Synthetic method of benzothiazine formaldehyde derivative
A technology of benzothiazine and synthesis method, which is applied in the field of synthesis of benzothiazine formaldehyde derivatives, can solve the problems of no literature reports, etc., and achieve the effects of simple method, cheap and easy-to-obtain environment, and high regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of synthetic method of benzothiazine formaldehyde derivative, comprises the following steps:
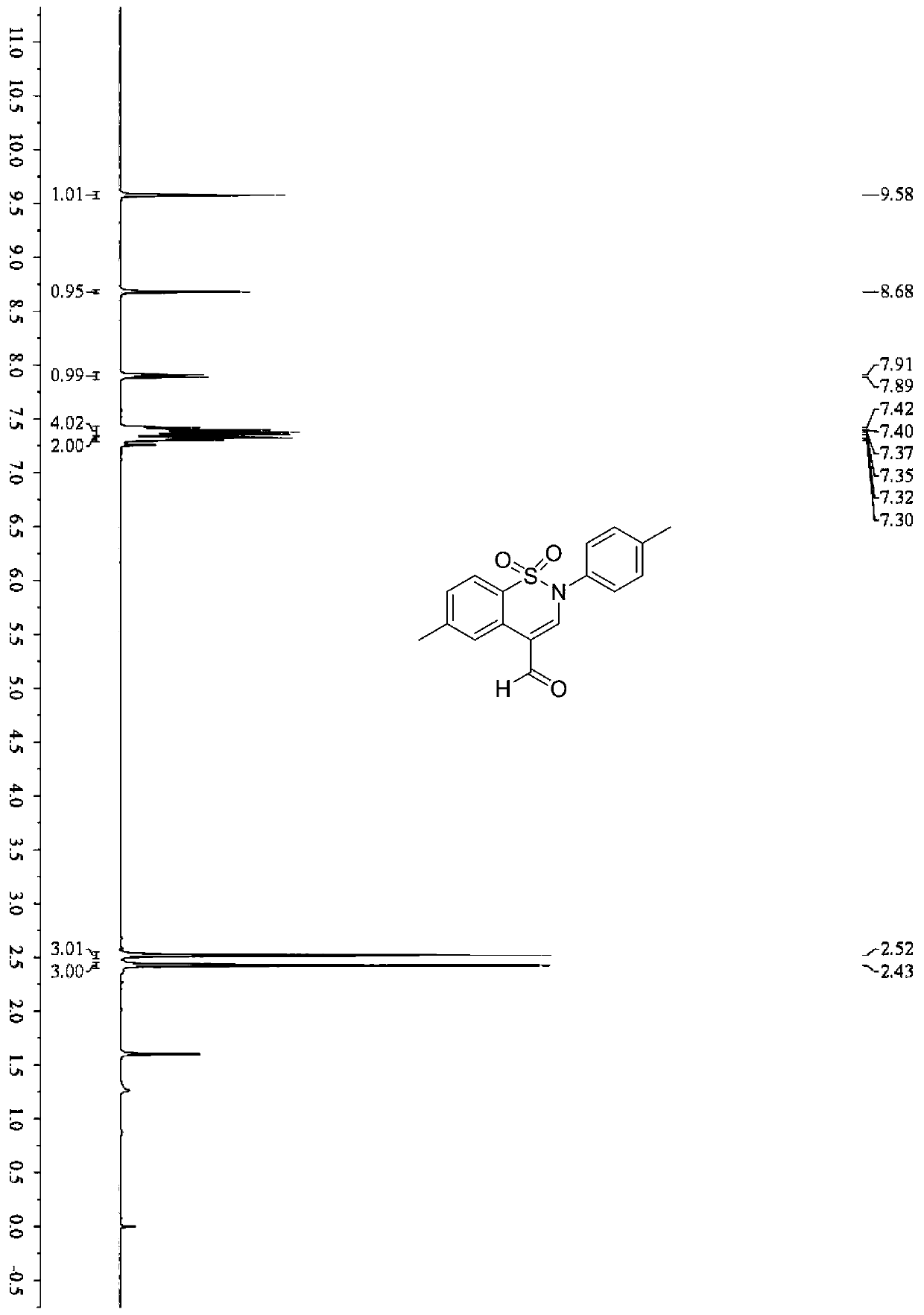
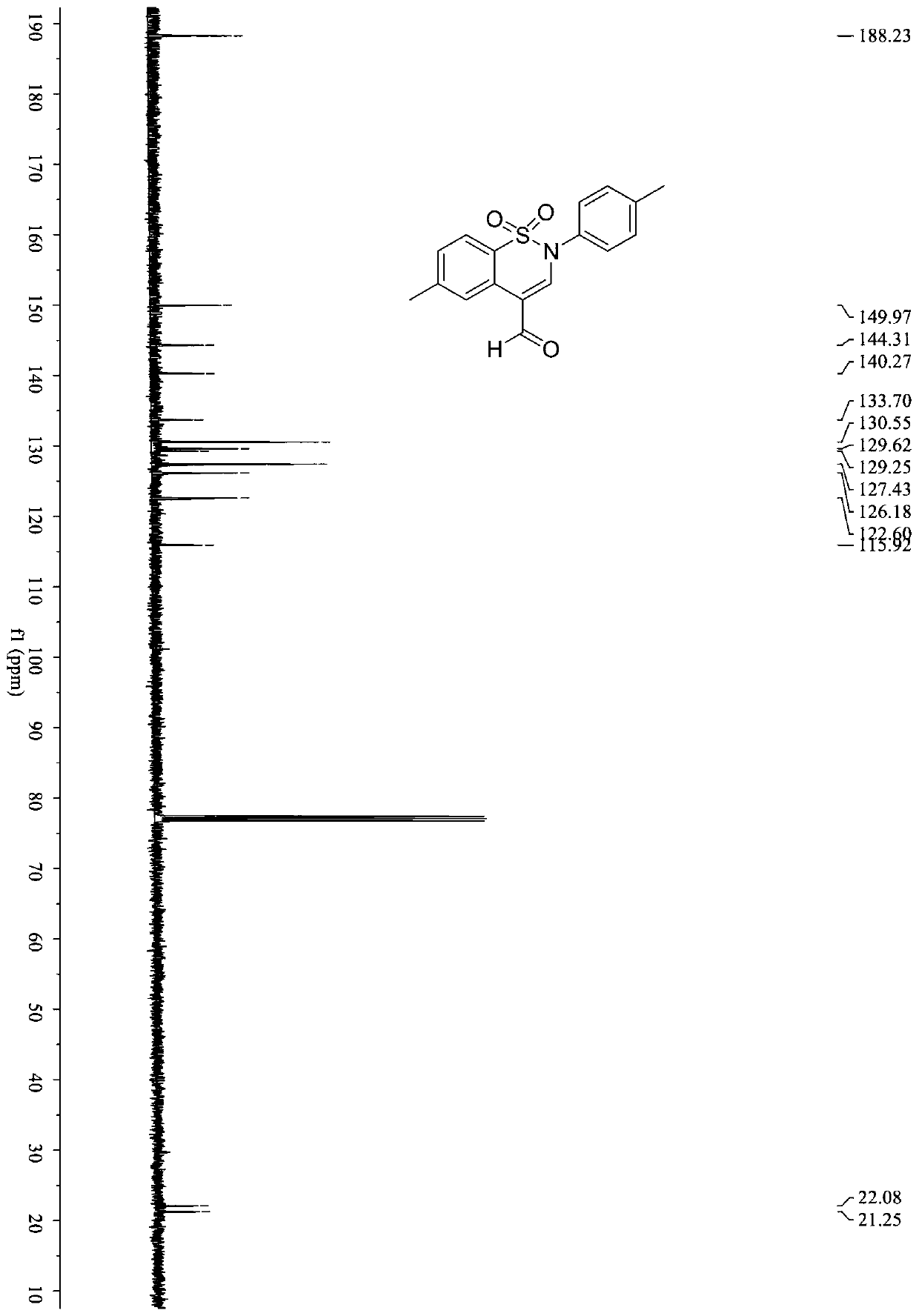
[0040] In a 5ml Snaite tube, add benzenesulfonylpropargylamine compound (0.1mmol, 30mg), EosinY (0.002mmol, 1.4mg), potassium carbonate (0.15mmol, 20.7mg), diphenyl disulfide (0.1 mmol, 21.8mg), 1,2-dichloroethane (1mL); the mixture was reacted at room temperature in the air for 24h under 2×30Wblue LEDs, and after the reaction was completed, it was spin-dried and further separated and purified by column chromatography (elution Liquid is a mixed solvent of petroleum ether and ethyl acetate, the volume ratio is 1:1), to obtain 23.5 mg of product (compound 1), and the yield is: 75%.
