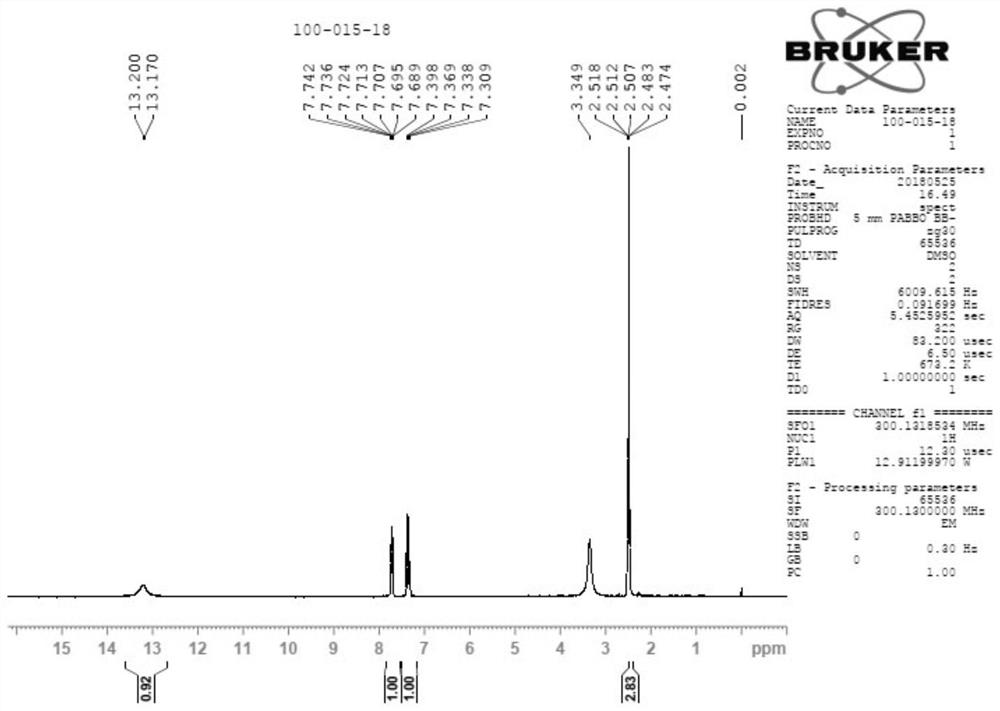
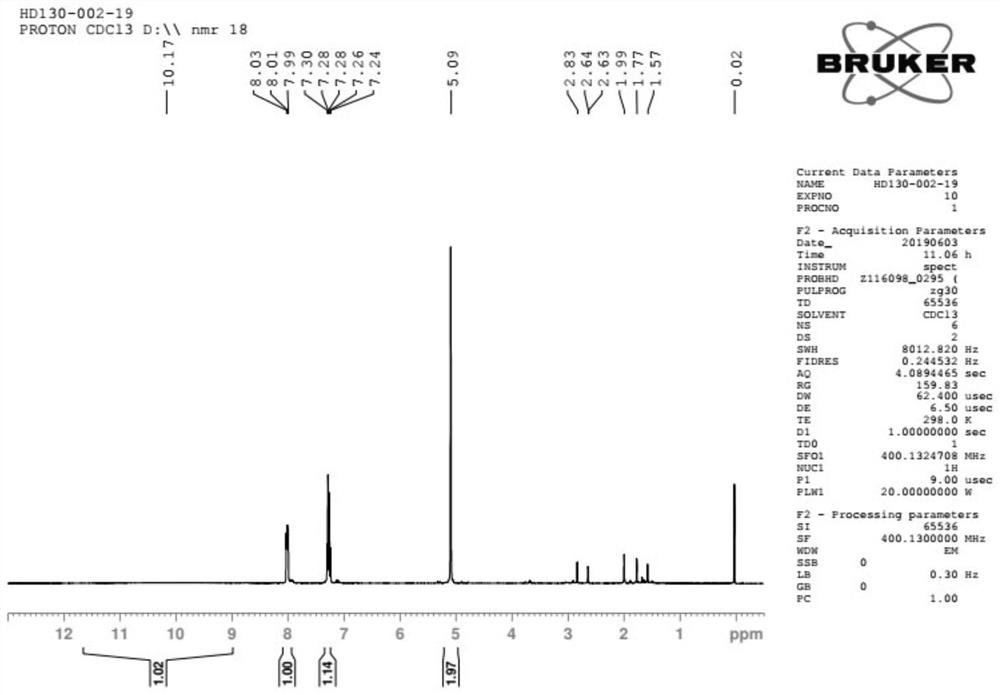
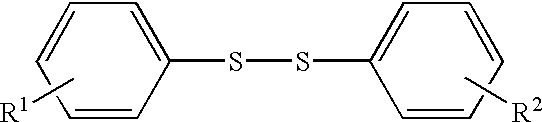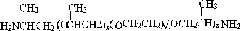Patents
Literature
80 results about "Diphenyl disulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
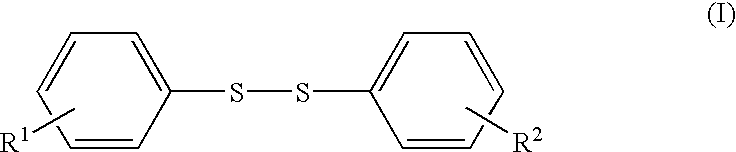
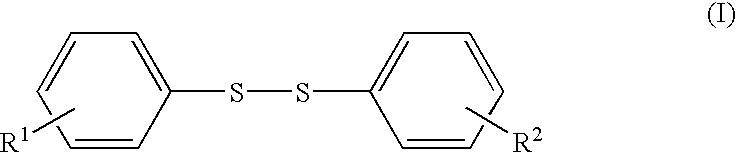
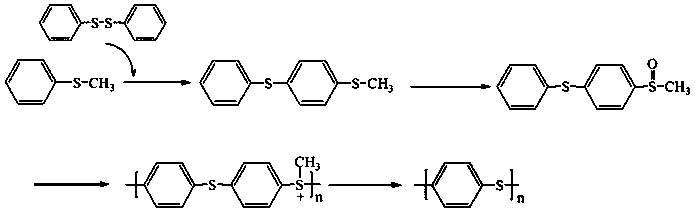
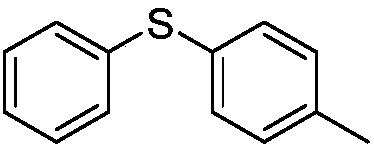
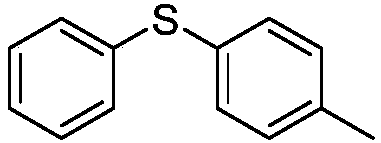
Diphenyl disulfide is the chemical compound with the formula (C₆H₅S)₂. This colorless crystalline material is often abbreviated Ph₂S₂. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiophenol is responsible for the disagreeable odour associated with this compound.
Cable jacket rubber and preparation method
ActiveCN101580606AScorch slowFast vulcanizationRubber insulatorsInsulated cablesTetramethylthiuram disulfideElectricity
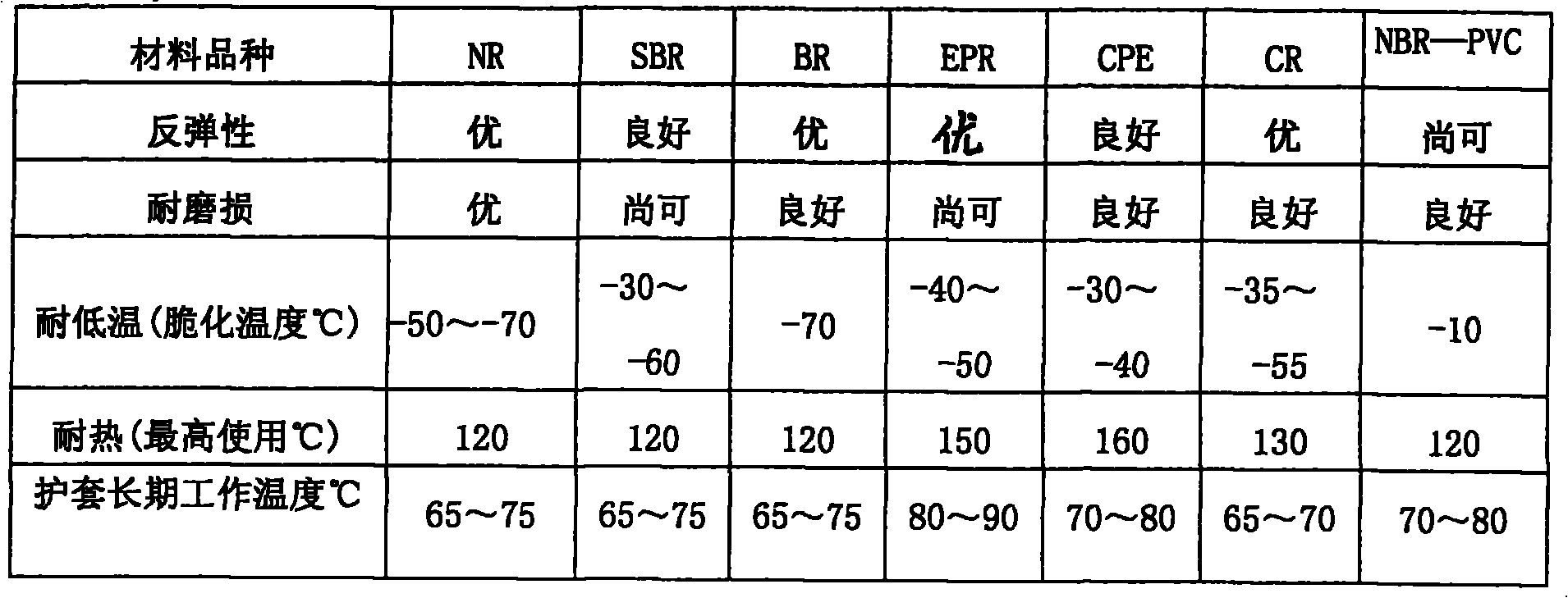
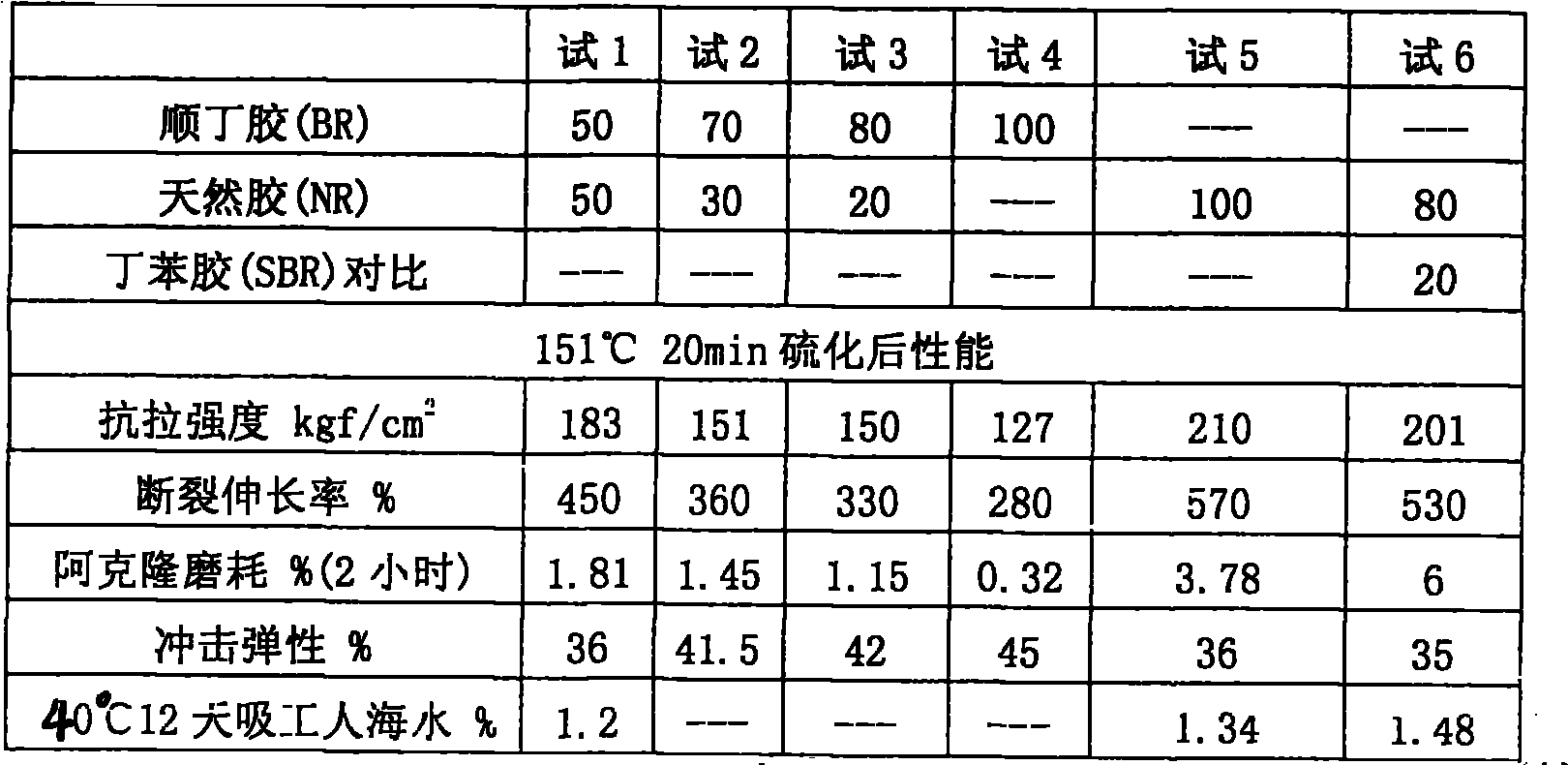
The invention relates to a cable jacket rubber and a preparation method, wherein the cable jacket rubber has high elastic resilience, softness and abrasive resistance at a low temperature. The cable jacket rubber is characterized in that a base material of the rubber is formed by mixing 30 to 50 weight percent of natural gum and 50 to 70 weight percent of cis-butadiene rubber; a vulcanizing system in a rubber compounding agent is a low-sulfur high-accelerant vulcanizing system, the addition of sulfur is 0.3 to 0.6 weight percent of that of the base material of the rubbere, the addition of the accelerant tetramethylthiuram disulfide is between 1.0 and 1.3 weight percent, the addition of the accelerant N-cyclohexyl-2-benzothiazole sulfonamide is between 1.8 and 2.2 weight percent, and the addition of the accelerant diphenyl disulfide benzothiazole is between 0.6 and 0.9 weight percent. The cable jacket rubber has wide range of working temperature, and can be bent and distorted randomly without cracking at a long-term usage temperature of between 50 DEG C below zero and 70 DEG C, the impact elasticity is high and can reach 42 percent, the specific elongation is more than 350 percent, and the Akron abrasion loss is less than 2 percent, thus the cable jacket rubber is particularly suitable for wind-power generated electricity tower tube cables.
