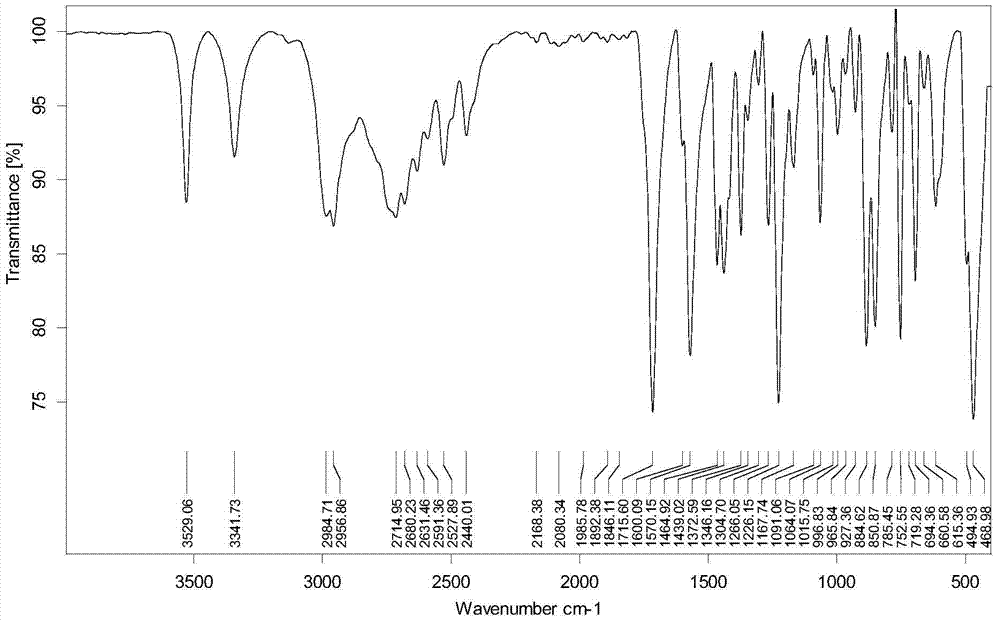
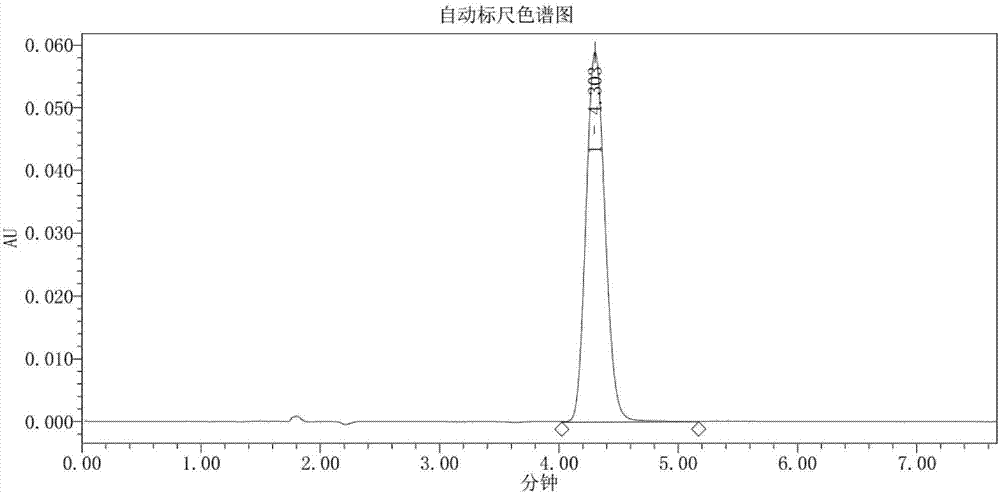
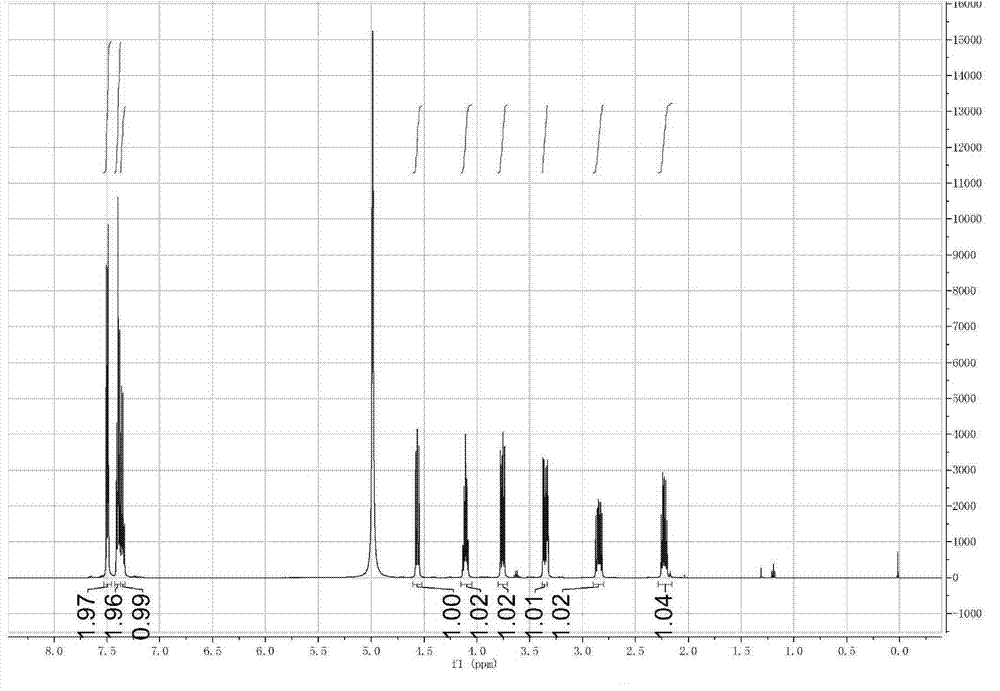
Synthesis method of (2S,4S)-4-thiophenyl-L-proline hydrochloride
A technology of proline hydrochloride and synthesis method, which is applied in the field of amino acid synthesis, can solve the problems of high cost, harsh conditions, and too long synthesis route, and achieve the effects of low requirements for instruments and equipment, mild reaction conditions, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 20 mL of methanol in a 50 mL round-bottomed flask, add 4 mL of thionyl chloride (55.7 mmol) dropwise under cooling in an ice-water bath, raise the temperature to room temperature and stir for 0.5 h, then add 4 g of L-hydroxyproline (30.5 mmol) in batches, React at room temperature for 2 hours and heat up to 45°C for about 5 hours (TLC detects that ninhydrin develops color until the raw material disappears, developing solvent CH 2 Cl 2 : MeOH=2:1 mixed volume fraction 1% acetic acid), the resulting reaction mixture L-hydroxyproline methyl ester was evaporated under reduced pressure to obtain 5.40 g of white solid, and the white solid L-hydroxyproline methyl ester was dissolved in Tetrahydrofuran: In 20mL mixed solvent with a water volume ratio of 1:1, under cooling in an ice-water bath, add 5.48g (65.2mmol) of solid sodium bicarbonate, slowly drop 7.2g (33mmol) of BOC anhydride into the solvent, and keep the reaction in the ice-water bath After 0.5 hours, the tempe...
Embodiment 2
