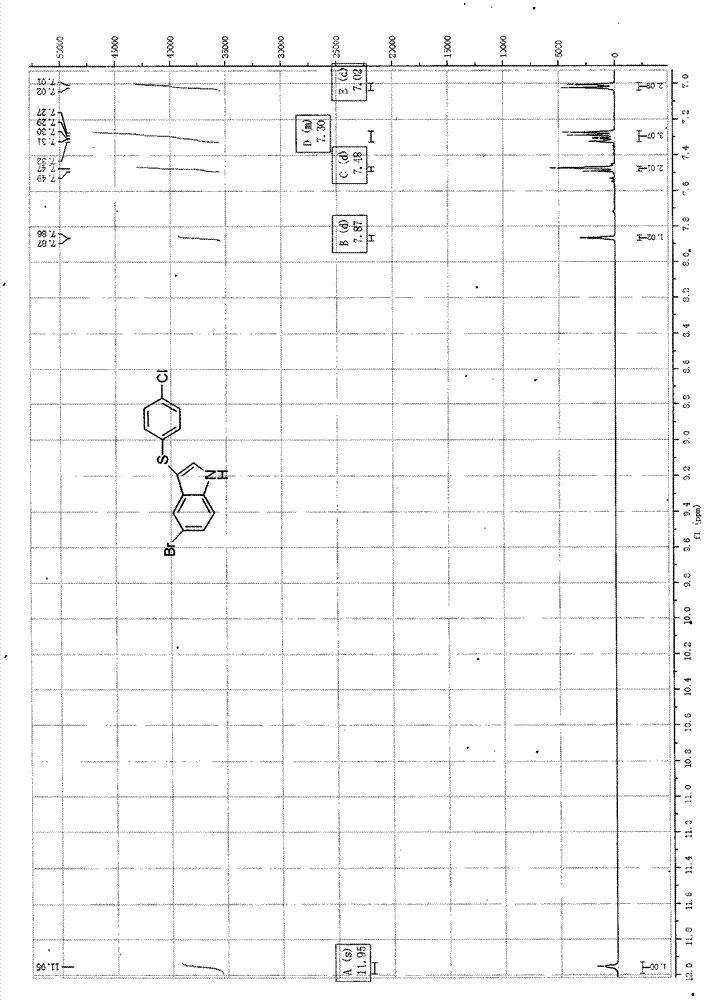
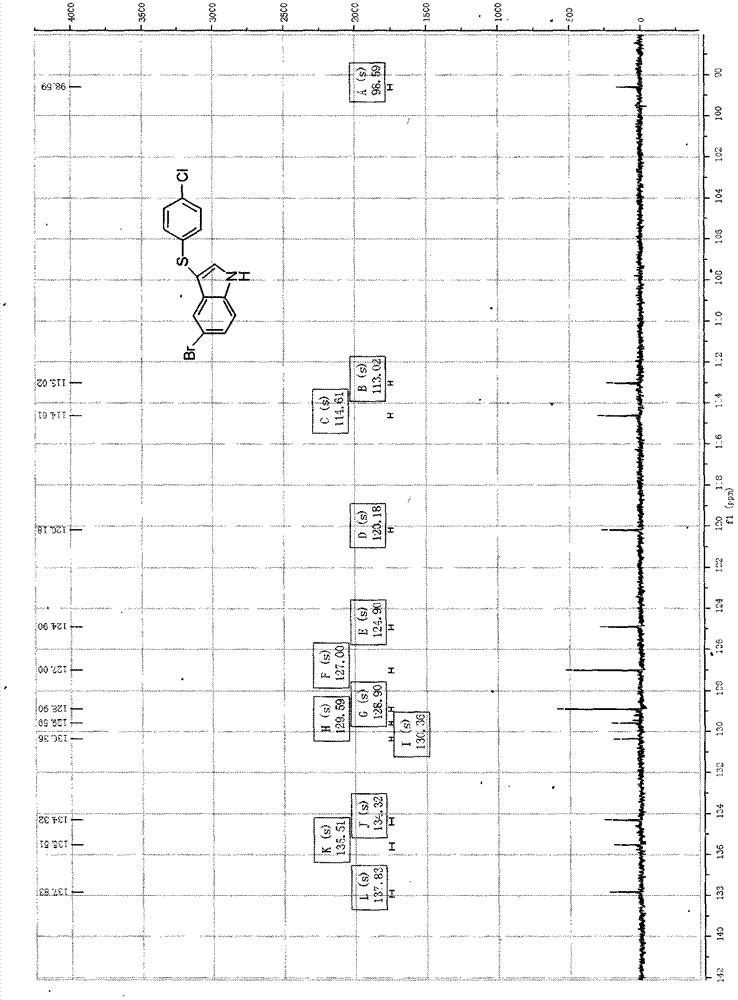
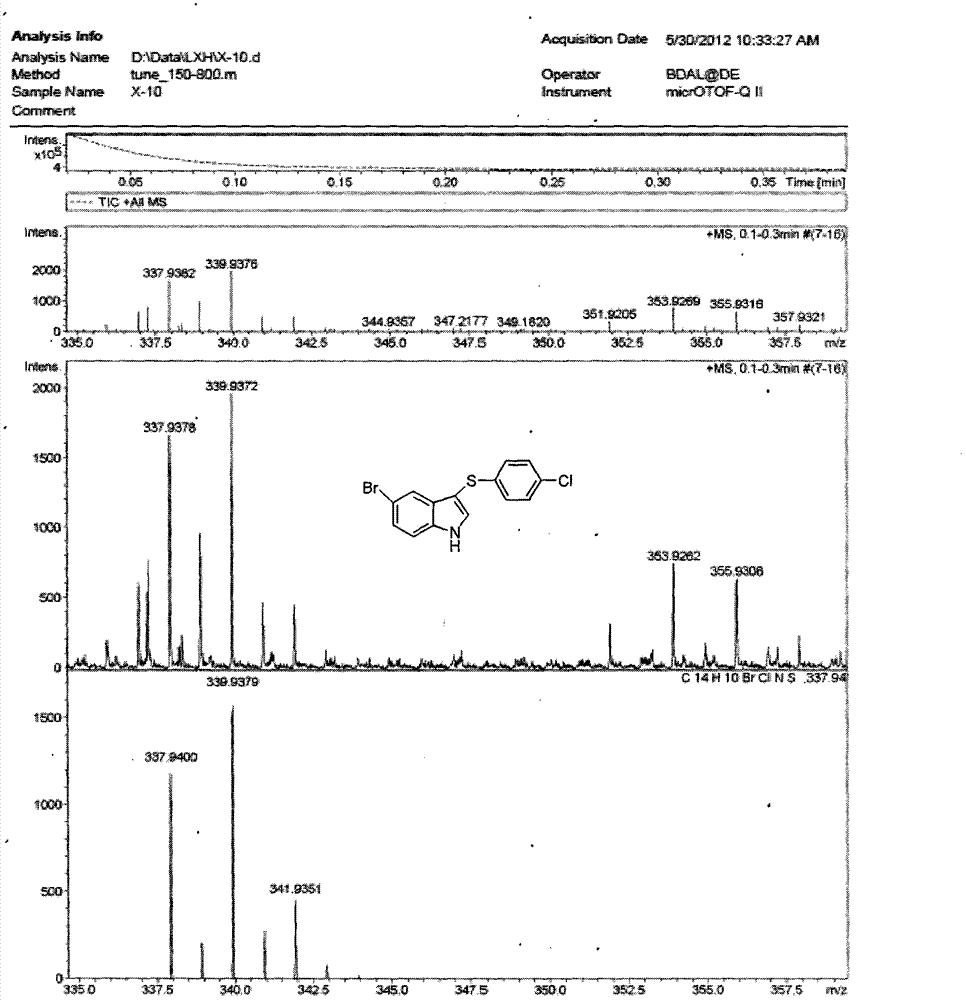
3-((4-chlorobenzene base) thio)-5-bromide-1-hydrogen-indole composite method
A synthetic method, the technology of chlorophenyl, which is applied in the field of synthesis of indole derivatives, can solve the problems of inability to convert technology into economic benefits, difficulty in expanding production scale, and difficulty in realizing large-scale reactions, so as to eliminate metal pollution , increase productivity, and expand the effect of production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Put 0.235mmol (0.046g) of 5-bromoindole and 0.940mmol (0.284g) of 4,4'-dichlorodiphenyl disulfide into a beaker, add 0.047mmol (0.012g) of tetra-n-butyl ammonium hydroxide, and then add Mixed solution of ethanol and water 21ml (V 乙醇 :V 水 = 1: 1.5) slightly heated and stirred, then the mixed solution was transferred to a 30ml stainless steel autoclave with a Teflon lining, heated to 130°C, and reacted for 48h under automatic pressure boosting, TLC tracking and determination of the disappearance of the reactant 5-bromoindole, Then it was naturally cooled to room temperature, and a yellow-brown solid was formed. The solid was collected by suction filtration, and the mother liquor was extracted with 170 ml of ethyl acetate. The organic layer was dried with anhydrous magnesium sulfate, and the solvent was evaporated to dryness to obtain a solid. The crude product is obtained by mixing the two solids. First pass through the column with petroleum ether with a boiling range o...
Embodiment 2