Owner:WUXI HUAMEI CABLE
Non-aqueous electrolyte and non-aqueous electrolyte secondary cell
InactiveUS20040091786A1Reduce gas volumeExcellent characteristicsNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsPhysical chemistryDiphenyl disulfide
A non-aqueous electrolyte containing propylene carbonate and 1,3-propanesultone as additives can reduce the amount of a gas evolved during storage at a high temperature of a non-aqueous electrolyte secondary cell comprising the electrolyte, a non-aqueous electrolyte containing at least one compound selected from the group consisting of vinylene carbonate, diphenyl disulfide, di-p-tolyldisulfide and bis(4-methoxyphenyl)disulfide as an additive can improve cycle characteristics of a non-aqueous electrolyte secondary cell comprising the electrolyte, and a non-aqueous electrolyte containing a combination of the above two types of additives can provide a non-aqueous electrolyte secondary cell exhibiting excellent retention of capacity and storage stability.
Owner:PANASONIC CORP +1
Non-aqueous secondary battery having enhanced discharge capacity retention
InactiveUS6866966B2High discharge capacity retentionLead-acid accumulatorsOrganic electrolyte cells1,4-ButanediolHalogen
A discharge capacity retention of a non-aqueous secondary battery is enhanced by incorporating into its non-aqueous electrolytic solution a small amount of a substituted diphenyldisulfide derivative in which each of the diphenyl groups has a substituent such as alkoxy, alkenyloxy, alkynyloxy, cycloalkyloxy, aryloxy, acyloxy, alkanesulfonyloxy, arylsulfonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy, halogen, CF3, CCl3, or CBr3. Preferably, a small amount of methyl 2-propylcarbonate, 2-propynyl methanesulfonate, 1,3-propanesultone, divinylsulfone, 1,4-butanediol dimethanesulfonate or cyclohexylbenzene is further incorporated.
Owner:UBE IND LTD
Sulfur solvent for cleaning sulphur deposition in wellhole
The invention relates to a sulfur solvent for cleaning sulphur deposition in the wellhole, which consists of the following components: 90 to 95 weight percent of host crystal, 0.5 to 3.5 weight percent of catalyst and 1.5 to 7.5 weight percent of corrosion inhibitor, wherein the host crystal is dialkyl disulfide or diphenyl disulfide; the catalyst is organic amine, lewis base or a mixture of the organic amine and lewis base; and the corrosion inhibitor is beta-diethylin phenyl ketone or beta-abietylamine phenyl ketone or a mixture of the beta-diethylin phenyl ketone or the beta-abietylamine phenyl ketone. The sulfur dissolving rate and the sulfur-bearing quantity of the invention are superior to the common physical solvent, such as aliphatic hydrocarbon, benzene, CS2 and the conventional chemical solvent. The sulfur solvent can be used for cleaning and relieving sulphur deposition blockage of the wellhole of the high-sulfur gas well.
Owner:SOUTHWEST PETROLEUM UNIV +1
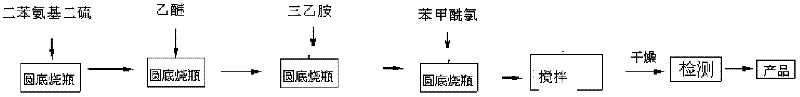
Method for preparing rubber peptizing agent, namely 2,2'-dibenzamido diphenyl disulfide compound
InactiveCN102304074AReduce acidityReduce viscosityHydropoly/poly sulfide preparationRoom temperatureBenzoyl chloride
The invention relates to a method for preparing a rubber peptizing agent, namely a 2,2'-dibenzamido diphenyl disulfide compound. The method comprises the following steps of: putting diphenyl disulfide, a deacid reagent, namely triethylamine, and a solvent in a reactor, stirring at room temperature, and slowly dropping benzoyl chloride, wherein the reaction time is 15-30 min; and obtaining the product by filtering, washing and drying after reacting. The mol ratio of the diphenyl disulfide to the deacid reagent to the benzoyl chloride to diethyl ether is (1.0-1.2):(2.0-2.2):(2.0-2.4):(50-70). According to the method disclosed by the invention, the nitrochlorobenzene is used as a raw material; the diphenyl disulfide is synthesized; the reaction speed is rapid; the reaction process is simple;and the purity of the obtained product is high.
Owner:LIAOCHENG UNIV
Method for preparing polyarylene sulfide
The present invention relates to a method for preparing polyarylene sulfide, in which the polyarylene sulfide is prepared by a polymerization reaction of reactants including a diiodo aromatic compound and a sulfur compound, the method including: further adding 0.01 to 10.0 wt. % of diphenyl disulfide with respect to the weight of the polyarylene sulfide to the reactants to form the polyarylene sulfide having a melting point of 265 to 320° C.The diphenyl disulfide included in the reactants according to the present invention costs far less than other conventional polymerization inhibitors to dramatically lower the production cost, and the polyarylene sulfide prepared using the diphenyl disulfide exhibits low iodine content and very excellence in thermal stability.
Owner:SK CHEM CO LTD
Method for preparing asymmetric disulfide
ActiveCN111777536AEfficient synthesisSimple and fast operationHydropoly/poly sulfide preparationPhenylsulfonamidePtru catalyst
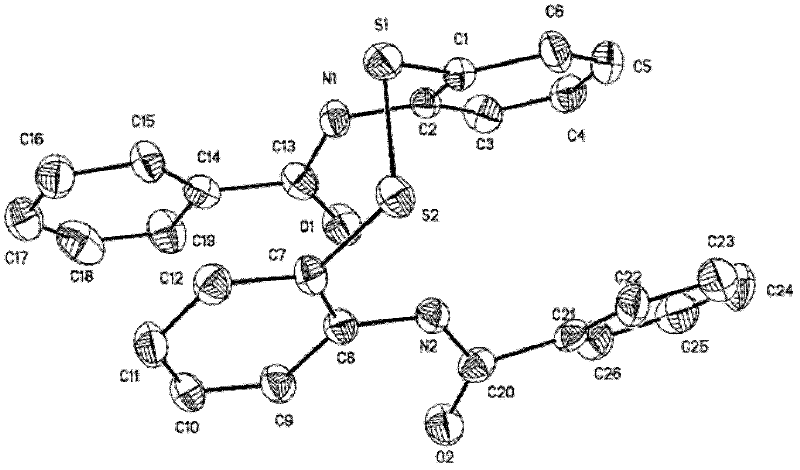
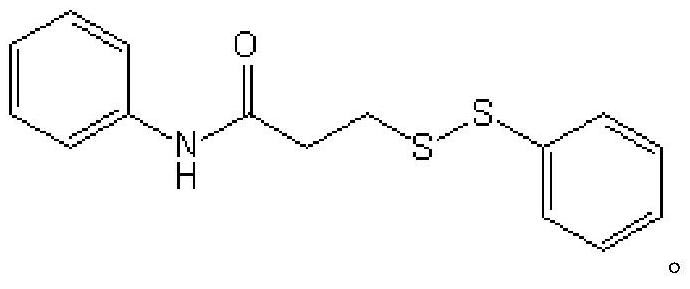
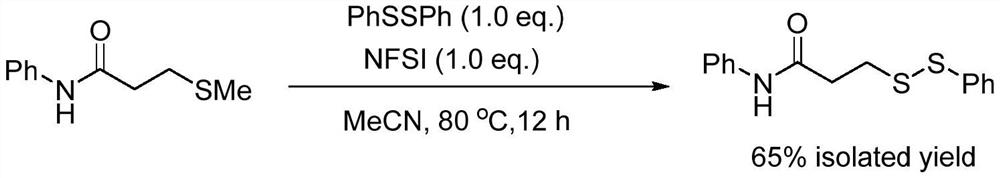
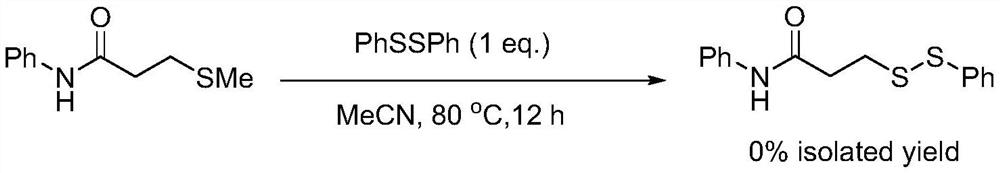
The invention relates to the technical field of fine chemical engineering, and discloses a method for preparing asymmetric disulfide. The preparation method comprises the following specific steps: taking 3-methylthio-N-phenylpropionamide and diphenyl disulfide as raw materials, taking N-fluorobisbenzenesulfonamide as an additive, and preferably carrying out a reaction in an acetonitrile solvent under a heating condition so as to obtain a target product, namely the symmetric disulfide N-phenyl-3-(phenyldithioalkyl)propionamide. The method is simple and convenient to operate and mild in reaction, 3-methylthio-N-phenylpropionamide and symmetric diphenyl disulfide are used as reaction raw materials, the asymmetric disulfide product is efficiently synthesized in one step, the use of a thiol rawmaterial with unpleasant smell or a transition metal catalyst is avoided, and the method has a potential application value.
