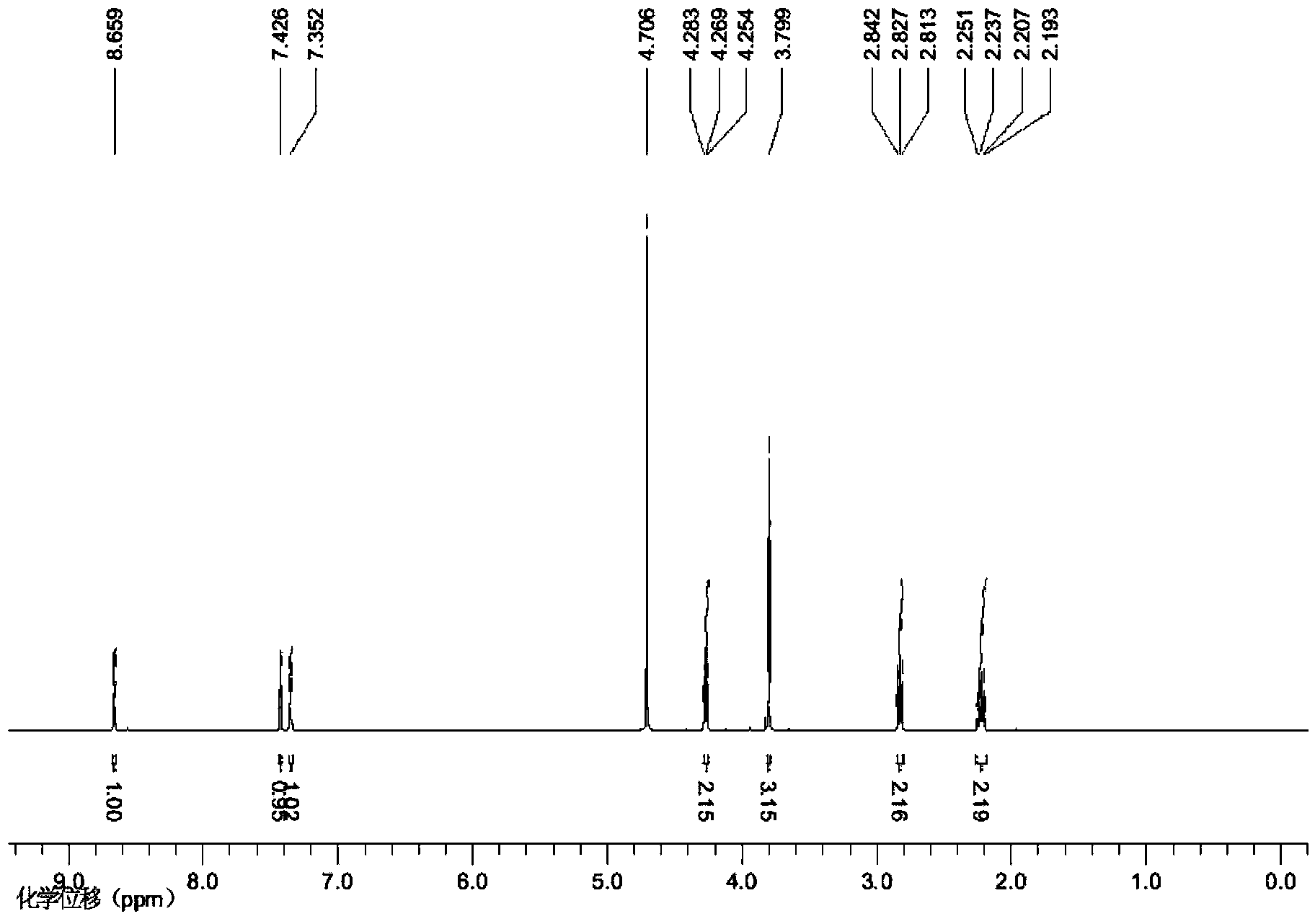
[0022] Example 1: 1-(3-sulfonic acid) propyl-3-methylimidazolium perrhenate ionic liquid
[0023] (1) Preparation of ionic liquids
[0024] 1) Dissolve N-methylimidazole and 1,3-propane sultone with a molar ratio of 1:1 in a certain amount of absolute ethanol, reflux at 50°C for 12 hours, cool, filter, and wash with ether, in Vacuum drying at 50°C for 8 hours to obtain ionic liquid intermediate A;
[0025] 2) Add the ionic liquid intermediate A and 37% concentrated hydrochloric acid obtained in step 1) with a molar ratio of 1:1 to a certain amount of toluene solution, and react at 70°C for 12 hours. After the reaction is complete, evaporate the toluene under reduced pressure , to obtain acidified ionic liquid intermediate B, that is, 1-(3-sulfonic acid) propyl-3-methylimidazolium chloride salt;
[0026] 3) Dissolve silver perrhenate in a certain amount of acetonitrile, and mix 1-(3-sulfonic acid) propyl-3-methylimidazolium chloride obtained in step 2) with silver perrhenate ...
Embodiment 2
[0033] Example 2: 1-(3-sulfonic acid) propyl-3-ethylimidazolium perrhenate ionic liquid
[0034] (1) Preparation of ionic liquids
[0035] 1) Dissolve N-ethylimidazole and 1,3-propane sultone with a molar ratio of 1:1 in a certain amount of absolute ethanol, reflux at 50°C for 8 hours, cool, filter, and wash with ether, in Vacuum drying at 50°C for 8 hours to obtain ionic liquid intermediate A;
[0036] 2) Add the ionic liquid intermediate A and 37% concentrated hydrochloric acid obtained in step 1) with a molar ratio of 1:1 to a certain amount of toluene solution, and react at 70°C for 12 hours. After the reaction is complete, evaporate the toluene under reduced pressure , to obtain acidified ionic liquid intermediate B, that is, 1-(3-sulfonic acid) propyl-3-ethylimidazolium chloride salt;
[0037] 3) Dissolve silver perrhenate in a certain amount of acetonitrile, and mix 1-(3-sulfonic acid) propyl-3-ethylimidazolium chloride obtained in step 2) with silver perrhenate at a ...
Embodiment 3
[0045] Example 3: 1-(3-sulfonic acid) propyl-3-butylimidazolium perrhenate ionic liquid
[0046] (1) Preparation of ionic liquids
[0047] 1) Dissolve N-butylimidazole and 1,3-propane sultone with a molar ratio of 1:1 in a certain amount of absolute ethanol, reflux at 50°C for 12 hours, cool, filter, and wash with ether, Vacuum drying at 50°C for 8 hours to obtain ionic liquid intermediate A;
[0048] 2) Add the ionic liquid intermediate A and 37% concentrated hydrochloric acid obtained in step 1) with a molar ratio of 1:1 to a certain amount of toluene solution, and react at 70°C for 12 hours. After the reaction is complete, evaporate the toluene under reduced pressure , to obtain acidified ionic liquid intermediate B, i.e. 1-(3-sulfonic acid) propyl-3-butylimidazolium chloride salt;
[0049] 3) Dissolve silver perrhenate in a certain amount of acetonitrile, and mix 1-(3-sulfonic acid) propyl-3-butylimidazolium chloride obtained in step 2) with silver perrhenate at a molar ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com