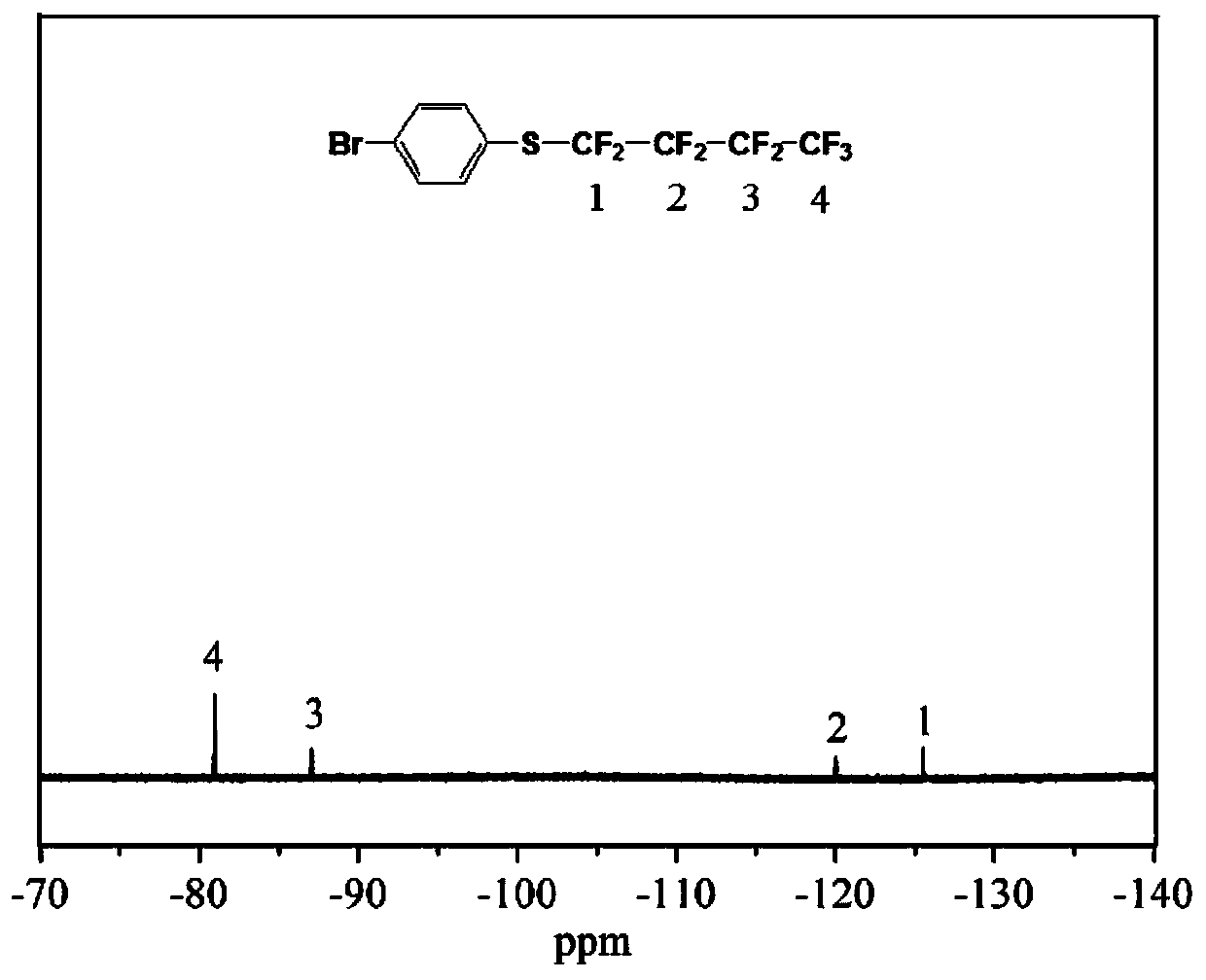
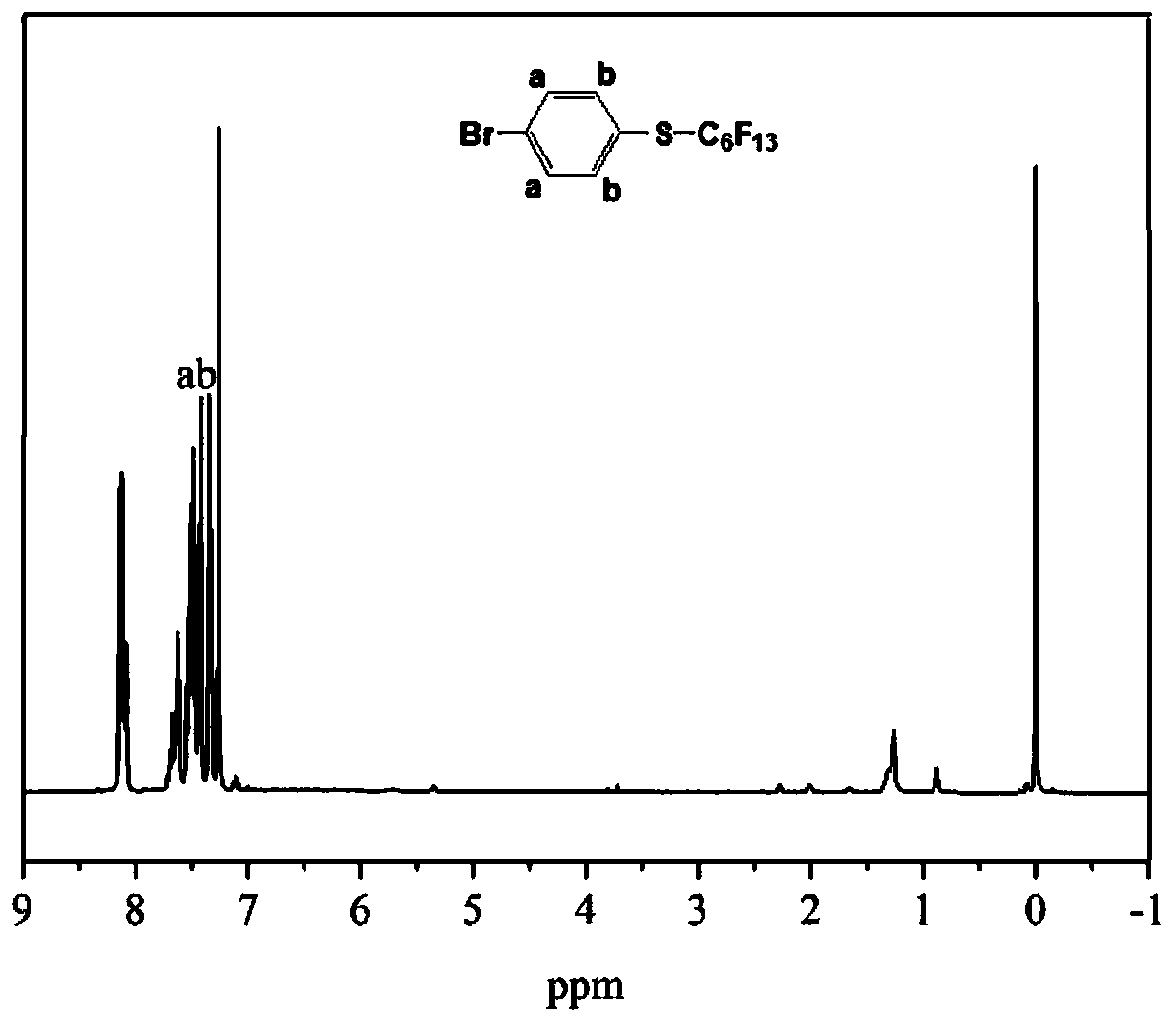
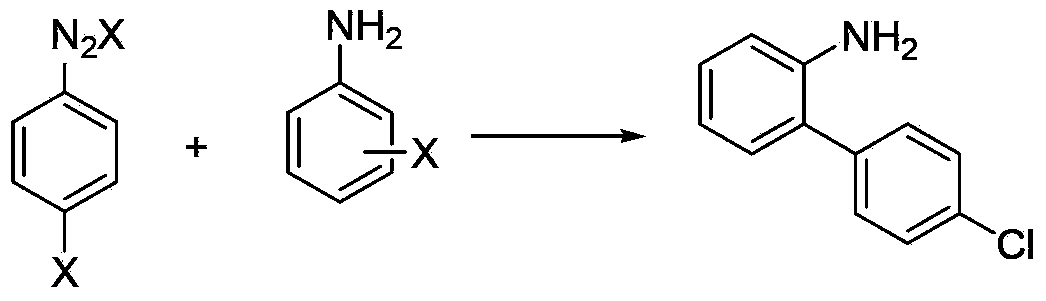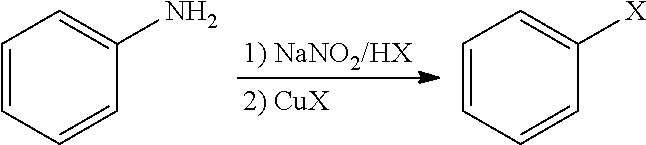Patents
Literature
91 results about "Halobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
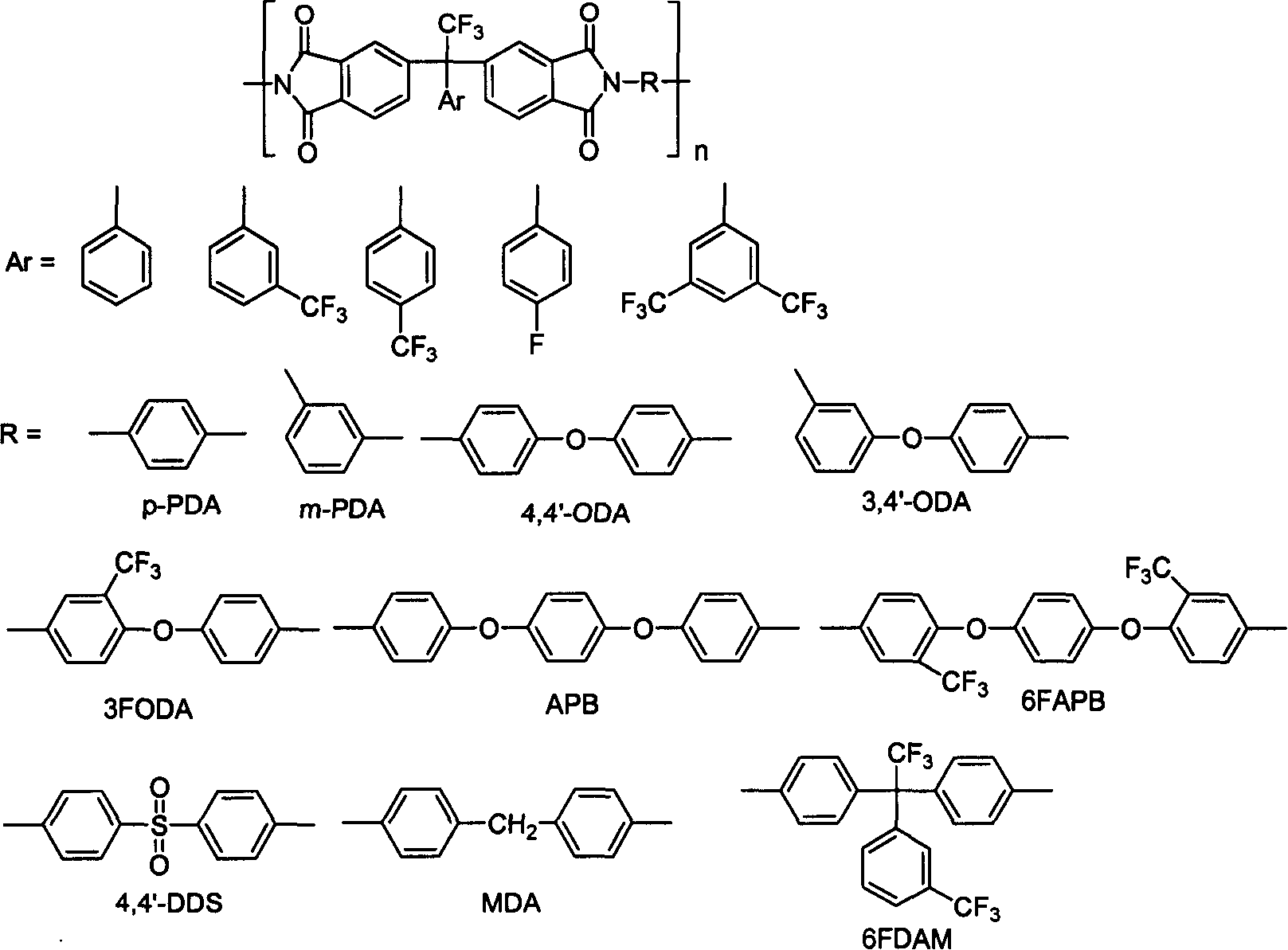
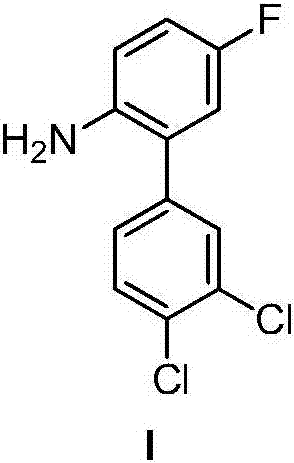
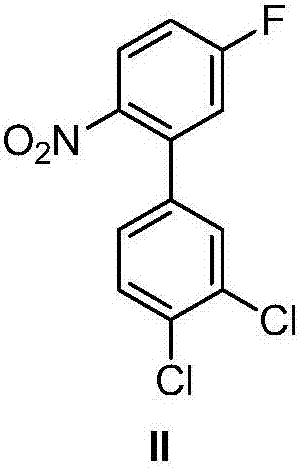
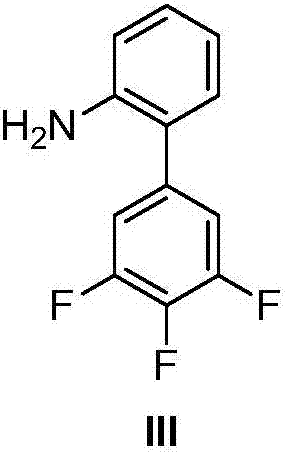
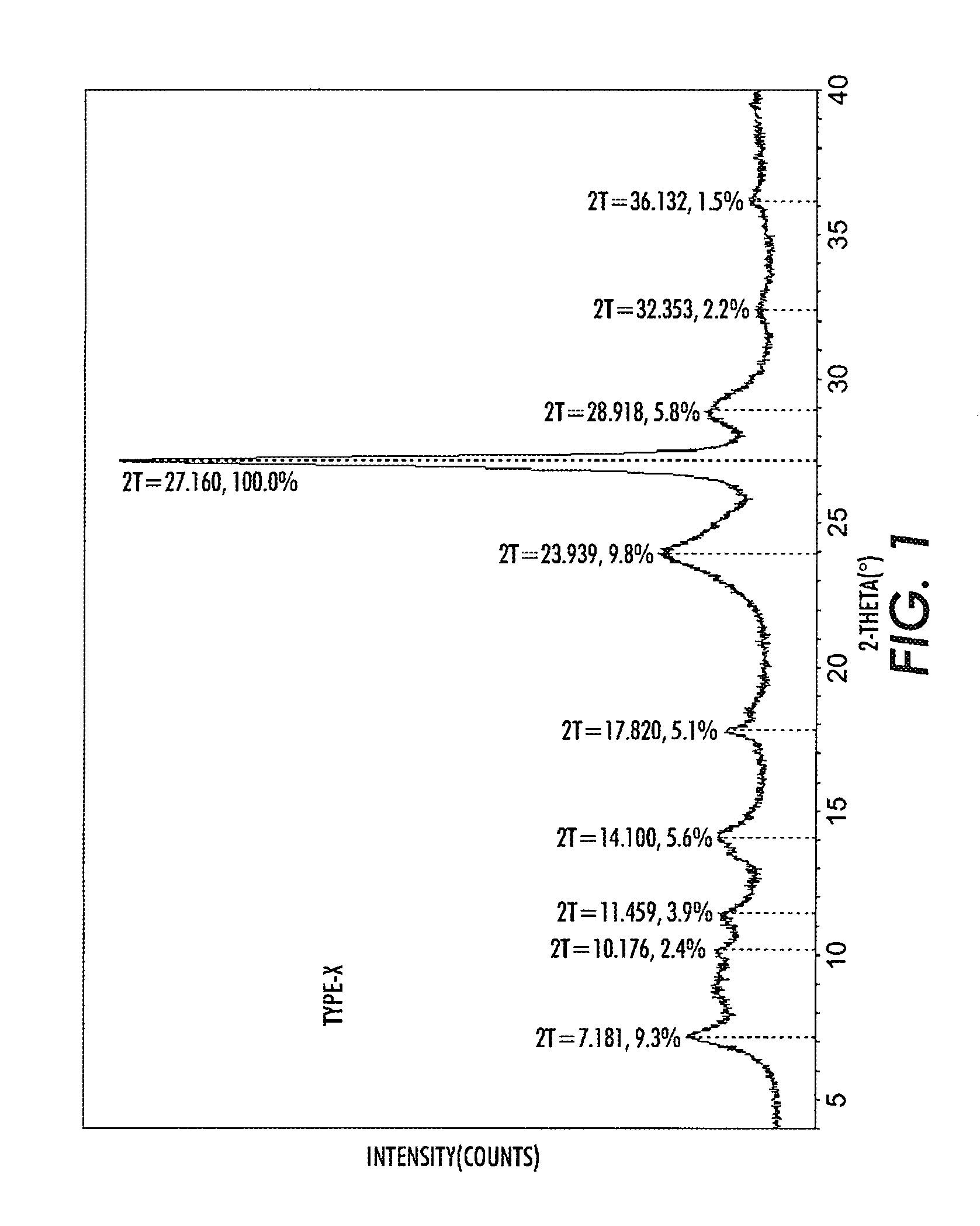
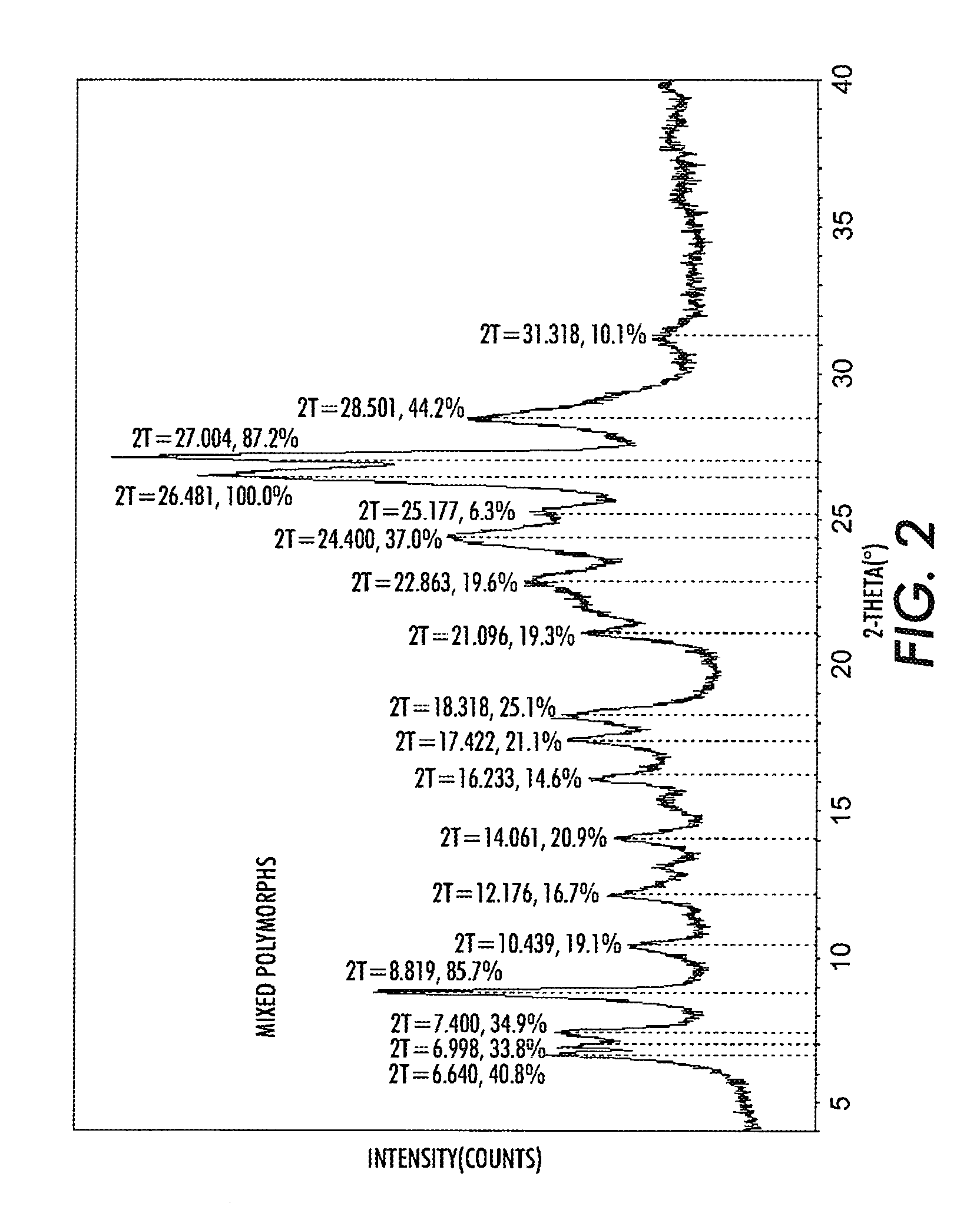
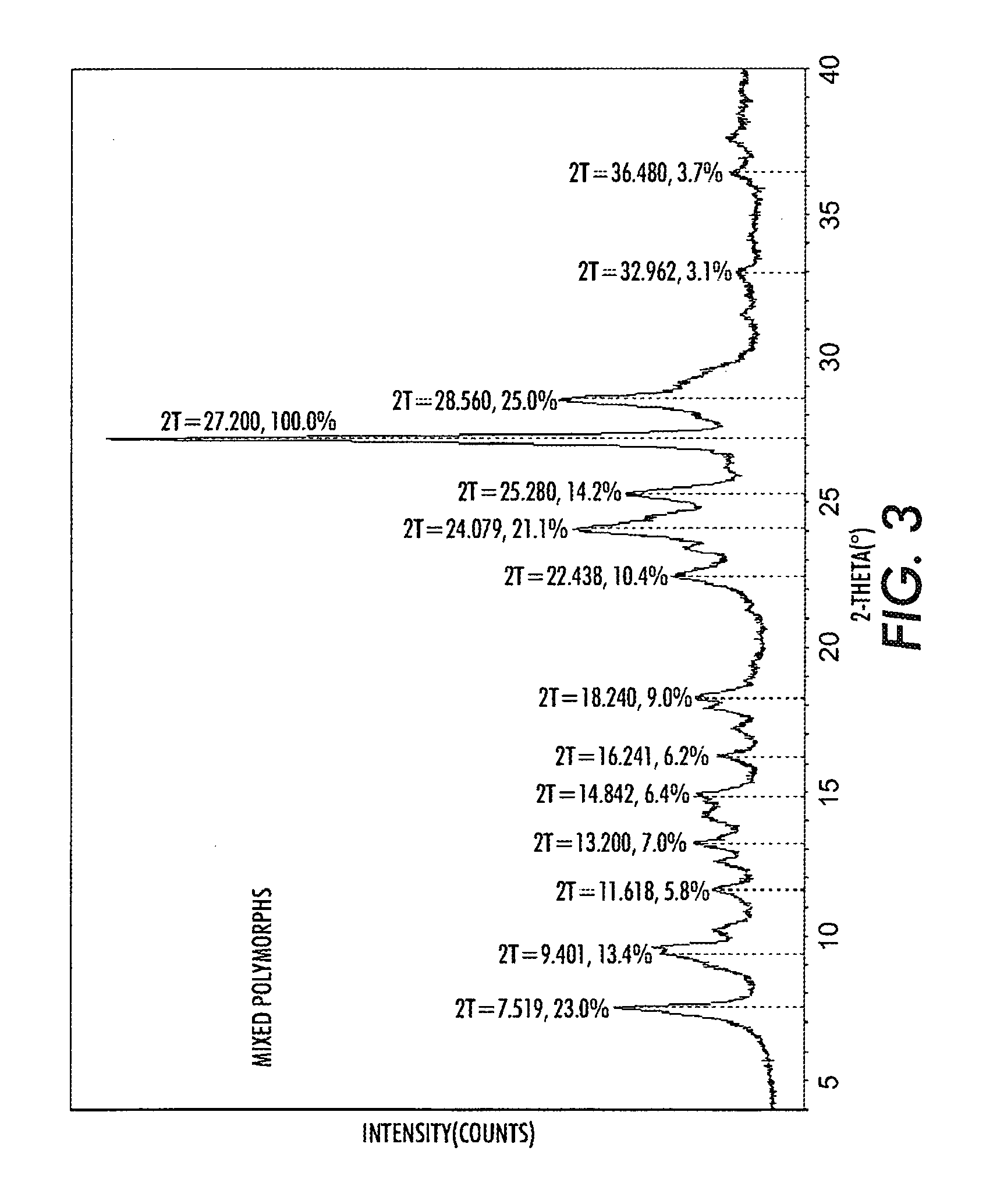
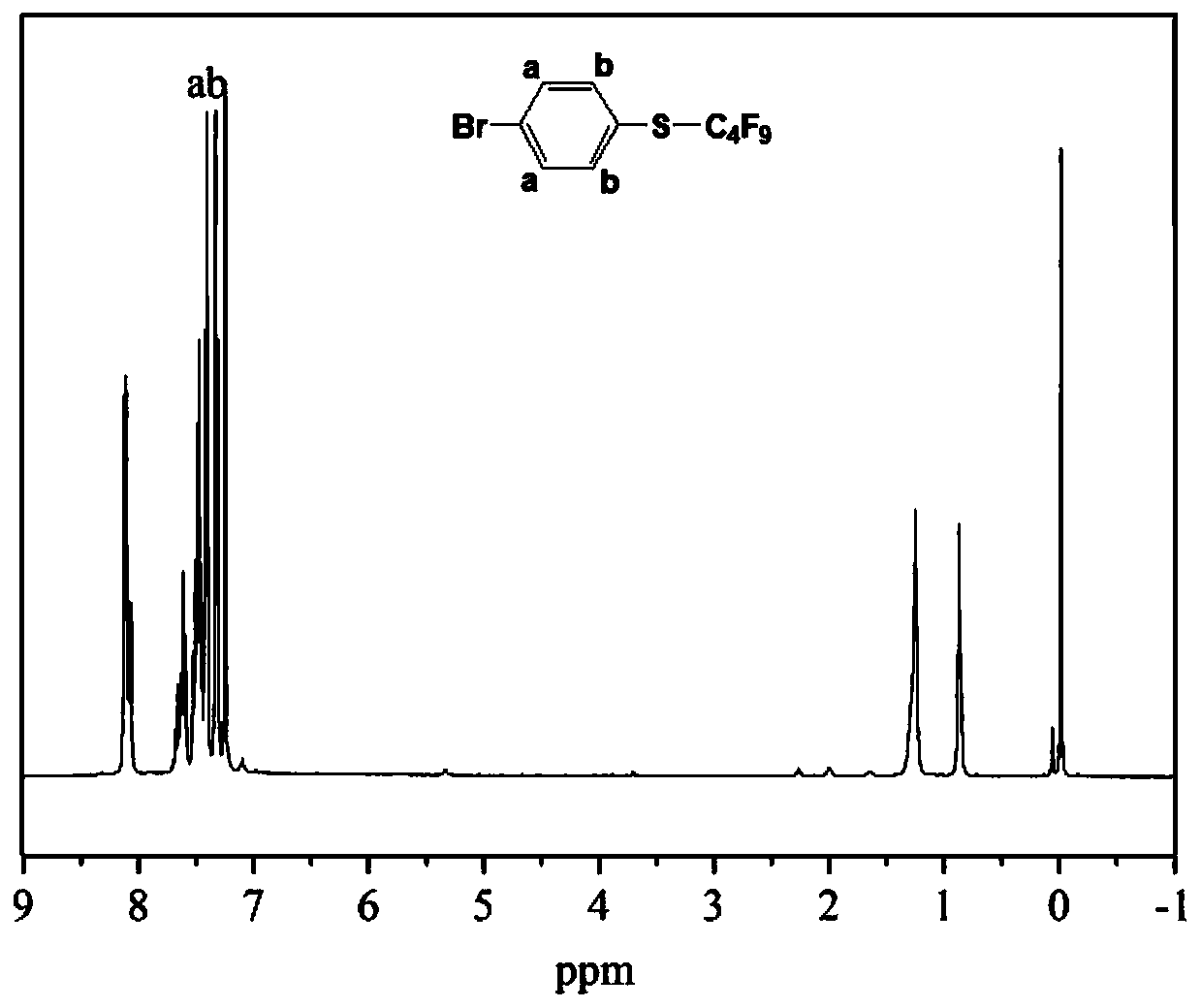
Halogenated benzene compound.
Electrolytes for lithium ion secondary batteries