Owner:CHANGZHOU UNIV
Non-aqueous electrolyte and non-aqueous electrolyte secondary battery
InactiveUS7132199B2Excellent characteristicsRaise the ratioNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsPhysical chemistryPropylene carbonate
A non-aqueous electrolyte containing propylene carbonate and 1,3-propanesultone as additives can reduce the amount of a gas evolved during storage at a high temperature of a non-aqueous electrolyte secondary cell comprising the electrolyte, a non-aqueous electrolyte containing at least one compound selected from the group consisting of vinylene carbonate, diphenyl disulfide, di-p-tolydisulfide and bis(4-methoxyphenyl)disulfide as comprising the electrolyte, and a non-aqueous electrolyte containing a combination of the above-two types of additives can provide a non-aqueous electrolyte secondary cell exhibiting excellent retention of capacity and storage stability.
Owner:PANASONIC CORP +1
2,2'-dibenzamido-diphenyl disulfide preparation method
ActiveCN104402786AHigh yieldEmission reductionHydropoly/poly sulfide preparationBisulfideWater vapor
The invention discloses a 2,2'-dibenzamido-diphenyl disulfide preparation method, and belongs to the technical field of synthesis of organic compounds. The preparation method comprises the following steps: preparing a 2,2'-diphenylamine disulfide from a sulfide and o-nitro halobenzene as raw materials by adopting a one-pot method, and performing acylation on the 2,2'-diphenylamine disulfide to obtain a 2,2'-dibenzamido-diphenyl disulfide. The process step that an intermediate product, namely 2-amino-4-chloro thiophenol, is separated and purified by adopting the processes of steam distillation, organic solvent extraction and the like in the prior art is omitted, the technological process is simplified, meanwhile, the yield of the intermediate product, namely the 2,2'-diphenylamine disulfide, is improved, and the yield of the 2,2'-dibenzamido-diphenyl disulfide is further improved. The preparation method has the advantages of being short in technological process, simple in operation, less in three wastes emission, environmental-friendly in the production process, high in yield, low in production cost, simple in equipment and small in investment, and facilitates industrial popularization and application.
Owner:HUANGHUAI UNIV
Triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton and preparation method thereof
InactiveCN103980181AReduce releaseHigh reactivityOrganic chemistryEpoxy resin coatingsSolubilityDiphenyl disulfide
The invention relates to an aromatic sulfonium salt compound and in particular relates to triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton as well as a preparation method and application thereof. The preparation method of the triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton comprises that 1-methyl-2-phenyl indole is taken as a raw material, diphenyl disulfide is taken as a sulfur source, a C-S bond coupling reaction is carried out for synthesizing aromatic sulfide, then a reaction is carried out on the aromatic sulfide and diaryl iodonium salt under the copper-catalyzed condition, so that the triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton is obtained. Ultraviolet absorption wavelength of the triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton can be more than 290nm in a red shift manner, the triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton can be applied to an ultraviolet curing composition, especially ultraviolet curing coating, as a positive ion photoinitiator and has good reaction activity, surface curability and solubility, and the special group, namely 1-methyl-2-phenyl indole, is introduced, so that photo-initiation performance of the triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton is effectively improved.
Owner:山西亮龙涂料有限公司
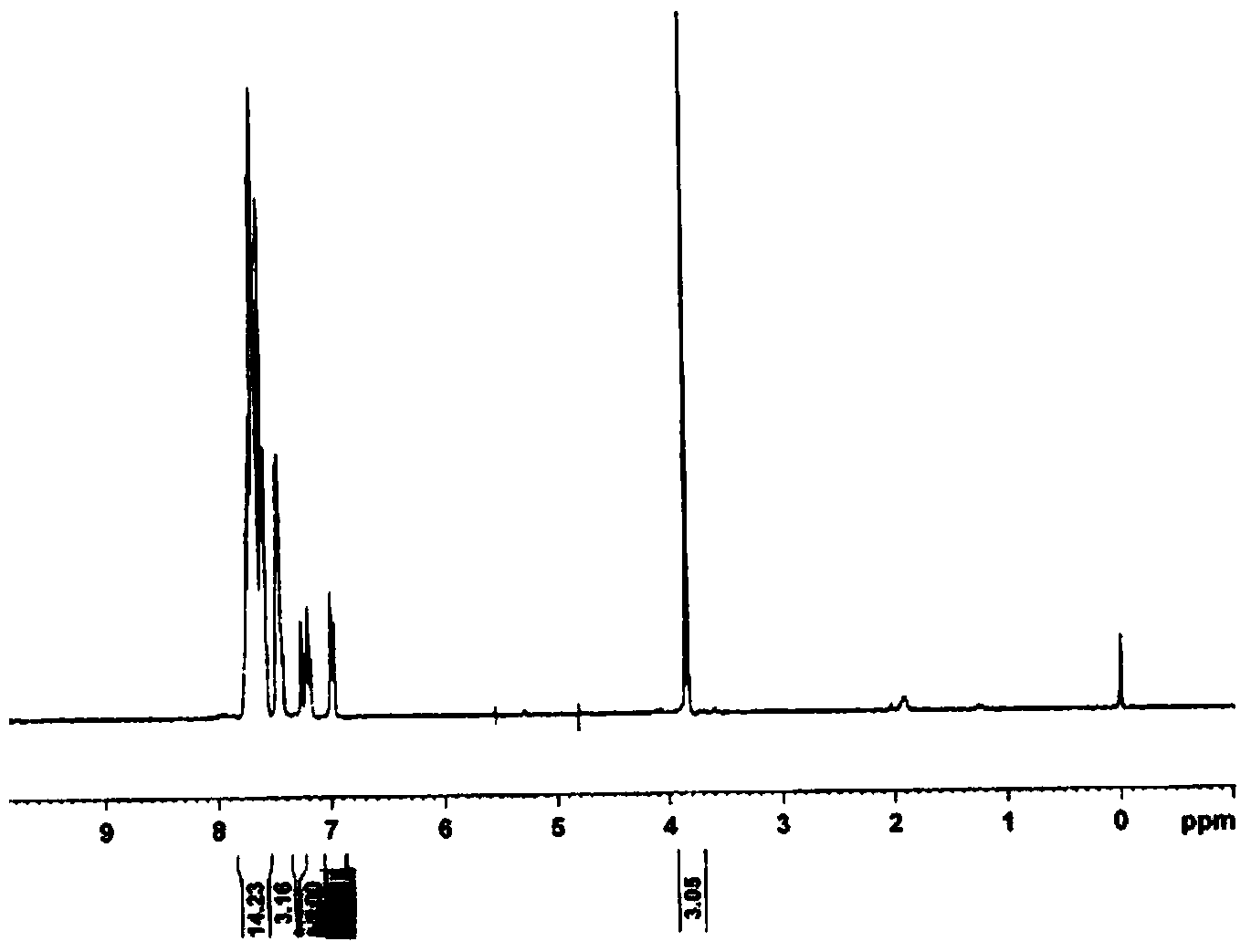
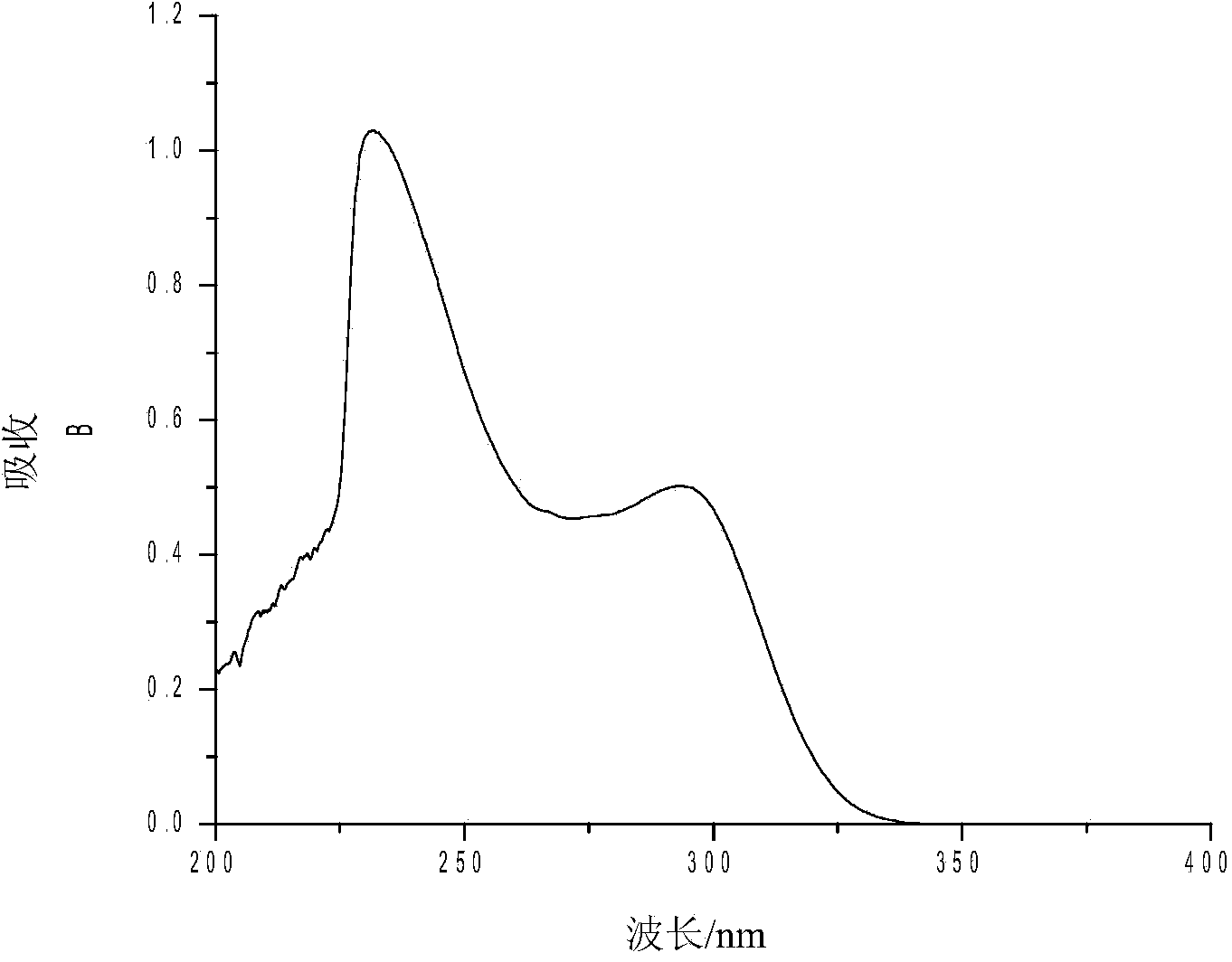
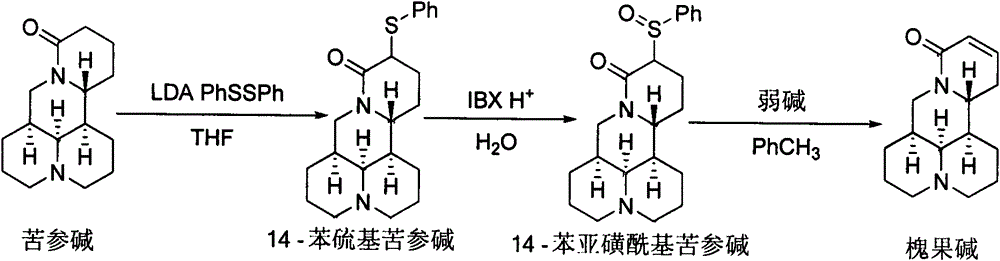
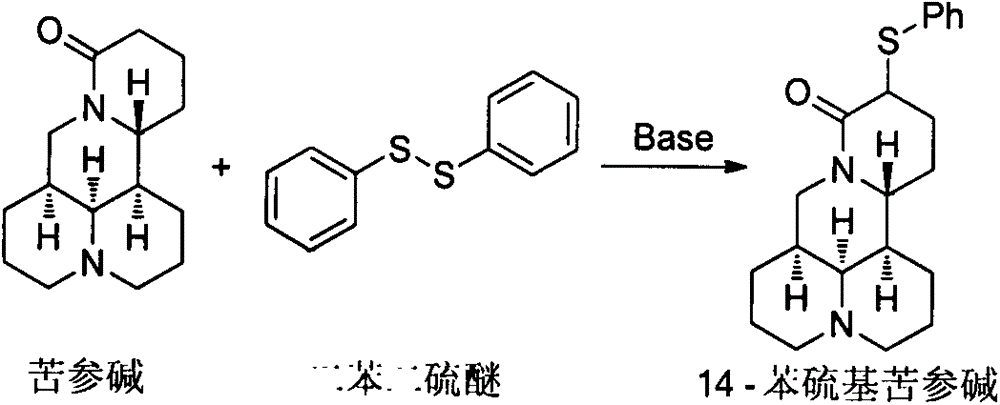
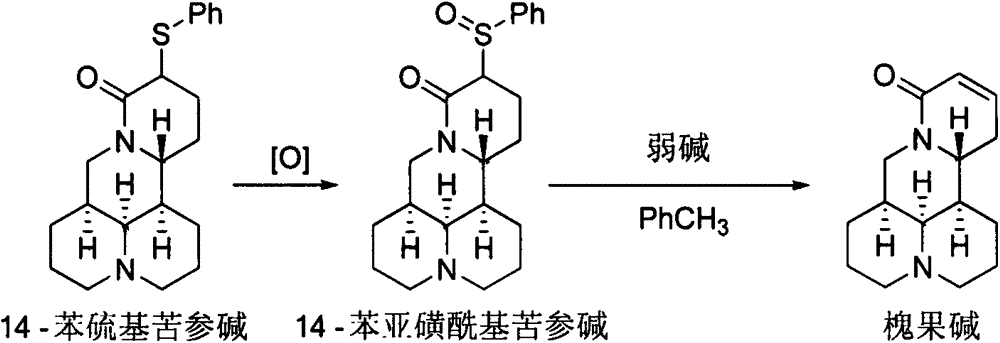
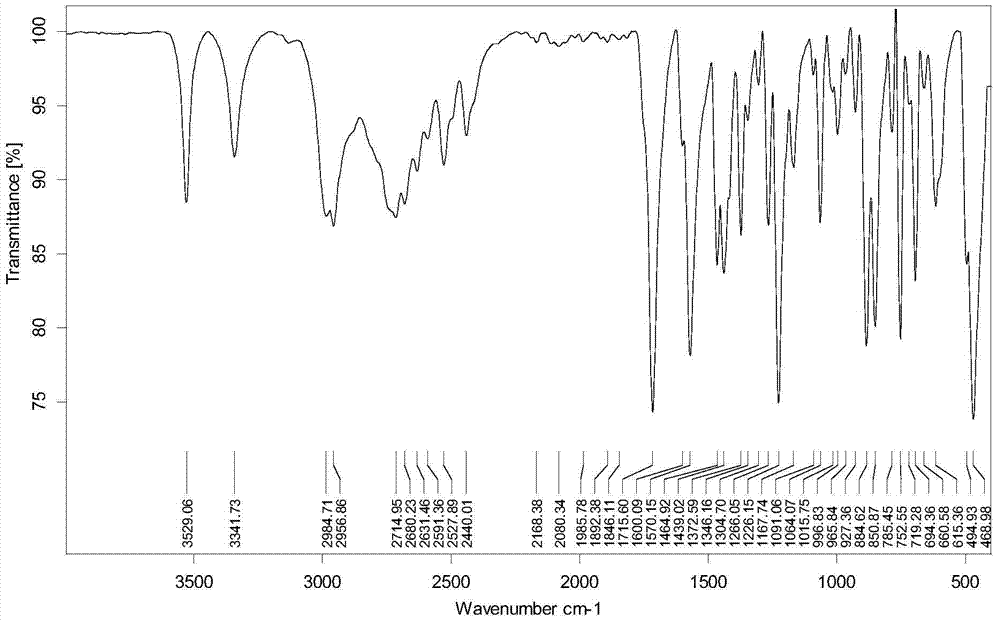
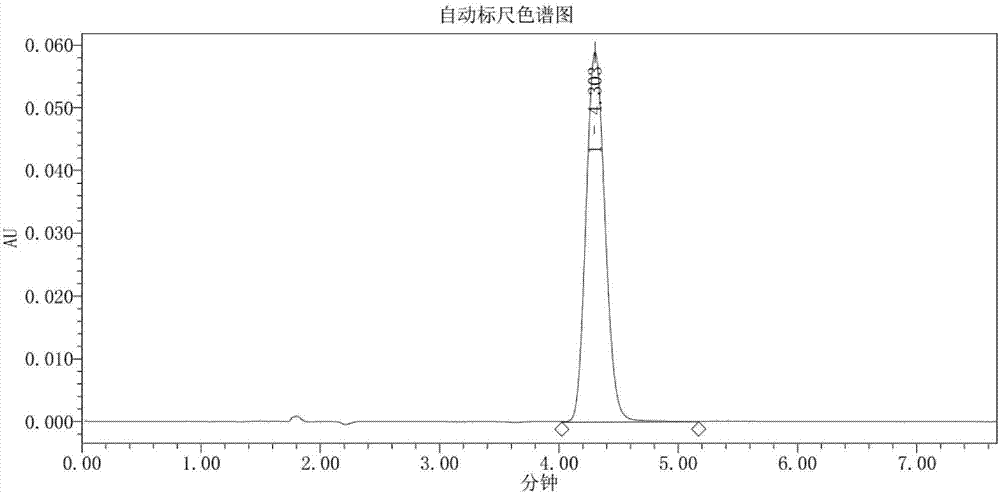
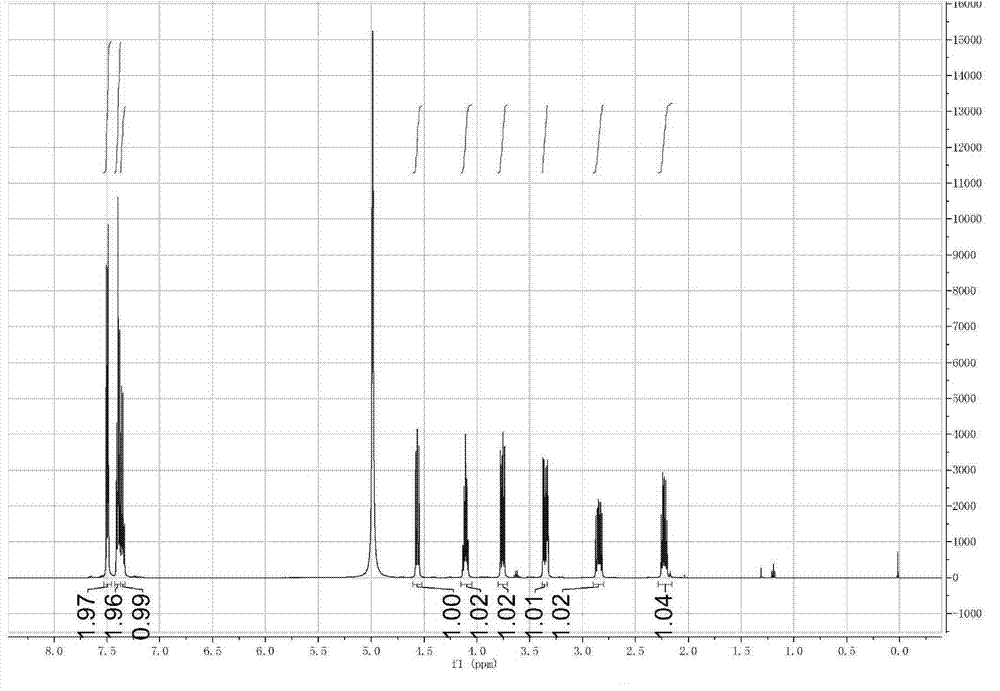
Method for synthesizing sophocarpine