[0041] Benzenesulfonyl propargylamine compound: R 1 is 4-Me; R 2 For 4-Me. (4-Me represents the position of the methyl group on the benzene ring)
[0042] The structural characterization data of the product obtained in this embodiment are as follows:
[0043] 1 H NMR (400MHz, CDCl 3 ...
Embodiment 2
[0049] The synthetic method of benzothiazine formaldehyde derivative comprises the following steps:
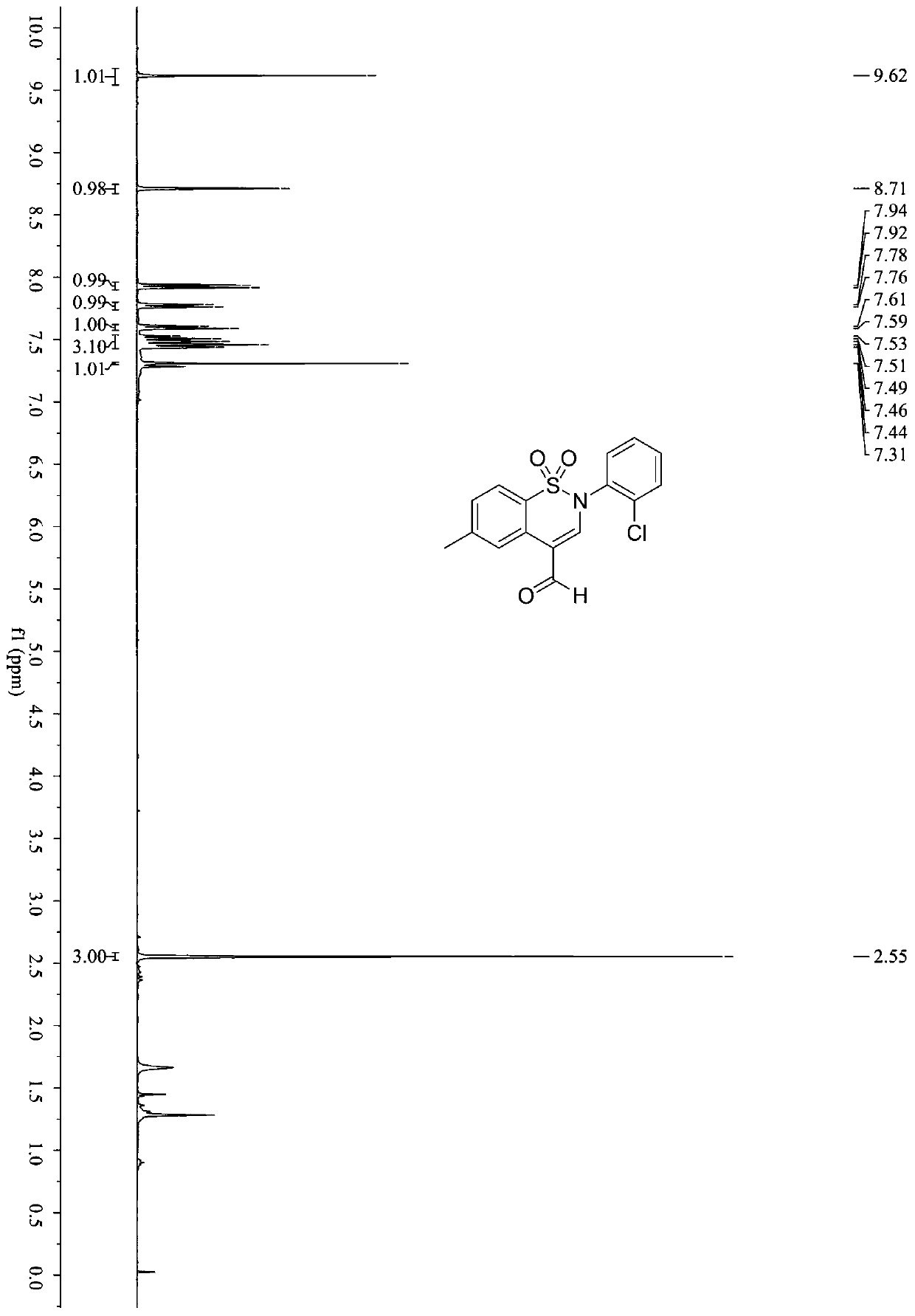
[0050] In a 5ml Snaite tube, add benzenesulfonylpropargylamine compound (0.1mmol, 30mg), EosinY (0.002mmol, 1.4mg), potassium carbonate (0.15mmol, 20.7mg), diphenyl disulfide (0.1 mmol, 21.8mg), 1,2-dichloroethane (1mL); the mixture was reacted at room temperature in the air for 24h under 2×30Wblue LEDs, and after the reaction was completed, it was spin-dried and further separated and purified by column chromatography to obtain the product (Compound 2) 16.3 mg, yield: 51%.
[0051] Benzenesulfonyl propargylamine compound: R 1 is 4-Me; R 2 For 2-Cl. (2-Cl represents the position of Cl on the benzene ring)
[0052] The structural characterization data of the product obtained in this embodiment are as follows:
[0053] 1 H NMR (400MHz, CDCl 3 )δ9.62(s,1H),8.71(s,1H),7.93(d,J=8.1Hz,1H),7.77(d,J=7.7Hz,1H),7.60(d,J=7.9Hz, 1H), 7.55–7.42(m,3H), 7.31(s,1H), 2.55(s,3H). ima...
Embodiment 3
[0059] The synthetic method of benzothiazine formaldehyde derivative comprises the following steps:
[0060] In a 5ml Snaite tube, add benzenesulfonylpropargylamine compound (0.1mmol, 30mg), EosinY (0.002mmol, 1.4mg), potassium carbonate (0.15mmol, 20.7mg), diphenyl disulfide (0.1 mmol, 21.8mg), 1,2-dichloroethane (1mL); the mixture was reacted at room temperature in the air for 24h under 2×30Wblue LEDs, and after the reaction was completed, it was spin-dried and further separated and purified by column chromatography to obtain the product (Compound 3) 22.2 mg, yield: 64%.
[0061] Benzenesulfonyl propargylamine compound: R 1 is 4-Me; R 2 It is 3-Me-4-Cl (representing the meta-position of the methyl group on the benzene ring and the para-position of chlorine on the benzene ring).
[0062] The structural characterization data of the product obtained in this embodiment are as follows:
[0063] 1 H NMR (400MHz, CDCl 3 )δ9.49(s,1H),8.56(s,1H),7.78(d,J=8.2Hz,1H),7.37(d,J=8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com