ActiveUS20060147808A1Improve propertiesNot prone to and explosionElectrolytic capacitorsOrganic electrolyte cellsBenzenePropane sultone
The present invention relates to additives for electrolytes of lithium ion secondary batteries that include one or more of the following: 1,3-propane sultone, succinic anhydride; ethenyl sulfonyl benzene, and halobenzene. It can also include biphenyl, cyclohexylbenzene; and vinylene carbonate. The weight of said 1,3-propane sultone is between 0.5 wt. % and 96.4 wt. %, said succinic anhydride is between 0.5 wt. % and 96.4 wt. %; said ethenyl sulfonyl benzene is between 0.5 wt. % and 95.2 wt. %; and said halobenzene is between 0.5 wt. % and 95.2 wt. % of the weight of the additive. Batteries with electrolytes containing said additives have improved over-charge characteristics and low temperature properties, and reduced gas generation during charging and discharging.
Owner:BYD CO LTD
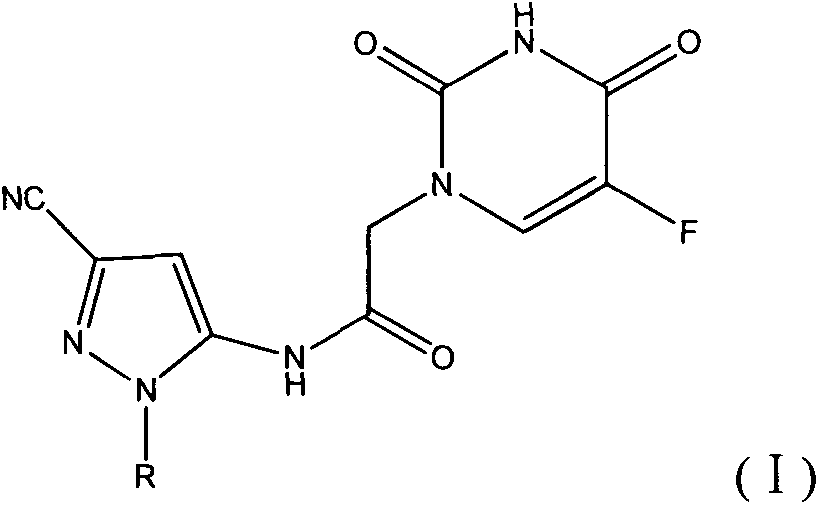
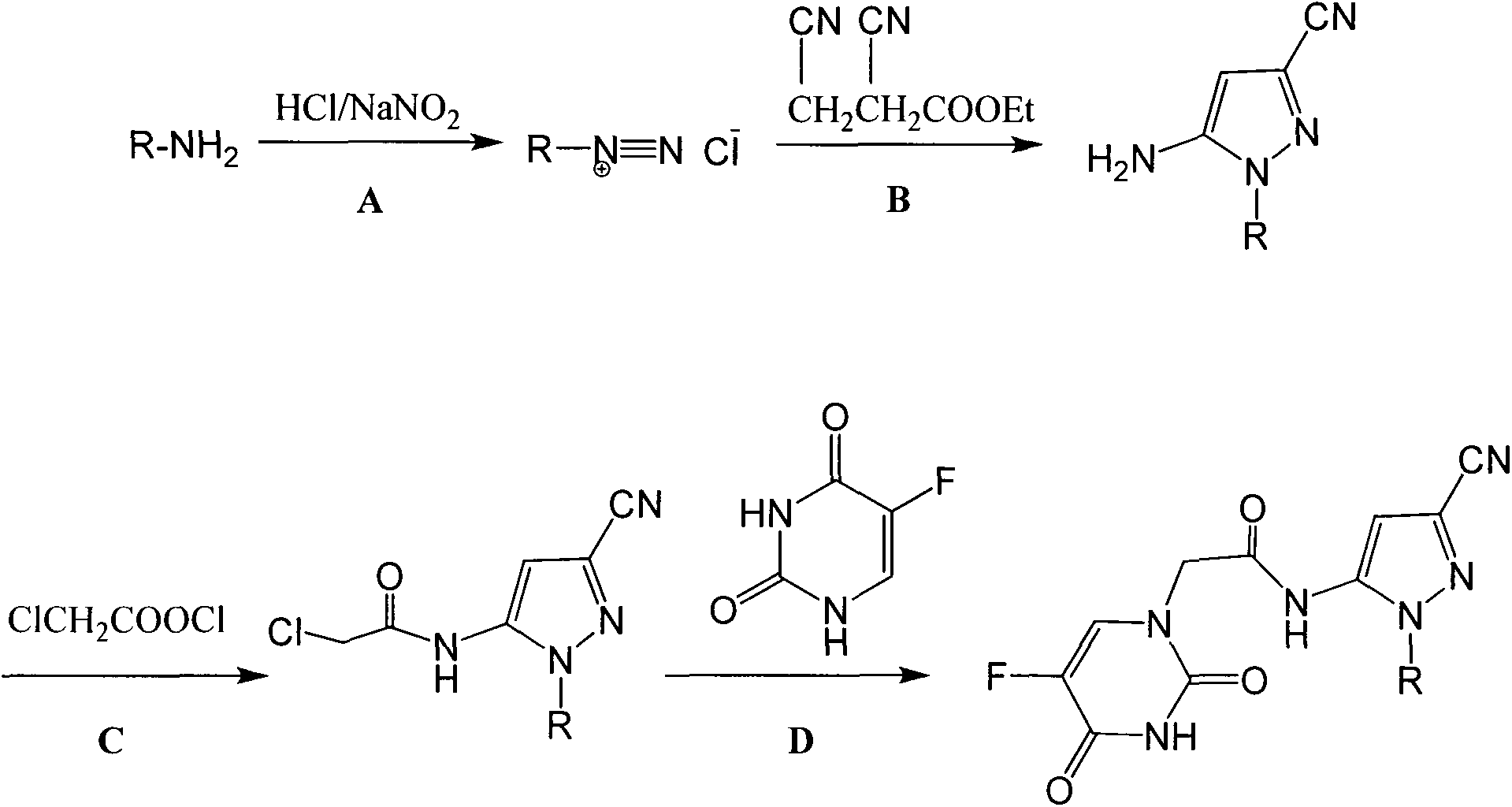
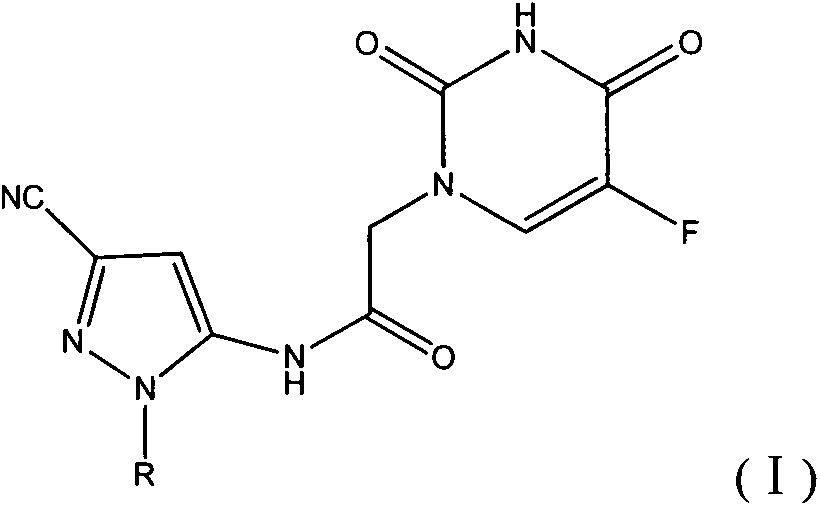
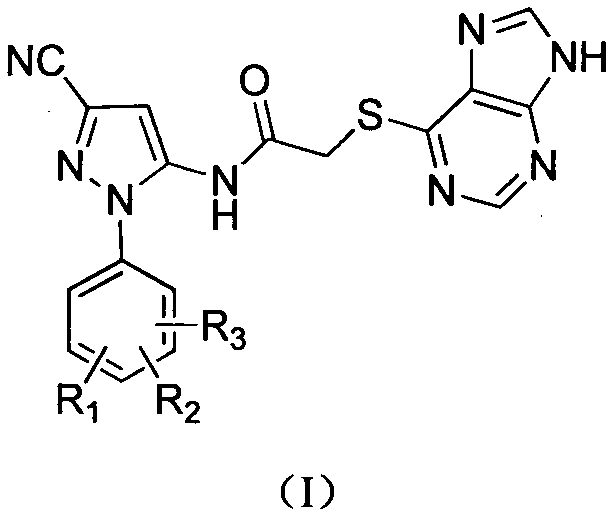
Halobenzene cyano pyrazol compound with insecticidal action as well as preparation method and application
The invention discloses a halobenzene cyano pyrazol compound with the insecticidal action as well as a preparation method and application. The halobenzene cyano pyrazol compound is a compound shown in a structural general formula (1) or a