The invention provides a new method for synthesizing sophocarpine. The method comprises the following steps: removing alpha-position hydrogen in amide in matrine by taking matrine as a raw material and taking lithium diisopropylamide (LDA) as alkali, then directly performing nucleophilic substitution reaction with diphenyl disulfide to obtain 14-thiophenyl matrine, oxidizing by IBX (o-iodyl-benzoic acid) in a water phase to obtain 14-phenylsulfinyl matrine and eliminating benzenesulfenic acid under the assistance of weak alkali to obtain sophocarpine. The method provided by the invention takes the step of oxidizing a nitrogen-containing thioether compound with IBX in a water phase to form sulfoxide as the key step for synthesizing sophocarpine. The method is characterized by high efficiency, environmental friendliness and easiness in operation. The synthesis route is as shown in the description.
Owner:NANKAI UNIV
Synthesis method of (2S,4S)-4-thiophenyl-L-proline hydrochloride
InactiveCN104844495ALow priceMild reaction conditionsOrganic chemistrySodium bicarbonateHydroxyproline
The invention discloses a synthesis method of (2S,4S)-4-thiophenyl-L-proline hydrochloride. The method comprises the following steps: adding L-oxyproline into a methanol-thionyl chloride reaction mixed solution, reacting, and separating to obtain a white solid L-oxyproline methyl ester; dissolving the L-oxyproline methyl ester in a tetrahydrofuran-water mixed solvent, adding a sodium bicarbonate solid and BOC acid anhydride to react, and separating to obtain colorless viscous grease BOC-L-oxyproline methyl ester; mixing the BOC-L-oxyproline methyl ester, diphenyl disulfide ether and tributyl phosphine in toluene, and reacting at 80-120 DEG C for 4-5 hours to obtain N-BOC-(2S,4S)-4-thiophenyl-L-proline methyl ester; and carrying out hydrolysis and recrystallization on the reaction solution to obtain the (2S,4S)-4-thiophenyl-L-proline pure product. The method has the advantages of low raw material price, mild reaction conditions, high selectivity, lower requirements for instruments and equipment, simple technical operation, high total yield, high product purity and simple purification process. The synthesis route has low pressure on environmental protection, and is more suitable for industrial mass production.
Owner:WUHAN UNIV OF TECH
Synthetic method of high-molecular-weight linear polyphenylene sulfide
The invention discloses a synthetic method of high-molecular-weight linear polyphenylene sulfide. The synthesis method comprises the following steps: synthesizing 4-(thiophenyl)thioanisole by taking polyphenylene sulfide and diphenyl disulfide as raw materials, further synthesizing 4-(sulfophenyl)methyl phenyl sulfoxide through oxidation by using hydrogen peroxide, then carrying out a cationic polymerization reaction on the 4-(sulfophenyl)methyl phenyl sulfoxide under the action of trifluoromethanesulfonic acid to synthesize a polymethyl[4-(thiophenyl)-phenyl]sulfonium trifluoromethanesulfonate; and finally, removing methyl by a nucleophilic reagent pyridine to obtain the polyphenylene sulfide. The polyphenylene sulfide synthesized by the method is high in purity and high in molecular weight and is in linear, the reaction system is mild in condition, operation of the whole process is relatively stable, and the method has an industrial application prospect.
Owner:TAIYUAN UNIV OF TECH
Method for preparing baloxavir intermediate and intermediate obtained by method
ActiveCN112062750AReduce usageSuitable for industrial productionOrganic chemistryChemical synthesisGrignard reagent
The invention belongs to the technical field of chemical synthesis, and provides a method for preparing a balosavir intermediate. The method comprises the following steps: (1) methylating 3,4-difluorobromobenzene to obtain a compound A-1; (2) reacting the compound A-1 with a Grignard reagent, then introducing carbon dioxide gas for reaction, and adding acid for acidification to obtain a compound A-2; (3) brominating the compound A-2 to obtain a compound A-3; (4) adding diphenyl disulfide and a reducing agent 1 into THF, dropwise adding methanol, dropwise adding the compound A-3, and reacting to obtain a compound A-4; (5) carrying out ring closing reaction on the compound A-4 in polyphosphoric acid to obtain a compound A-5; and (6) reducing the compound A-5 into alcohol by adopting a reducing agent 2 to obtain a compound A-6. According to the method, highly toxic and foul thiophenol is prevented from being used while expensive reagents are prevented from being used, so that the processcost is reduced. The invention also provides an intermediate compound prepared by the method.