[0039] Take 125mL of methanol in a 250mL round-bottomed flask, add 25mL of thionyl chloride (348.2mmol) dropwise under cooling in an ice-water bath, raise the temperature to room temperature and stir for 0.5h, then add 25g (190.7mmol) of L-hydroxyproline in batches, React at room temperature for 2 hours and raise the temperature to 45°C for about 7 hours (TLC detects that ninhydrin develops color until the raw material disappears, developing solvent CH 2 Cl 2 : MeOH=2:1 mixed volume fraction 1% acetic acid), the resulting reaction mixture L-hydroxyproline methyl ester was evaporated under reduced pressure to obtain 34.42g of white solid, and L-hydroxyproline methyl ester was dissolved in tetrahydrofuran: Add 34.25 g (407.5 mmol) of solid sodium bicarbonate to 120 mL of a mixed solvent with a water volume ratio of 1:1, and add 34.25 g (407.5 mmol) of solid sodium bicarbonate under cooling in an ice-water bath. Slowly add 45 g (206.25 mmol) of BOC anhydride into the solvent, and...
Embodiment 3
[0041] Take 250mL of methanol in a 500mL round-bottomed flask, add 50mL of thionyl chloride (696.25mmol) dropwise under cooling in an ice-water bath, raise the temperature to room temperature and stir for 0.5h, then add 50g (381.25mmol) of L-hydroxyproline in batches, React at room temperature for 2 hours and heat up to 45°C for overnight reaction (TLC detects that ninhydrin develops color until the raw material disappears, developing solvent CH 2 Cl 2 : MeOH=2:1 mixed volume fraction of 1% acetic acid), the resulting reaction mixture L-hydroxyproline methyl ester was evaporated under reduced pressure to obtain 69.4g of white solid (there was a solvent that was not evaporated to dryness), and the white solid L- Hydroxyproline methyl ester was dissolved in tetrahydrofuran: water in 250mL mixed solvent with a volume ratio of 1:1, under cooling in an ice-water bath, 68.5g (815mmol) of sodium bicarbonate solid was added, and 90g (412.5mmol) of BOC anhydride was slowly added dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com