[0048] Put 3.760mmol (0.736g) of 5-bromoindole and 0.940mmol (0.284g) of 4,4'-dichlorodiphenyl disulfide into a beaker, add 0.752mmol (0.192g) of tetra-n-butyl ammonium hydroxide, and then add Mixed solution of ethanol and water 21ml (V 乙醇 :V 水 = 1: 1.5) slightly heated and stirred, then the mixed solution was transferred to a 30ml stainless steel autoclave with a Teflon lining, heated to 130°C, and reacted for 48h under automatic pressure boosting, TLC tracking and determination of the disappearance of the reactant 5-bromoindole, Then it was naturally cooled to room temperature, and a yellow-brown solid was formed. The solid was collected by suction filtration, and the mother liquor was extracted with 170 ml of ethyl acetate. The organic layer was dried with anhydrous magnesium sulfate, and the solvent was evaporated to dryness to obtain a solid. The crude product is obtained by mixing the two solids. First pass through the column with petroleum ether with a boiling range o...
Embodiment 3
[0050] Put 0.470mmol (0.092g) of 5-bromoindole and 0.940mmol (0.284g) of 4,4'-dichlorodiphenyl disulfide into a beaker, add 0.094mmol (0.024g) of tetra-n-butyl ammonium hydroxide, and then add Mixed solution of ethanol and water 21ml (V 乙醇 :V 水 = 1: 1.5) slightly heated and stirred, then the mixed solution was transferred to a 30ml stainless steel autoclave with a Teflon lining, heated to 130°C, and reacted for 48h under automatic pressure boosting, TLC tracking and determination of the disappearance of the reactant 5-bromoindole, Then it was naturally cooled to room temperature, and a yellow-brown solid was formed. The solid was collected by suction filtration, and the mother liquor was extracted with 170 ml of ethyl acetate. The organic layer was dried with anhydrous magnesium sulfate, and the solvent was evaporated to dryness to obtain a solid. The crude product is obtained by mixing the two solids. First pass through the column with petroleum ether with a boiling range o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com