Owner:HEADING NANJING PHARMTECH CO LTD
High-strength cold-resistant aramid fiber synchronous belt and preparation method thereof
The invention relates to the field of aramid fibers, particularly to a high-strength cold-resistant aramid fiber synchronous belt and a preparation method thereof. The high-strength cold-resistant aramid fiber synchronous belt is prepared from aramid short fibers, nylon short fibers, glass fiber core wires, ferroferric oxide, phenolic resin, N-2,2-dibenzamido diphenyl disulfide, calcium borate, silicon dioxide, aluminum oxide, bentonite, sodium carboxymethyl cellulose and silicone rubber. According to the method, the performance of all the raw materials is fully considered, fiber sorting is guided under an external magnetic field through the magnetic materials, the synergistic effect among the raw material components is effectively enhanced, good use of the synchronous belt at the low temperature is guaranteed, and the use time of the synchronous belt in the low-temperature environment is prolonged.
Owner:NINGBO FULONG SYNCHRONOUS BELT
Method for preparing polyarylene sulfide
InactiveUS8859720B2Reduce contentImprove thermal stabilityChemical/physical/physico-chemical stationary reactorsSulfurIodine
The present invention relates to a method for preparing polyarylene sulfide, in which the polyarylene sulfide is prepared by a polymerization reaction of reactants including a diiodo aromatic compound and a sulfur compound, the method including: further adding 0.01 to 10.0 wt. % of diphenyl disulfide with respect to the weight of the polyarylene sulfide to the reactants to form the polyarylene sulfide having a melting point of 265 to 320° C.The diphenyl disulfide included in the reactants according to the present invention costs far less than other conventional polymerization inhibitors to dramatically lower the production cost, and the polyarylene sulfide prepared using the diphenyl disulfide exhibits low iodine content and very excellence in thermal stability.
Owner:SK CHEM CO LTD
Preparation method for rubber peptizer DBD
InactiveCN104892475AGood peptization effectKeep healthyHydropoly/poly sulfide preparationProduction rateToxic material
The invention relates to the technical field of synthesis of chemical raw materials, in particular to a preparation method for a rubber peptizer DBD. The preparation method comprises the following steps: synthesizing 2-aminobenzothiazole; synthesizing 2,2'-disulfide diphenylamine; synthesizing 2,2'-dibenzoylamino diphenyl disulfide. The rubber prepared with the preparation method is good in peptization effect; no toxic substances generate during preparation; the body health of production workers is ensured; the technological process is simple; the production cost is low; the productivity is high.
Owner:LIAOCHENG KINGE SYNTHETIC MATERIAL
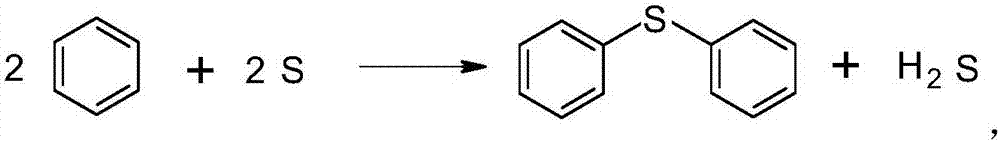
Synthetic method of diphenyl sulfide
InactiveCN106946752ALow costSuitable for industrial productionSulfide preparationChlorobenzeneOrganic layer
The invention discloses a synthesis method of diphenyl sulfide. According to the method, the molar ratio of benzene, Lewis acid catalyst and sulfur is 3.0~3.6:1.0~3.0:1.0. After mixing benzene and catalyst, the temperature is raised to 50~70°C, sulfur is slowly added, and the hydrogen sulfide gas generated by the reaction is Pour into the sodium hydroxide solution, reflux until the reaction is complete after adding the sulfur, then cool to room temperature, and slowly add the synthetic material dropwise to 5-15% dilute hydrochloric acid for pickling, leave the upper layer organic layer after stratification Benzene is removed by heating and precipitating to obtain diphenyl sulfide, and benzene is recovered and used mechanically. The invention uses benzene and sulfur as raw materials, significantly reduces the cost, produces less waste gas and waste water, is environmentally friendly, and reaches the international standard. More than 98%, suitable for industrial production.
Owner:LIANYUNGANG ZHICHENG CHEM
Composite metal photocatalysis system and preparation method and application thereof
ActiveCN110975940AFast transferEfficient transferOrganic-compounds/hydrides/coordination-complexes catalystsOrganic free radical generationPtru catalystCycloaddition
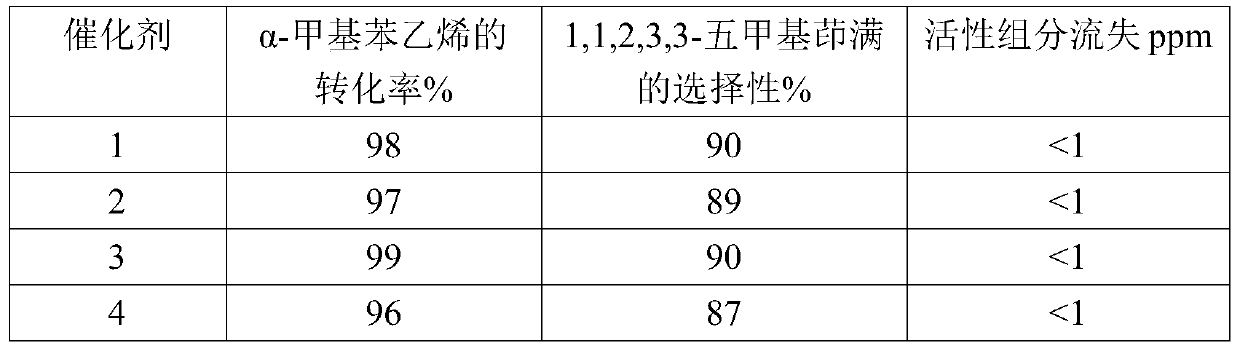
The invention discloses a composite metal photocatalysis system which is represented by Ni-M-X-Y-Z, wherein M is selected from the group consisting of Al, Ag, Bi, Cd, Ge, Sb and Sn; x is selected fromthe group consisting of triphenylphosphine, triethylphosphine, tributylphosphine, tri-tert-butylphosphine and tricyclohexylphosphine; Y is selected from the group consisting of carbon nanorods, molecular sieves, ordered mesoporous carbon and silicon dioxide; Z is selected from the group consisting of diphenyl disulfide, dicumyl peroxide, hydrazine and 2,4-dinitrophenylhydrazine; the mass fractionof Ni is 10-20%; the mass fraction of M is 20-30%; the mass fraction of X is 20-40%; the mass fraction of Y is 15-40%,; and the mass fraction of Z is 10-30%. The catalytic system is used for preparing 1,1,2,3,3-pentamethylindane through free radical cycloaddition of alpha-methylstyrene and 2-methyl-2-butene, an conversion rate is larger than 90%, and selectivity is larger than 85%; and a catalystis stable and not prone to loss in the free radical cycloaddition reaction. A method provided by the invention is easy to operate and good in economic benefit.
Owner:WANHUA CHEM GRP CO LTD
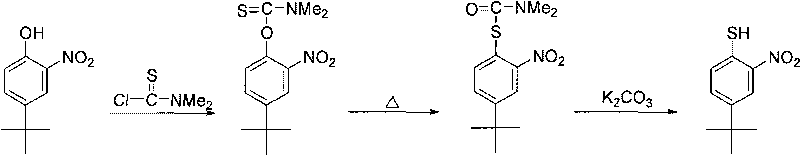
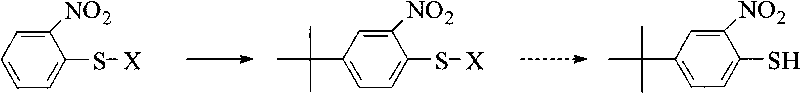
Method for preparing p-tert-butyl o-nitrothiophenol
The invention relates to the synthesis field of chemical intermediates and discloses a method for directly preparing p-tert-butyl o-nitrothiophenol. In the method, with o-nitrothiophenol or 2,2'-binitro diphenyl disulfide as the raw material, the p-tert-butyl o-nitrothiophenol and the derivatives thereof are obtained through tert-butylation reaction. The method is characterized by easily obtained raw materials, short reaction route, higher relative reaction yield and easy realization of large-scale production.
Owner:北京金源化学集团有限公司
Resveratrol synthesis preparation method
InactiveCN104326880AMild reaction conditionsHigh yieldOrganic compound preparationGroup 5/15 element organic compoundsXylyleneFood additive
The invention relates to a resveratrol preparation method which belongs to the field of food additives and preparing methods thereof. The resveratrol synthesis preparation method comprises the following steps: (1) using 4-methoxybenzyl bromide, triphenyl phosphine and xylene as raw materials for synthesis of triphenyl 4-methoxybenzyl phosphorus bromide (compound I); (2) using the compound I, tetrahydrofuran and 3, 5-dimethoxy benzaldehyde as raw materials for synthesis of a cis / trans-3, 4, 5-trimethoxy-1, 2-diphenylethene mixture (compounds II); (3) using the compound II, tetrahydrofuran and diphenyl disulfide as raw materials for synthesis of trans-3, 4, 5-trimethoxy-1, 2-diphenylethene (compound III); and (4) using the compound III, boron tribromide and methylene chloride as raw materials for synthesis of trans-3, 4, 5-trihydroxy-1, 2-diphenylethene (compound IV), namely resveratrol. The resveratrol synthesis preparation method has the advantages of mild reaction conditions, high yield (on the basis of the 4-methoxybenzyl bromide, the total yield is 66%), high purity (more than 98%) and the like.
