Triaryl sulfonium salt containing 1-methyl-2-phenyl indole skeleton and preparation method thereof
A phenylindole, triaryl technology, applied in triaryl sulfonium salt and its preparation, the application field of ultraviolet curing coatings, can solve the problems of cracking, low solubility, etc., to reduce the release amount and the degree of molecular conjugation The effect of high, good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put a magnetic stirring bar in a two-necked flask equipped with a condenser, add diphenyl disulfide (1.1mmol) and 1-methyl-2-phenylindole (2mmol) respectively, drop DMSO until just dissolved, Cuprous iodide (0.1 mmol) was added, and the temperature was raised to 110° C., followed by reaction for 6 hours. After the reaction, the solvent was removed by rotary evaporation, and column chromatography was carried out to obtain 1-methyl-2-phenyl-3-phenylindole sulfide as white crystals. The yield was 78%, and the melting point was 102- 104°C. 1H NMR (300MHz, CDCl3): 3.75(s, CH3, 3H), 7.01-7.19(m, ArH, 7H), 7.22-7.26(m, ArH, 1H), 7.32-7.37(m, ArH, 5H), 7.41 (m, ArH, 1H).
Embodiment 2
[0022] A magnetic stirring bar was placed in a two-necked flask equipped with a condenser, and diphenyl iodide trifluoromethanesulfonate (1.1 mmol) and 1-methyl-2-phenyl-3-phenylsulfide ind Indole (1mmol), add 1,1,2,2-tetrachloroethane dropwise until just dissolved, then add cuprous iodide (0.1mmol), copper powder (0.05mmol), rise to 110°C, react for 2.5 hours . After the reaction liquid was cooled to room temperature, the solvent was spun off, and the mixture was subjected to column chromatography using dichloromethane and ethyl acetate as eluents successively to obtain compound (I) as white crystals.
[0023]
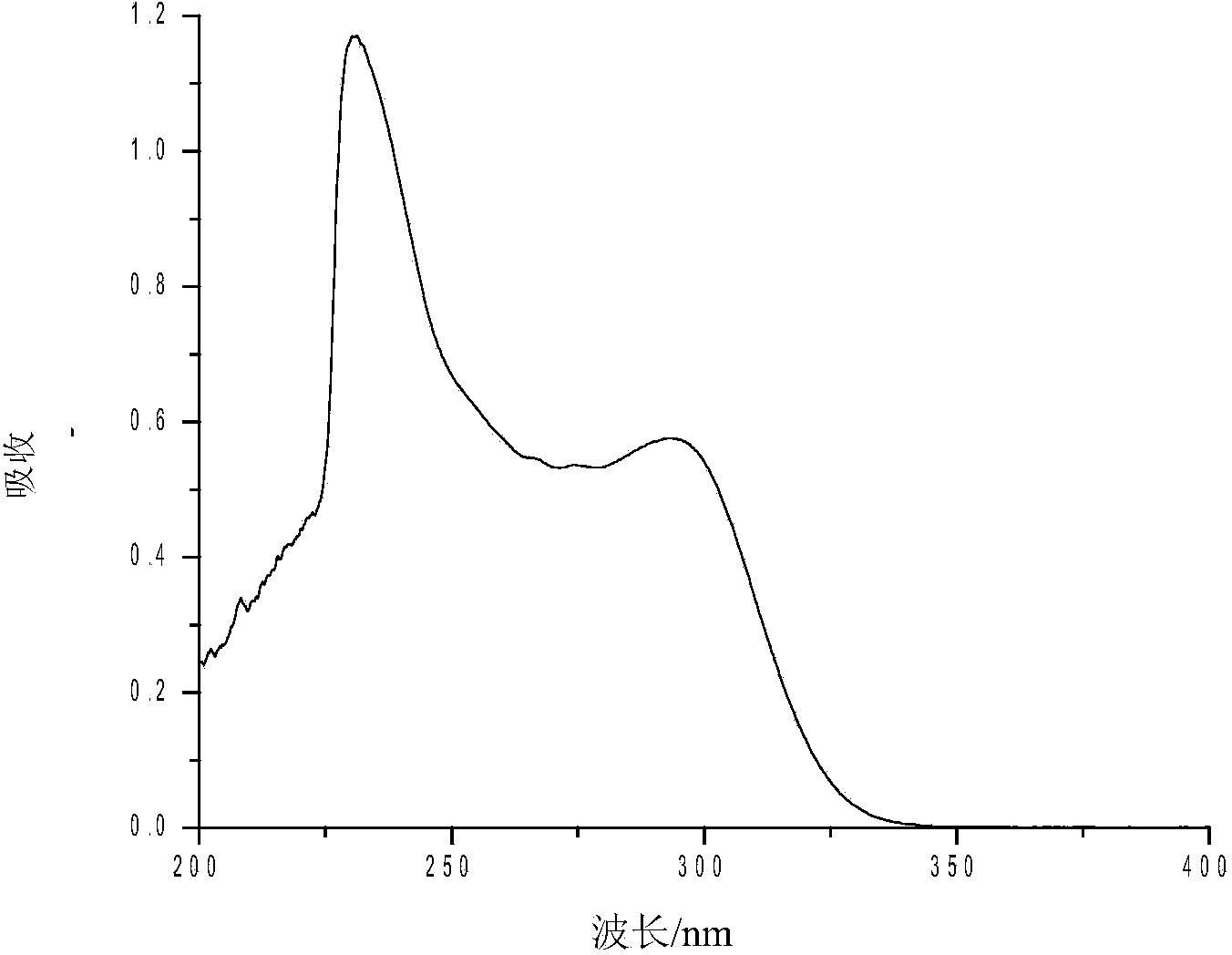
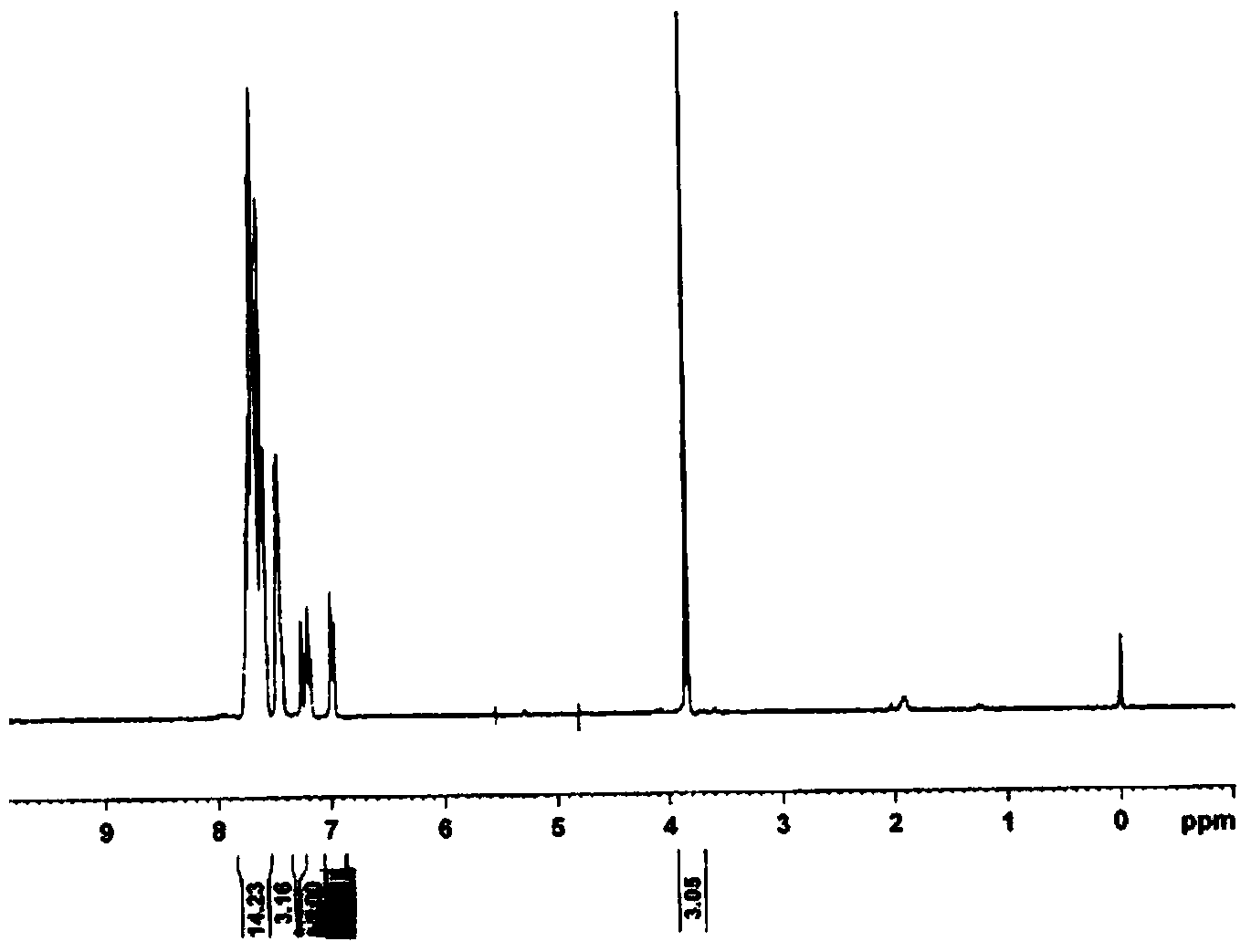
[0024] Compound (I): white crystals, yield 61.3%, melting point 204-205°C. UV spectrum see figure 1 ; H NMR spectrum see figure 2 ; 1 HNMR (300MHz, CDCl 3 ):3.83(s,N-CH 3 ,3H), 7.17~7.19(m,ArH,4H), 7.22~7.44(m,ArH,5H), 7.45~7.64(m,ArH,5H), 7.67~7.73(m,ArH,5H).
