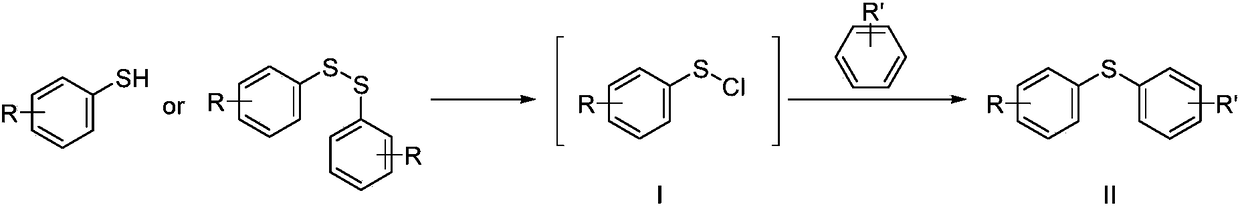
One-pot synthesis method for substituted diphenyl sulfide
A technology for diphenyl sulfide and diphenyl disulfide, which is applied to the field of one-pot synthesis of substituted diphenyl sulfide, can solve the problems of narrow application range of diphenyl sulfide, little industrial production, and high price, and achieves a reaction equipment Low requirements, avoid distillation process and the formation of high boilers, the effect of easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
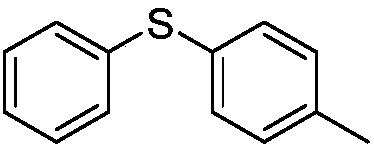
[0036]Add 21.8g of diphenyl disulfide and 50mL of dichloroethane into the three-neck flask, cool down to 5-10°C, slowly add 13.4g of sulfuryl chloride dropwise, while controlling the internal temperature at 5-10°C, and the dropwise addition is completed in about 4 hours. Raise the temperature to 25-30°C to react for 2 hours, and remove 10 mL of dichloroethane under normal pressure. Cool in an ice bath to 5-10°C, add 31.9g of aluminum trichloride, keep stirring at this temperature for 1 hour, then slowly add 19.32g of toluene dropwise, the dropwise addition is completed in about 1 hour, and keep warm and separate for 3 hours after dropping. Slowly add the reaction solution into ice water to quench, extract the water phase with dichloroethane, combine the organic phases to recover the dichloroethane from the desolvation under reduced pressure, and collect the dichloroethane at 60-80pa under reduced pressure to collect the fraction at 90-95°C to obtain The color liqui...
Embodiment 2
[0038]
[0039] Add 21.8g of thiophenol and 50mL of dichloroethane into the three-neck flask, cool down to 5-10°C, slowly add 26.7g of sulfuryl chloride dropwise, while controlling the internal temperature at 5-10°C, and complete the dropwise addition in about 2 hours. Raise the temperature to 25-30°C to react for 2 hours, and remove 10-15 mL of dichloroethane under normal pressure. Cool in an ice bath to 5-10°C, add 31.6g of aluminum trichloride, keep stirring at this temperature for 1 hour, then slowly add 19.1g of toluene dropwise, the dropwise addition is completed in about 1 hour, and keep warm and separate for 3 hours after dropping. Slowly add the reaction solution into ice water to quench, extract the water phase with dichloroethane, combine the organic phases to recover the dichloroethane from the desolvation under reduced pressure, and collect the dichloroethane at 60-80pa under reduced pressure to collect the fraction at 90-95°C to obtain Color liquid 35.4g, yiel...
Embodiment 3
[0041]
[0042] Add 21.8g of diphenyl disulfide and 50mL of dichloroethane into the three-neck flask, cool down to 5-10°C, slowly add 13.4g of sulfuryl chloride dropwise, while controlling the internal temperature at 5-10°C, and the dropwise addition is completed in about 2 hours. Raise the temperature to 25-30°C to react for 2 hours, and remove 10-15 mL of dichloroethane under normal pressure. Cool in an ice bath to 5-10°C, add 31.9g of aluminum trichloride, keep stirring at this temperature for 1 hour, then slowly add 20.1g of fluorobenzene dropwise, the dropwise addition is completed in about 1 hour, and keep warm and separate for 3 hours after dropping. Slowly add the reaction solution into ice water to quench, extract the water phase with dichloroethane, combine the organic phases to recover the dichloroethane from desolvation under reduced pressure, and collect the dichloroethane at 60-80pa under reduced pressure to collect the fraction at 68-73°C to obtain The color ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com