Owner:西安莹朴生物科技股份有限公司
Preparation method of diphenyl disulfide compounds
ActiveCN111763163AImprove conversion rateLow costOrganic compound preparationMagnesium organic compoundsGrignard reagentDiphenyl disulfide
The invention discloses a preparation method of diphenyl disulfide compounds. The preparation method comprises the following steps: stirring an isopropyl magnesium halide Grignard reagent and a substituted halogen benzene compound in an organic solvent at -78 DEG C to -20 DEG C for 30-90 minutes to obtain a thoroughly halogen-magnesium exchanged substituted phenyl Grignard reagent; and adding dichlorodisulfide into the reaction system, slowly heating to room temperature after the reaction is finished, quenching the reaction by using a saturated ammonium chloride aqueous solution, extracting byusing ethyl acetate or diethyl ether, drying by using anhydrous magnesium sulfate, and concentrating the organic phase to obtain the diphenyl disulfide compounds. According to the method, the diphenyl disulfide compounds are prepared by taking the phenyl Grignard reagent as a raw material through a one-pot method, and has the following advantages: the synthetic route is short, the preparation process is simple, the cost is low, the operation is easy, the yield is excellent, and the industrial production is easy.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
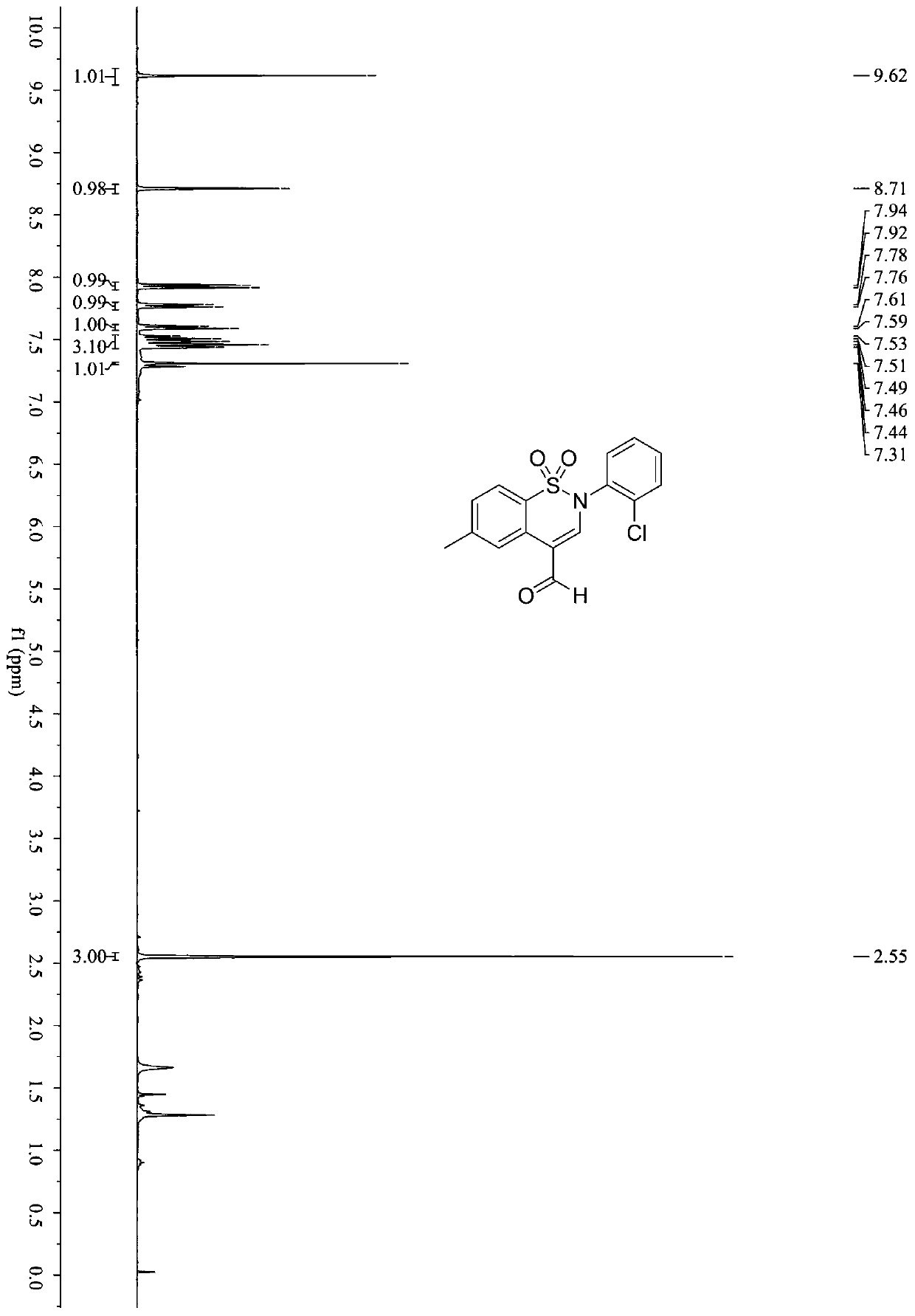
Synthetic method of benzothiazine formaldehyde derivative
The invention belongs to the technical field of benzothiazide derivatives, and discloses a synthetic method of a benzothiazide formaldehyde derivative. The synthesis method comprises the following steps: in the system of an organic solvent, an alkaline compound, a photocatalyst and an additive, carrying out a reaction on a benzenesulfonyl propargylamine derivative in an aerobic environment throughillumination to obtain a benzothiazine formaldehyde derivative, wherein the additive is more than one of diphenyl disulfide and thiophenol. According to the method, the benzothiazine compound is prepared by utilizing the carbon oxidation reaction of alkyne. The method is simple, convenient, efficient and high in regioselectivity, the used raw materials are simple and easy to obtain, no extra organic oxidizing agent needs to be added, oxygen in the air is used as an oxygen source and is environmentally friendly, cheap and easy to obtain. In addition, the whole operation process is simple and feasible, steps are simple and products are easy to purify.
Owner:SOUTH CHINA UNIV OF TECH
Rubber peptizer intermediate 2,2'-dinitro-diphenyl disulfide and preparation method thereof
InactiveCN102558000AImprove responseHigh purityHydropoly/poly sulfide preparationPolymer scienceVulcanization
The invention provides diphenyl disulfide type vulcanized peptizer intermediate, namely 2,2'-dinitro-diphenyl disulfide and a preparation method thereof. The preparation method is characterized in that ortho-nitrochlorobenzene, industrial sodium sulfide and sulfur powder are adopted as raw materials, and the 2,2'-dinitro-diphenyl disulfide is synthesized in ethanol. The preparation method has the advantages of simple reaction process and high purity of obtained products.
Owner:LIAOCHENG UNIV
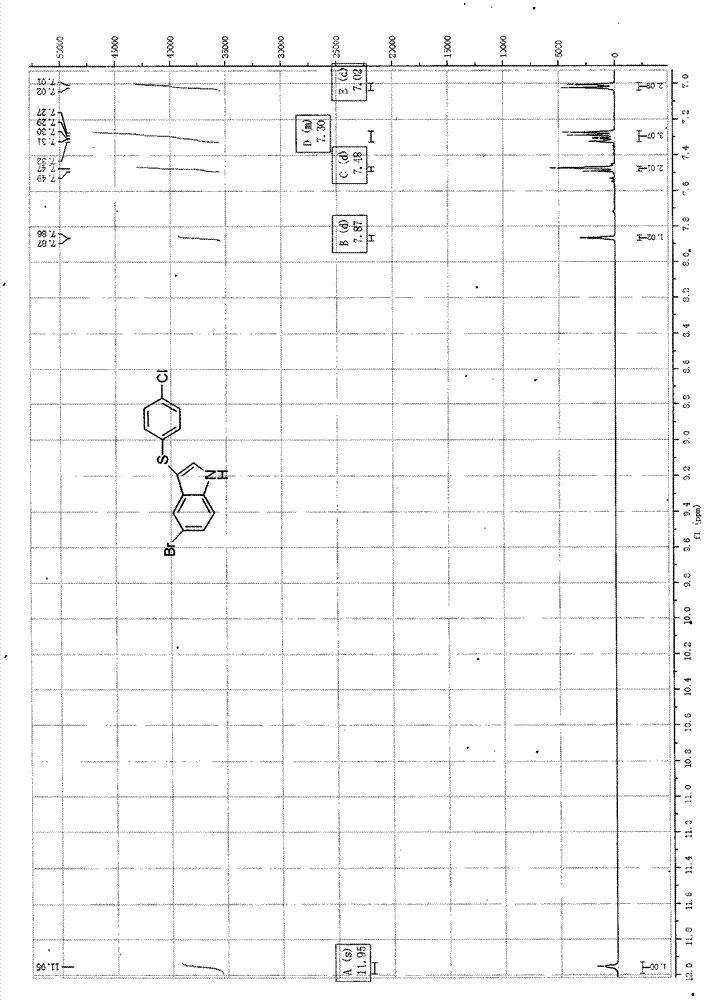
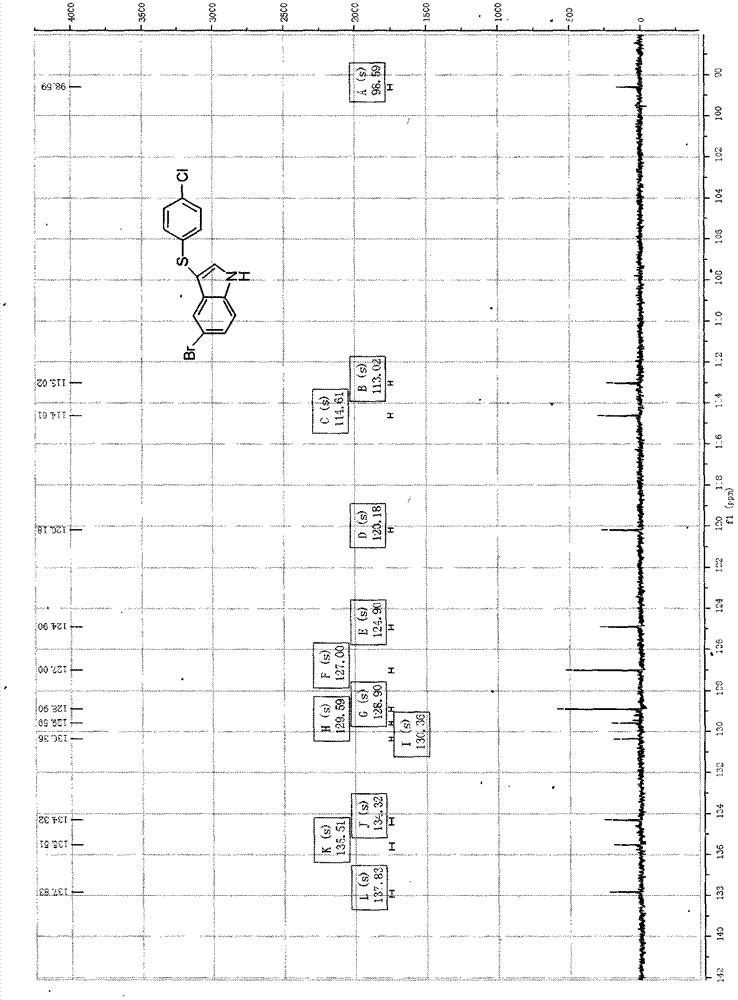
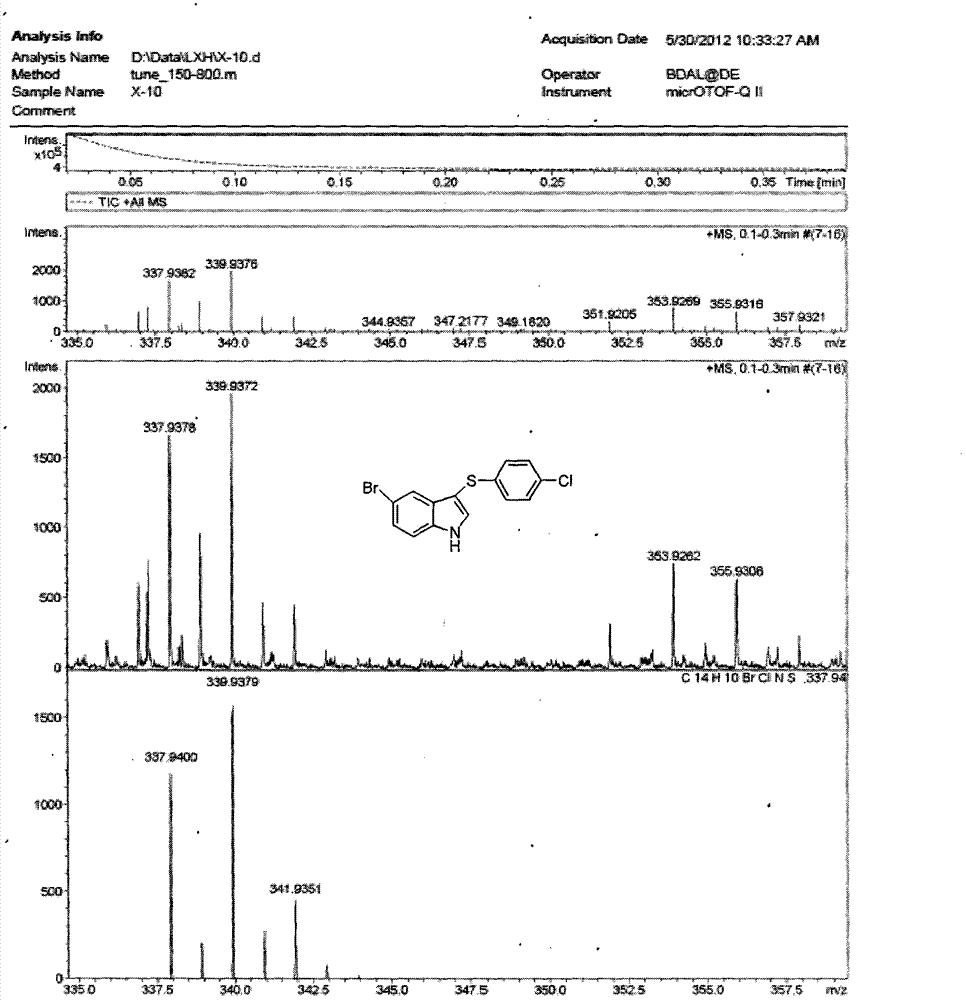
3-((4-chlorobenzene base) thio)-5-bromide-1-hydrogen-indole composite method
The invention provides a 3-((4-chlorobenzene base) thio)-5-bromide-1-hydrogen-indole composite method. 5-bromine indole and 4, 4 '-dichloro diphenyl disulfide ether serve as raw materials and are dissolved in an alcohol and water mixed solvent, an reaction is performed for 30 to 60 hours in the presence of strong base under the 80 to 140 DEG C and 5 to 15 pressure conditions, and the purified 3-((4-chlorobenzene base) thio)-5- bromide-1-hydrogen-indole is obtained through aftertreatment. According to the 3-((4-chlorobenzene base) thio)-5- bromide-1-hydrogen-indole composite method, the reaction conditions are mild, metal does not need to be added for metal catalysis in the reaction process, the gas protection is not required, and accordingly the difficulty of the treatment after the reaction is reduced, the reaction cost is greatly reduced, the pollution to the environment is reduced, and emissions of the waste are reduced.