pharmaceutically acceptable salt thereof. The compound has the advantages of little dosage, good insecticidal effect, simpleness in process method, low cost and wide market prospect.
Owner:NANJING UNIV OF TECH
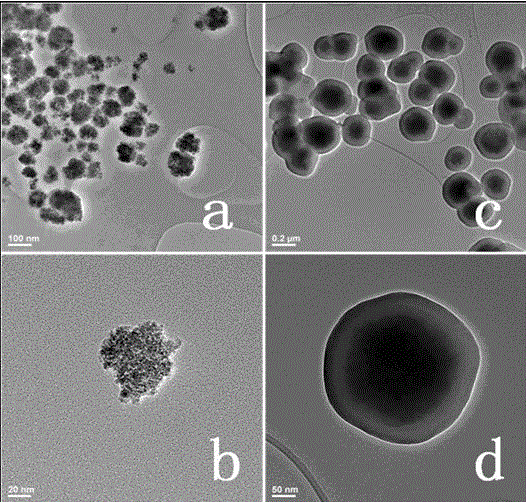
Magnetic palladium composite catalyst, and preparation method and use thereof
InactiveCN104785301ADecrease the solid loadImprove separation efficiencyCarboxylic acid nitrile preparationOrganic compound preparationMicrosphereSuzuki reaction
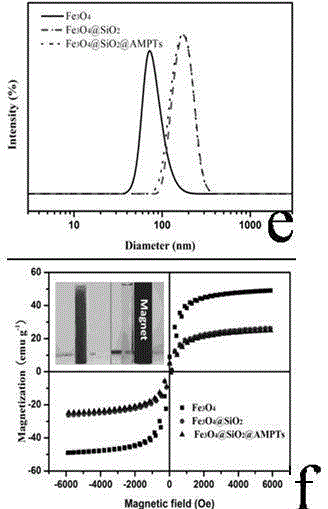
The invention discloses a magnetic palladium composite catalyst, and a preparation method and a use thereof. The magnetic palladium composite catalyst adopts superparamagnetic Fe3O4@SiO2 microspheres as a carrier, the surface is covalently modified with supported amino Pd (II) particles, and the catalyst is obtained through the steps of sequentially preparing Fe3O4 nanoparticles, preparing core-shell structured magnetic nanometer microspheres Fe3O4@SiO2 and preparing a Fe3O4@SiO2 supported amino ligand. The magnetic amino palladium composite catalyst can be applied in a Suzuki reaction, can realize approaching equal conversion of halobenzene with an electron donating group or an electron withdrawing group, and has unobvious decreasing activity after the catalyst is continuously used 5 times due to no obvious decrease of the supported amount of an active ingredient.