Embodiment 3
[0026] A magnetic stirrer was placed in a two-necked flask equipped with a condenser, and bis-(4-methylphenyl)iodotrifluoromethanesulfonate (1.1 mmol) and 1-methyl-2-phenyl-3- Phenylsulfide indole (1mmol), add 1,1,2,2-tetrachloroethane dropwise until just dissolved, then add cuprous iodide (0.1mmol), copper powder (0.05mmol), raise to 110°C Afterwards, react for 2.5 hours. After the reaction, the solvent was spun off, and the mixture was sequentially separated by column chromatography using dichloromethane and ethyl acetate as eluents to obtain compound (II) as pale yellow crystals.
[0027]
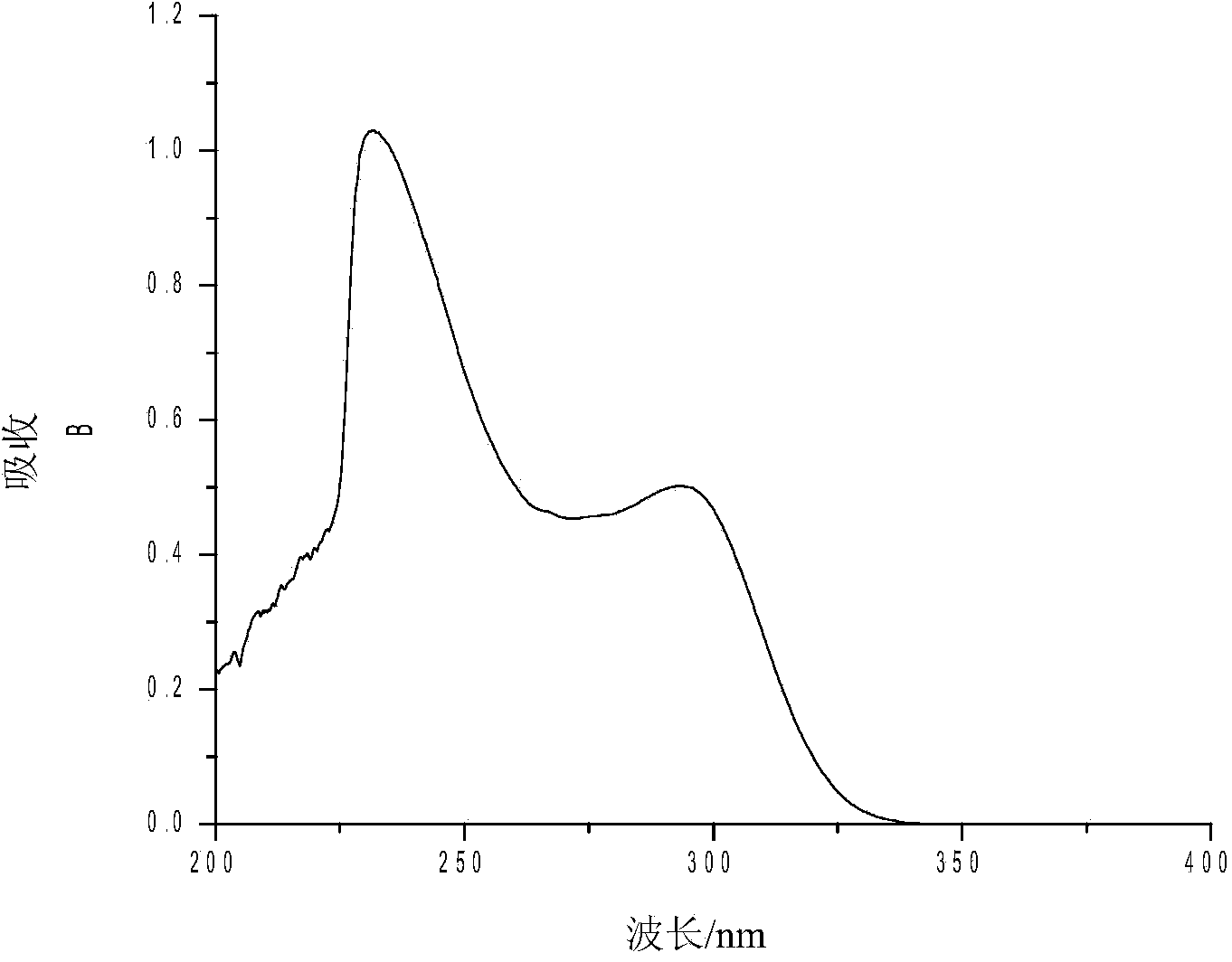
[0028] Compound (II): Pale yellow crystal, yield 62.0%, melting point 174-176°C. UV spectrum see image 3 ; H NMR spectrum Figure 4 : 1 HNMR (600MHz, CDCl 3 ):2.47(s,-CH 3 ,3H),3.84(s,N-CH 3 ,3H), 7.00~7.22(m,ArH,4H), 7.23~7.45(m,ArH,4H), 7.46~7.51(m,ArH,5H), 7.55~7.65(m,ArH,5H).
[0029] The following examples are the curing performance of compound (I) or compound (II) of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com