Owner:WENZHOU UNIVERSITY
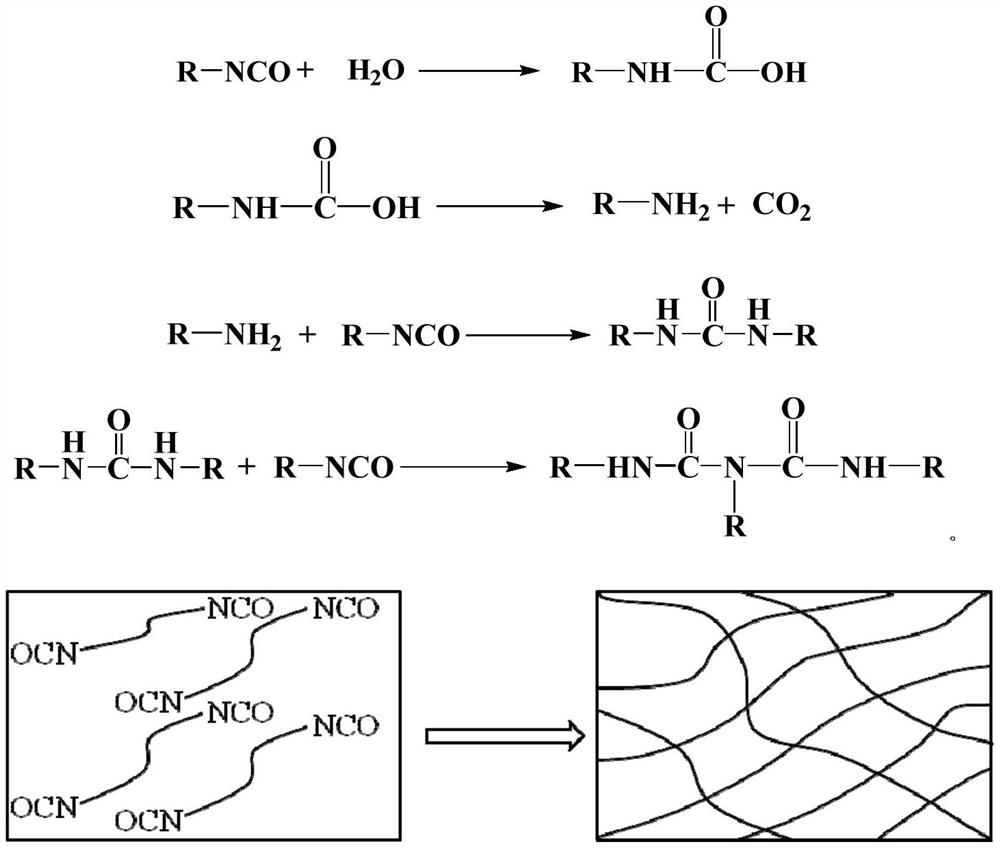
Heat-detachable polyurethane hot melt adhesive, preparation raw materials, and preparation method and bonding method of heat-detachable polyurethane hot melt adhesive
PendingCN114044868AReduce bond strengthWill not automatically disengagePolyureas/polyurethane adhesivesAdhesive processes with adhesive heatingAdhesive cementPolymer science
The invention belongs to the field of polyurethane adhesives, and relates to a heat-detachable polyurethane hot melt adhesive, preparation raw materials, and a preparation method and a bonding method of the heat-detachable polyurethane hot melt adhesive. The preparation raw materials for the detachable polyurethane hot melt adhesive comprise a polyol compound, a polyisocyanate compound, a sulfur-containing compound and a catalyst in a mass ratio of 100: (35-120): (2.5-150): (0.1-2), wherein the sulfur-containing compound is at least one selected from 2,2-dithiodiethanol, 4,4-dihydroxy diphenyl disulfide, 4,4-diaminodiphenyl disulfide and liquid polysulfide resin. The polyurethane hot melt adhesive prepared from the raw materials can be completely detached at a high temperature and cannot be automatically separated at the high temperature, so the aim that a bonded part can be detached under a heating condition is perfectly fulfilled.
Owner:XIAMEN WELDTONE TECH CO LTD
Preparation method of aryl monothioether compound
ActiveCN111635343AReduce manufacturing costQuick responseMercapto/sulfide group formation/introductionSulfide preparationHalohydrocarbonGrignard reagent
The invention discloses a preparation method of an aryl monothioether compound. The method comprises the following steps: reacting halogenated hydrocarbon with an isopropyl magnesium halide Grignard reagent at -20 DEG C for 30 minutes until a halogen-magnesium exchange reaction is completely completed; cooling a reaction solution to -78 DEG C, slowly dropwise adding a tetrahydrofuran solution of asubstituted diphenyl disulfide compound into the newly prepared Grignard reagent, maintaining the concentration of the reactants to be 0.5-1 mmol / mL, carrying out a stirring reaction for 1 hour in anorganic solvent at -78 DEG C, and then slowly heating the reaction solution to room temperature; and carrying out a quenching reaction by using a saturated ammonium chloride solution, extracting an organic phase by using ethyl acetate or diethyl ether, drying the organic phase by using anhydrous magnesium sulfate, and concentrating the organic phase to obtain the aryl thioether compound. The method is simple in preparation process, low in cost, high in speed, easy to operate and small in environmental pollution, groups sensitive to Grignard reagents can also be tolerated, and high yield is obtained.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Methods for nucleophilic fluoromethylation
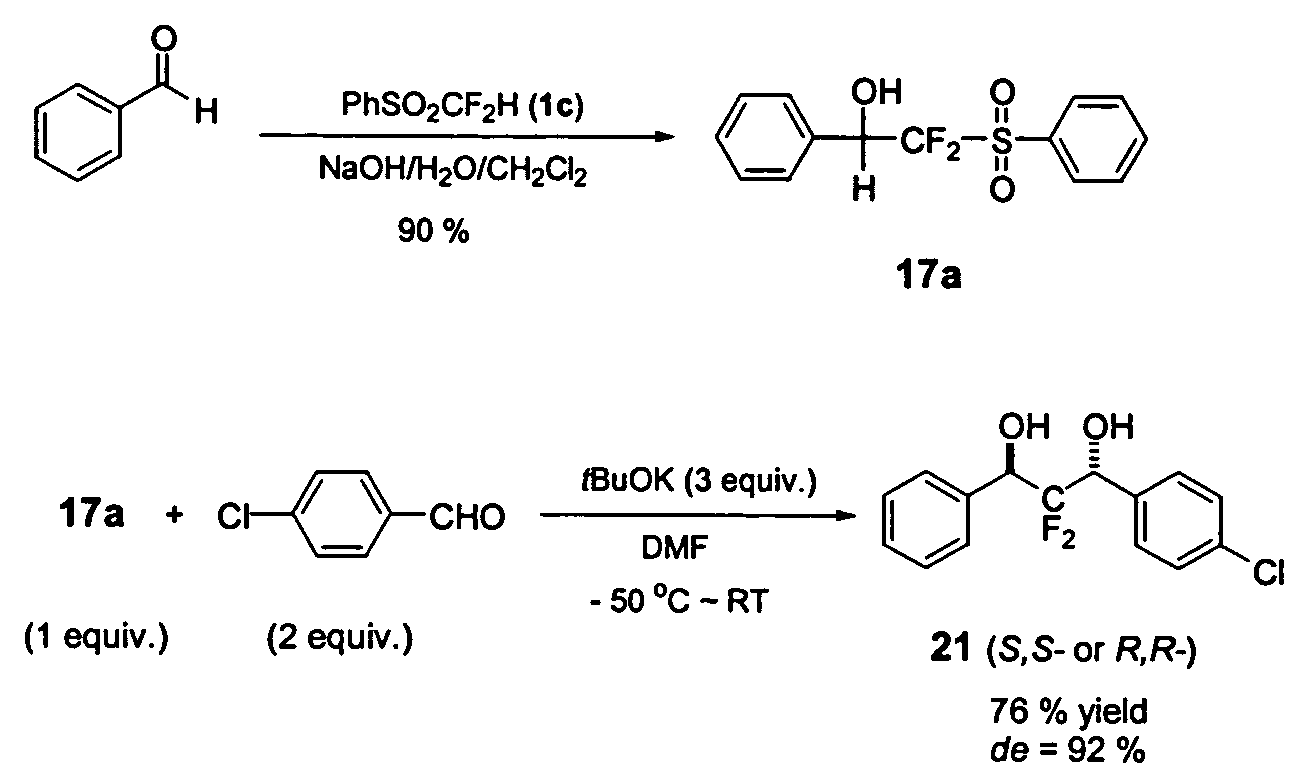
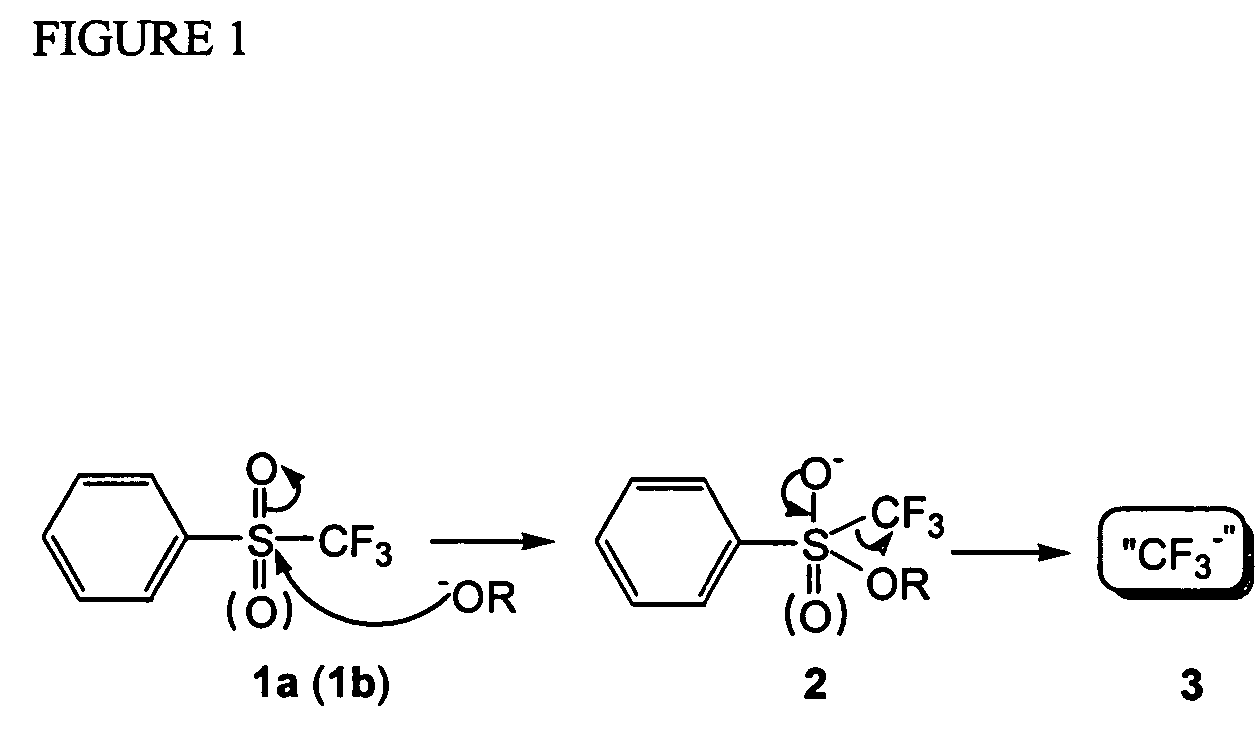
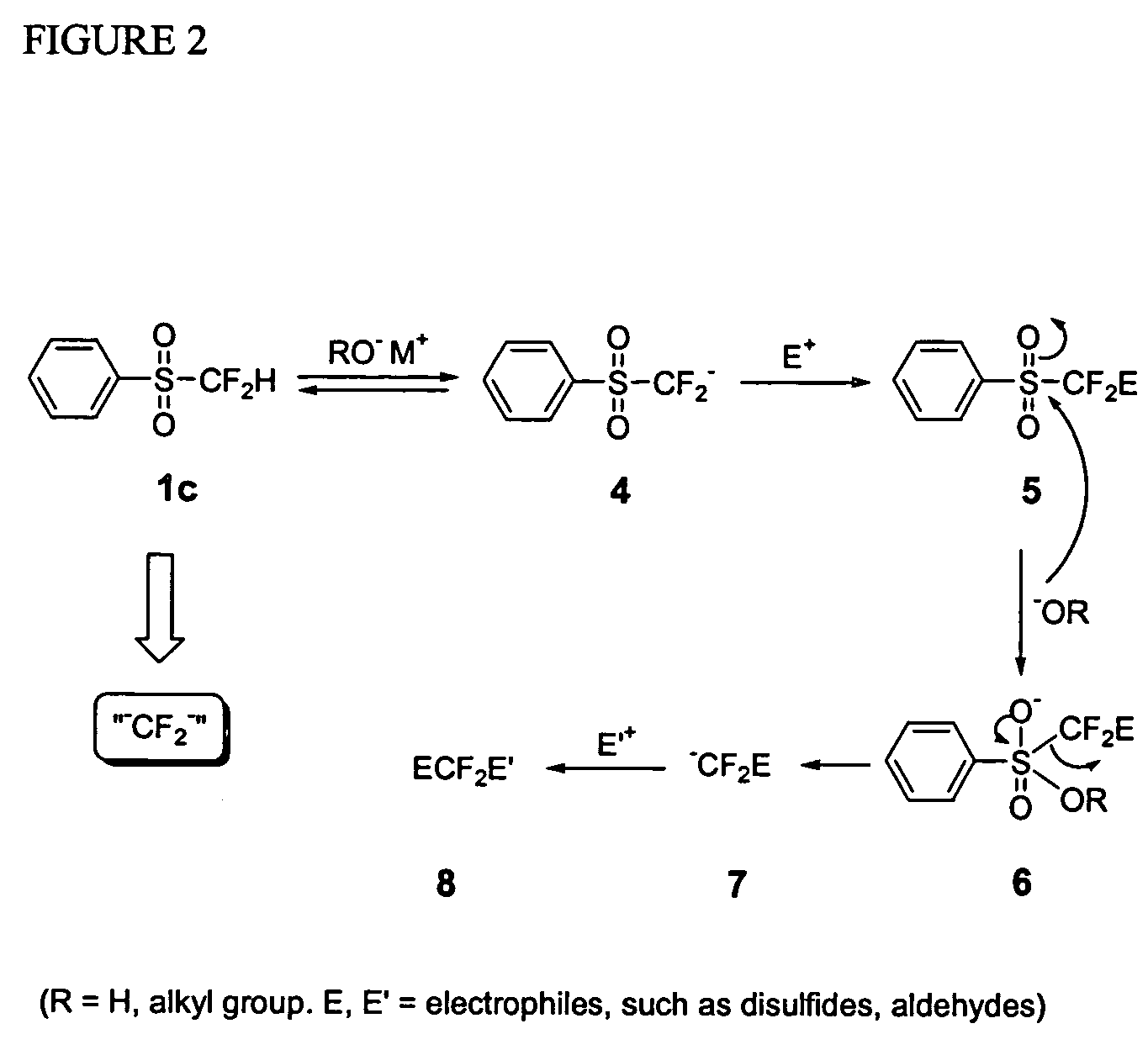
A novel, convenient and efficient method for trifluoromethylation of substrate compounds is disclosed. Particularly, alkoxide and hydroxide induced nucleophilic trifluoromethylation of carbonyl compounds, disulfides and other electrophiles, using phenyl trifluoromethyl sulfone PhSO2CF3 (or sulfoxide PhSOCF3) is disclosed. A method of both symmetrical and unsymmetrical anti-2,2-difluoropropan-1,3-diols with high diastereoselectivity (up to 94% de) is disclosed using difluoromethyl phenyl sulfone. This unusual type of high diastereoselectivity was obtained via an intramolecular charge-charge repulsion effect rather than the traditional steric control (based on the Cram's rule). Thus, difluoromethyl phenyl sulfone can be used as a novel difluoromethylene dianion species (“−CF2−”), which can couple two electrophiles (such as diphenyl disulfide or non-enolizable aldehydes) to give new difluoromethylenated products.