Owner:SUZHOU TOKIND CHEM CO LTD
Fluoro-aromatic organic tetracarboxylic dianhydride and its preparation method and use
InactiveCN1605587AImprove performanceImprove heat stabilityLiquid crystal compositionsOrganic chemistryAcetic anhydrideStrong acids
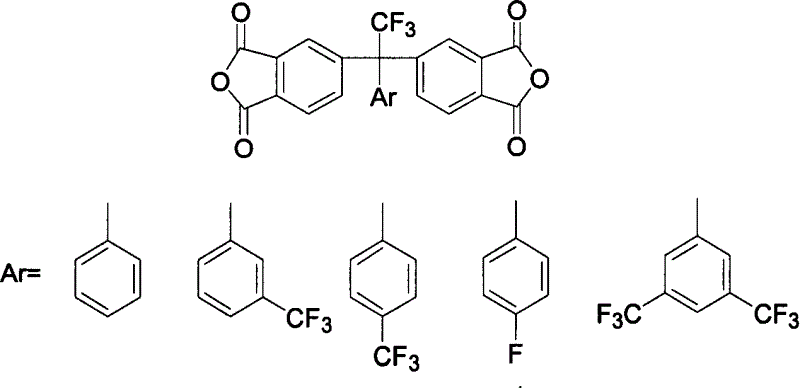
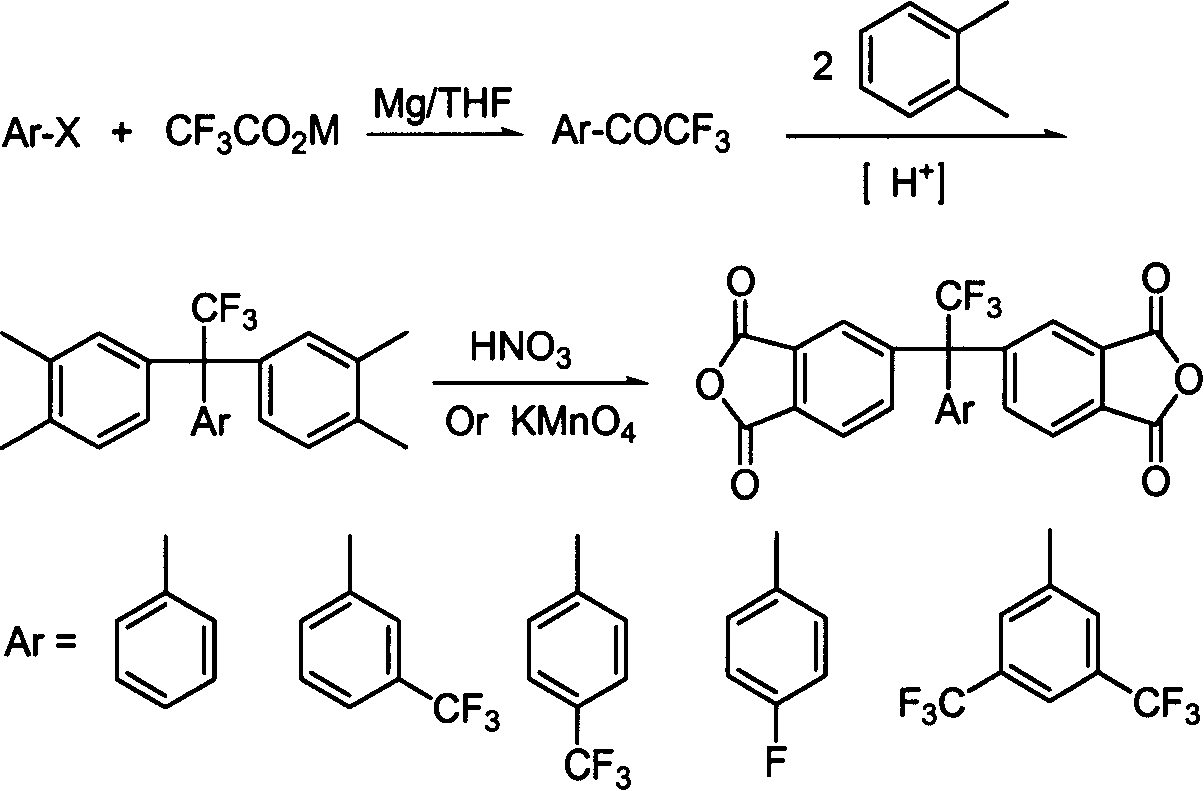
The present invention relates to the preparation process and use of fluoro aromatic organic tetraacid dianhydride and its derivative. The present invention prepares fluoro aromatic organic tetrahydric dianhydride and its derivative through the Grignard reaction between metal trifluoroacetate and substituted halogeno benzene to produce alpha, alpha, alpha-trifluoromethyl phenyil ketone; the condensation reaction of the alpha, alpha, alpha-trifluoromethyl phenyil ketone and one-xylene under the action of strong acid to obtain fluoro aromatic tetramethyl compound; the high temperature nitric acid oxidation or KMnO4 oxidation of the fluoro aromatic tetramethyl compound to obtain fluoro aromatic organic tetracid; and the final dewatering. Fluoro aromatic organic tetrahydric dianhydride and its derivative may be prepared into fluoro aromatic polyimide with excellent comprehensive performance through condensation with aromatic organic diamine and subsequent imination.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method of substituted thiophenol
A process for preparing substituted benzenethiol includes such steps as reaction between substituted halobenzene and thiohydrogenating reagent in non-protonic polar solvent while stirring, acidifying to pH=1-6, and distilling.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing amino biaromatic compound
ActiveCN105985248ANo small cost advantageBreakthrough noveltyAmino preparation from aminesOrganic compound preparationPtru catalystFormate
The invention relates to a method for synthesizing an amino biaromatic compound. The method comprises the following steps: reacting a nitroaromatic formic acid compound, an alkali and a solvent, removing water, adding substituted halobenzene, a catalyst and a ligand, and reacting; or reacting prepared dry nitroaromatic formate, substituted halobenzene, the catalyst, the ligand and the solvent; and extracting the obtained reaction product with the solvent, concentrating the obtained extract, and purifying the obtained concentrate to obtain the amino biaromatic compound. Compared with the prior art for synthesizing the amino biaromatic compound through two steps comprising coupling and reducing (such as hydrogenating), the method for synthesizing the amino biaromatic compound from cheap and easily available nitroaromatic formic acid and substituted halobenzene in the high-boiling point solvent under the action of the catalyst through a one-step reaction has the advantages of novel reaction, high safety, high yield, high efficiency, low cost and industrialization realization.
Owner:SHANGHAI XIAOMING DETECTION TECH SERVICE CO LTD
Method for synthesizing nitrobiphenyl compound
InactiveCN103360261AAtom economyEasy to getOrganic chemistryOrganic compound preparationPurification methodsNitrobenzene
The invention relates to a method for synthesizing a nitrobiphenyl compound, and particularly relates to a method for synthesizing a nitrobiphenyl compound through C-H activation. The method is characterized in that chemical products, namely nitrobenzene and substituted halobenzene which are easy to obtain on the market, are used as raw materials, the synthesis of a key intermediate, namely 2-nitrobiphenyl of boscalid and analogues of the 2-nitrobiphenyl is realized by using a C-H activation means, and the purification of the intermediate is realized by using purification method-recrystallization. The method has atom economy and great potential and advantages in terms of industrialization. Compared with a conventional method, the method has the advantages that the two raw materials adopted by the method are easy to obtain and have the price advantages.
Owner:TAIZHOU BAILLY CHEM CO LTD
Electrolytes for lithium ion secondary batteries
ActiveUS7833661B2Improve propertiesReduce gas volumeElectrolytic capacitorsOrganic electrolyte cellsBenzenePropane sultone
The present invention relates to additives for electrolytes of lithium ion secondary batteries that include one or more of the following: 1,3-propane sultone, succinic anhydride; ethenyl sulfonyl benzene, and halobenzene. It can also include biphenyl, cyclohexylbenzene; and vinylene carbonate. The weight of said 1,3-propane sultone is between 0.5 wt. % and 96.4 wt. %, said succinic anhydride is between 0.5 wt. % and 96.4 wt. %; said ethenyl sulfonyl benzene is between 0.5 wt. % and 95.2 wt. %; and said halobenzene is between 0.5 wt. % and 95.2 wt. % of the weight of the additive. Batteries with electrolytes containing said additives have improved over-charge characteristics and low temperature properties, and reduced gas generation during charging and discharging.
Owner:BYD CO LTD
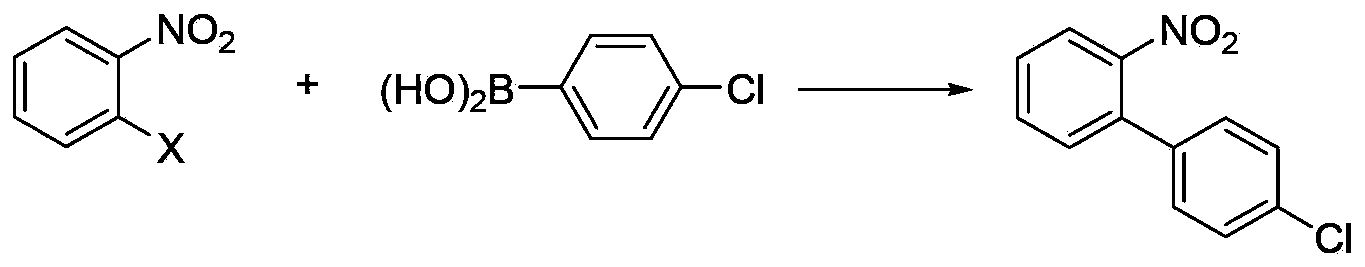
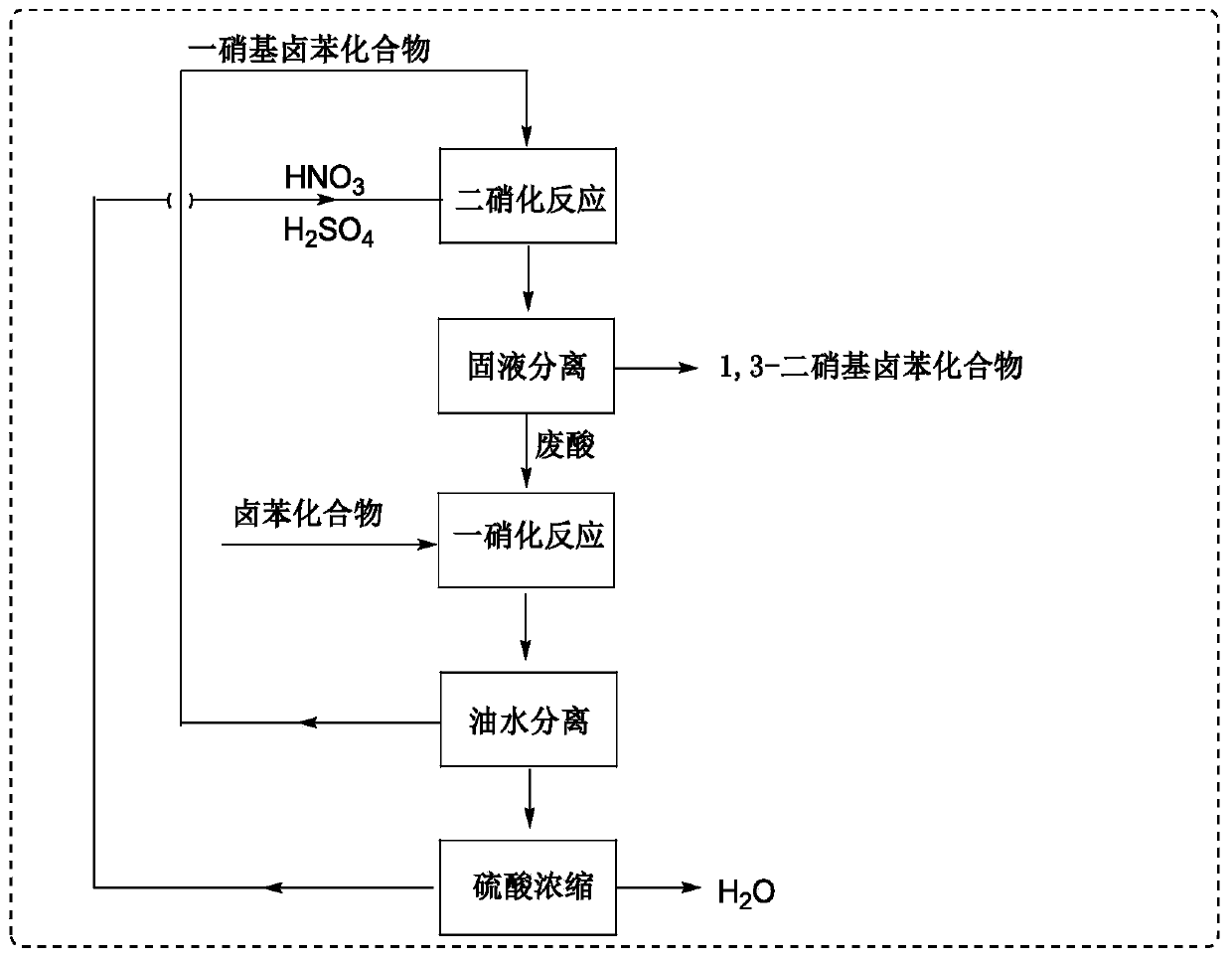
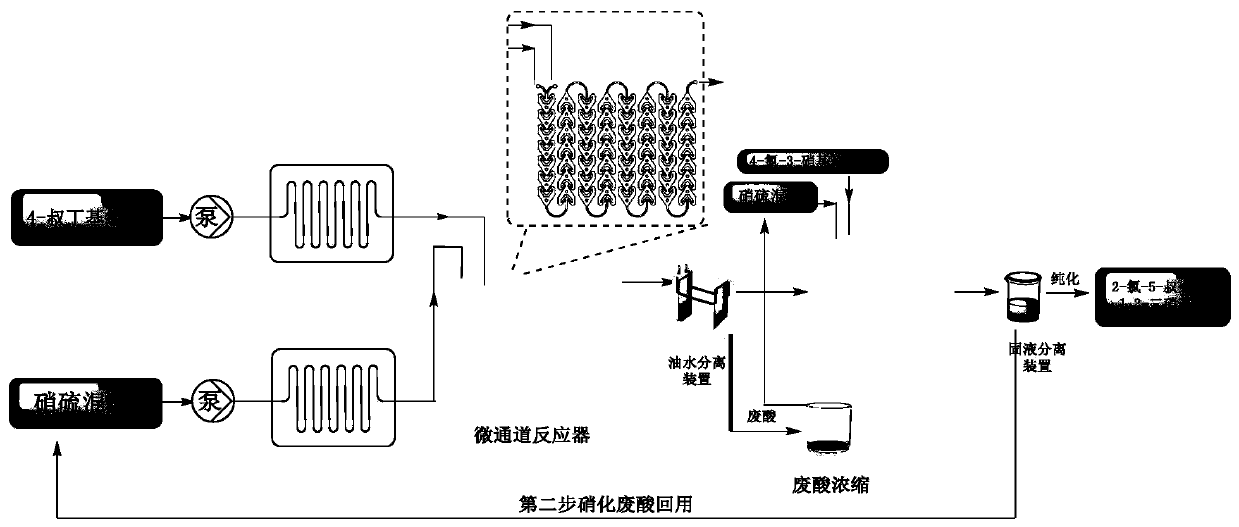
Method for synthesizing 1,3-dinitrohalobenzene compound
ActiveCN109970566ADropping time is longLong reaction heatSequential/parallel process reactionsChemical/physical/physico-chemical microreactorsMicroreactorNitration
The invention provides a method for synthesizing a 1,3-dinitrohalobenzene compound. The method comprises the following steps that A) a halogenated benzene compoundand mixed acid of nitric acid and sulfuric acid are subjected to a first nitrification reaction in a first-stage continuous flow microreactor, and oil-water separation is conducted to obtain a mononitrohalobenzene compound and first waste acid; B) the mononitrohalobenzene compound is drained into a second-stage continuous flow microreactor, a second nitrification reaction is conducted with the mixed acid of the nitric acid and the sulfuric acid, a generated nitration mixture is quenched at the outlet of the second-stage continuous flow microreactor, and filtering is conducted to obtain the 1,3-dinitrohalobenzene compound and second waste acid; C) the second waste acid is recycled to the first-stage continuous flow microreactor, a third nitration reaction with the halobenzene compound is carried out, the oil-water separation is conducted to obtain the mononitrohalobenzene compound and third waste acid, and steps B) and C) are repeated; the halobenzene compound has a structure shown in the formula I. The reaction time is short, the waste acid is less, and continuous production is achieved.