Owner:UNIV OF SOUTHERN CALIFORNIA
One-pot synthesis method for substituted diphenyl sulfide
InactiveCN108997180AEasy to quantitative controlHigh reaction safetySulfide preparationSulfonyl chlorideDistillation
The invention relates to a one-pot synthesis method for substituted diphenyl sulfide. The process comprises the following steps: adding thiophenol or diphenyl disulfide and derivatives thereof into asolvent, dropwise adding sulfonyl chloride for a reaction so as to obtain a substituted benzene sulfuryl chloride solution, removing a part of the solvent at a normal pressure, adding Lewis acid, thendropwise adding substituted benzene for a Friedel-Crafts reaction, and carrying out distillation or recrystallization so as to obtain the substituted diphenyl sulfide. The method provided by the invention has the advantages of high safety, simple and convenient raw material recovery, greatly-reducedpurifying difficulty, easily-available raw materials, simple unit operation, low requirements on reaction equipment, mild reaction conditions, high yield and content and applicability to industrial production; and the content of the finally obtained substituted diphenyl sulfide product generally reaches 98% or more.
Owner:ZHEJIANG YANGFAN NEW MATERIALS
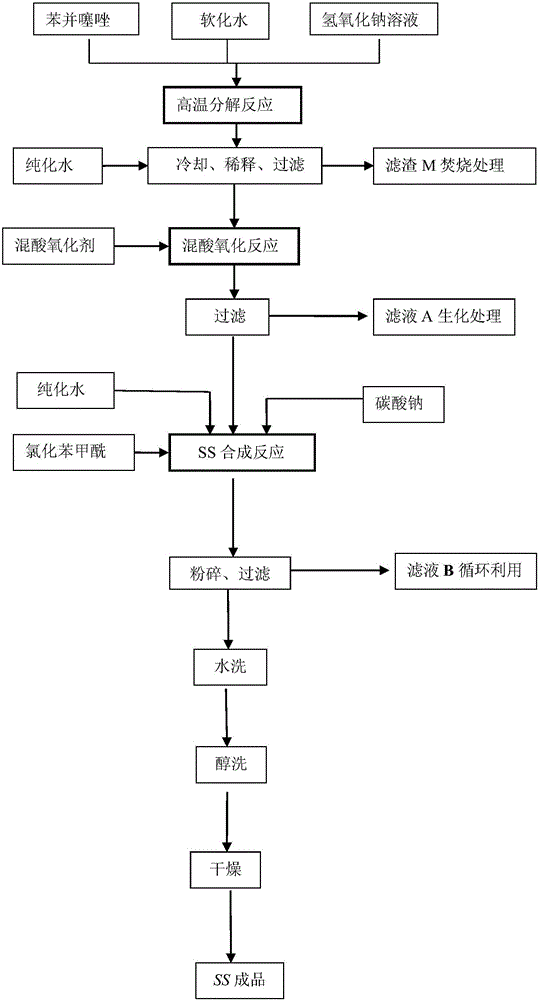
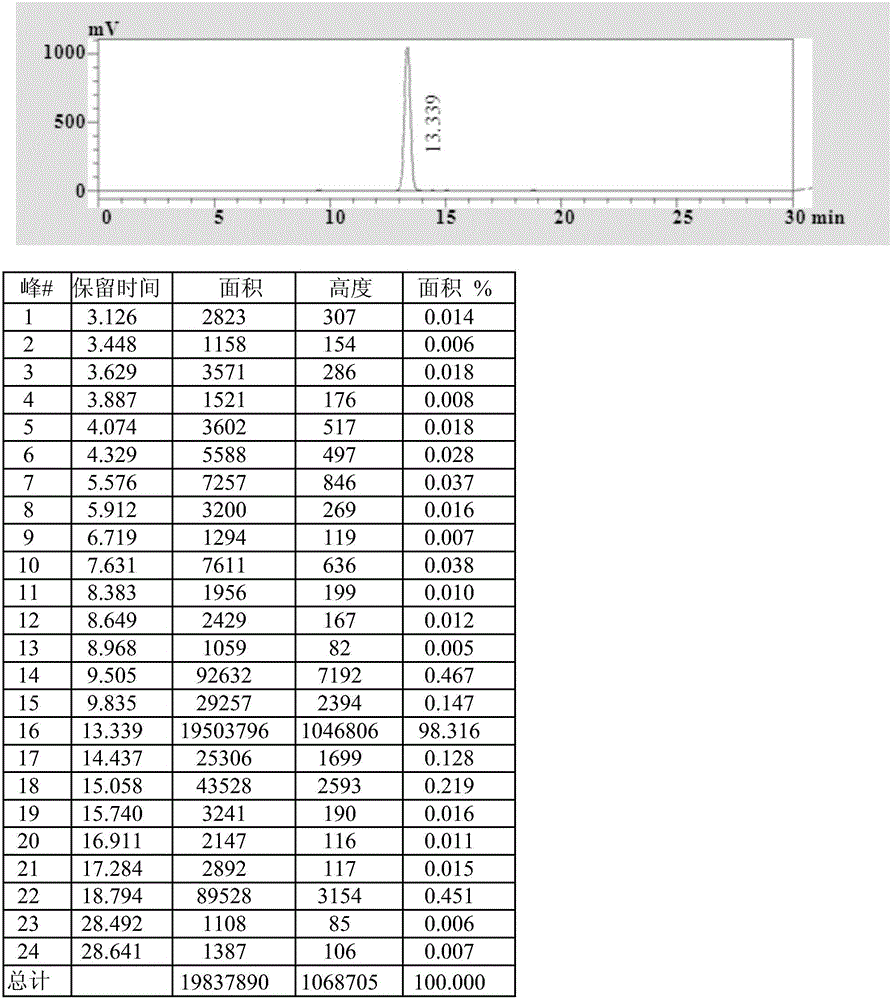
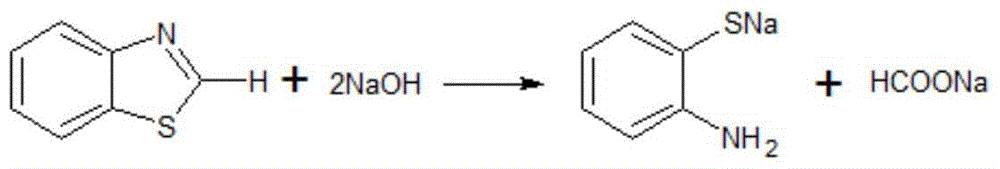
Rubber peptizer SS preparation method
ActiveCN105712913ASimple processEasy to operateOrganic compound preparationHydropoly/poly sulfide preparationDecompositionDiphenyl disulfide
The invention provides a rubber peptizer SS preparation method. The rubber peptizer SS preparation method includes the steps of subjecting benzothiazole and sodium hydroxide to high-temperature decomposition reaction so as to prepare a sodium aminothiophenol solution; dropwise adding a mixed acid solution prepared from water, sulfuric acid and hydrogen peroxide into the sodium aminothiophenol solution at 35-40 DEG C prior to oxidation reaction, and filtering to obtain DS (2,2'-diphenylamine disulphide) after the oxidation reaction is completed; adding the DS and the water into a reaction kettle, adding sodium carbonate, stirring to increase the temperature to 90-95 DEG C, dropwise adding benzoyl chloride for acylation reaction, cooling after the reaction is completed, performing solid-liquid separation and alcohol washing, and drying at 50 DEG C so as to obtain the SS (2,2'-dibenzoyl diphenyl disulfide). The rubber peptizer SS preparation method has the advantages that the raw materials are free of toxicity, cheap and easily available, and a technological process is simple and easy to operate; toxic solvents are unused, and accordingly a preparation workshop is low in toxicity and less polluted; high yield and high quality are achieved, so that the rubber peptizer SS is promising in market prospect; since the alcohol solvents can be recycled, overall cost is low and rubber peptizer SS purity can be above 98%.
Owner:WILLING NEW MATERIALS TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com