Owner:SHANDONG HIMILE CHEM TECH
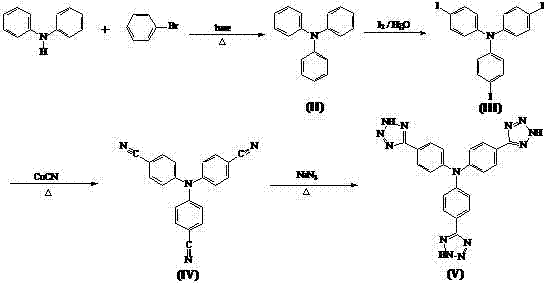
Synthesis method of tris-(4-tetrazolyl-phenyl)amine
The invention relates to a synthesis method of tris-(4-tetrazolyl-phenyl)amine, belonging to the field of material chemistry. The technical scheme of the invention is as follows: reacting diphenylamine with halo-benzene under a basic condition to obtain triphenylamine, carrying out substitution reaction on triphenylamine with iodine to generate tris-(4-iodophenyl)amine, then reacting tris-(4-iodophenyl)amine with CuCN to generate tris-(4-cyanophenyl)amine, and finally reacting tris-(4-cyanophenyl)amine with sodium azide to generate tris-(4-tetrazolyl-phenyl)amine. The porous material built by reacting obtained tris-(4-tetrazolyl-phenyl)amine with a metal has the stability of carboxylic acid porous frames, achieves intra-pore functionalization, and has pores in a mesoporous size. The adsorption capacity with respect to specific sulfur-containing small molecules shows that the porous material has potential application value in developing high-performance petrochemical products.
Owner:扬州三友合成化工有限公司
2,2'-dibenzamido-diphenyl disulfide preparation method
ActiveCN104402786AHigh yieldEmission reductionHydropoly/poly sulfide preparationBisulfideWater vapor
The invention discloses a 2,2'-dibenzamido-diphenyl disulfide preparation method, and belongs to the technical field of synthesis of organic compounds. The preparation method comprises the following steps: preparing a 2,2'-diphenylamine disulfide from a sulfide and o-nitro halobenzene as raw materials by adopting a one-pot method, and performing acylation on the 2,2'-diphenylamine disulfide to obtain a 2,2'-dibenzamido-diphenyl disulfide. The process step that an intermediate product, namely 2-amino-4-chloro thiophenol, is separated and purified by adopting the processes of steam distillation, organic solvent extraction and the like in the prior art is omitted, the technological process is simplified, meanwhile, the yield of the intermediate product, namely the 2,2'-diphenylamine disulfide, is improved, and the yield of the 2,2'-dibenzamido-diphenyl disulfide is further improved. The preparation method has the advantages of being short in technological process, simple in operation, less in three wastes emission, environmental-friendly in the production process, high in yield, low in production cost, simple in equipment and small in investment, and facilitates industrial popularization and application.
Owner:HUANGHUAI UNIV
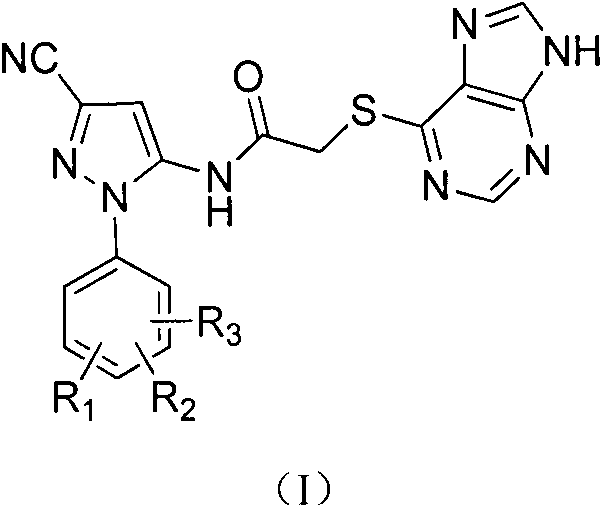
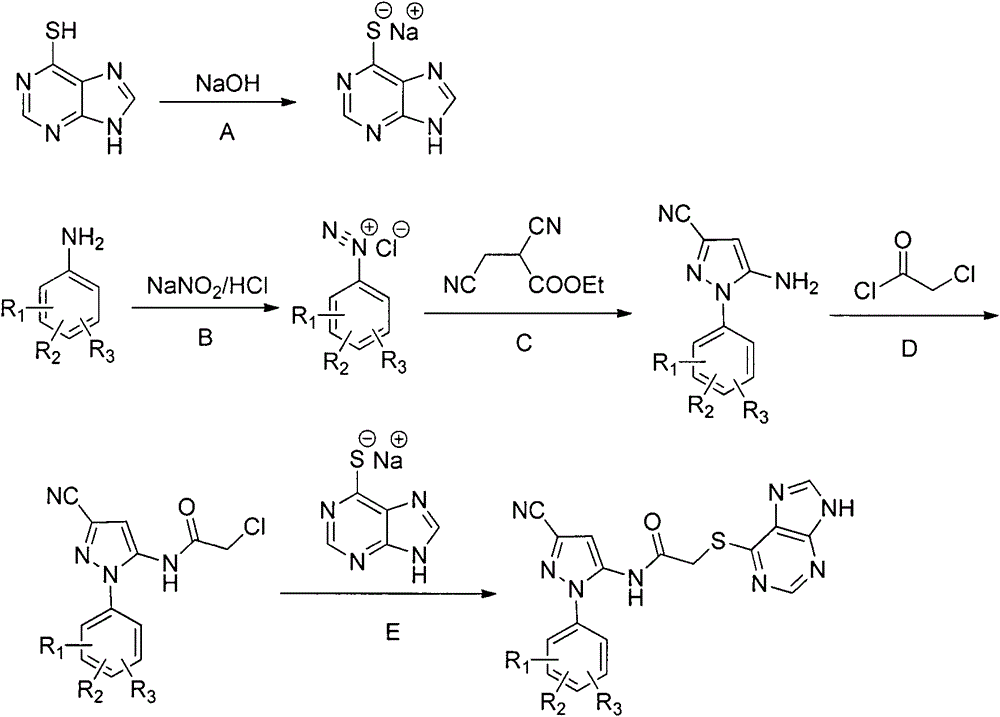
Halobenzene cyanopyrazole compound containing purine structure as well as preparation method and application
The invention discloses a halobenzene cyanopyrazole compound containing a purine structure as well as a preparation method and an application. The halobenzene cyanopyrazole compound is a compound with a general structure (I) as shown in the specification or a pharmaceutically acceptable salt thereof. The compound is low in dosage, good in insecticidal efficacy, simple in technique, low in cost and wide in market prospect.
Owner:NANJING UNIV OF TECH
Process for the manufacture of dihalodiphenylsulfones
ActiveUS20140039222A1High yieldHigh regional selectivityOrganic chemistryOrganic compound preparationRegioselectivitySulfone
A process for the preparation of dihalodiphenylsulfones_such as 4,4′-dichlorodiphenyl sulfone or 4,4′-bis-(4-chlorophenylsulfonyl)biphenyl with high regioselectivity, at low temperature and in the absence of toxic reagents by reacting together at least one acid, at least one fluorinated anhydride and at least one halobenzene. The invented process is particularly suited for the manufacture of 4,4′-dichlorodiphenyl sulfone.
Owner:SOLVAY SPECIALTY POLYMERS USA LLC
O-aminobiphenyl compound synthesis method
ActiveCN107488113AFew reaction stepsReduce processOrganic compound preparationAmino compound preparationSynthesis methodsFormate
The invention relates to an o-aminobiphenyl compound synthesis method, which comprises: carrying out a reaction on an o-nitroaromatic formic acid compound, an alkali and a solvent, removing water, adding substituted halobenzene, a catalyst and a ligand, carrying out a reaction, or carrying out a reaction on a pre-prepared o-nitroaromatic formate, substituted halobenzene, a catalyst, a ligand and a solvent, and carrying out post-treatment to obtain the o-aminobiphenyl compound. According to the present invention, the o-aminobiphenyl compound is synthesized by using the easily available or easily prepared o-nitroaromatic formate and the substituted halobenzene as the raw material through the one-step method without the use of hydrogen and other hazardous reducing agents, such that the coupling and nitro reduction continuous reaction is achieved, and the advantages of new reaction, safety, high efficiency, simple operation and low cost are provided.
Owner:SHANGHAI XIAOMING DETECTION TECH SERVICE CO LTD
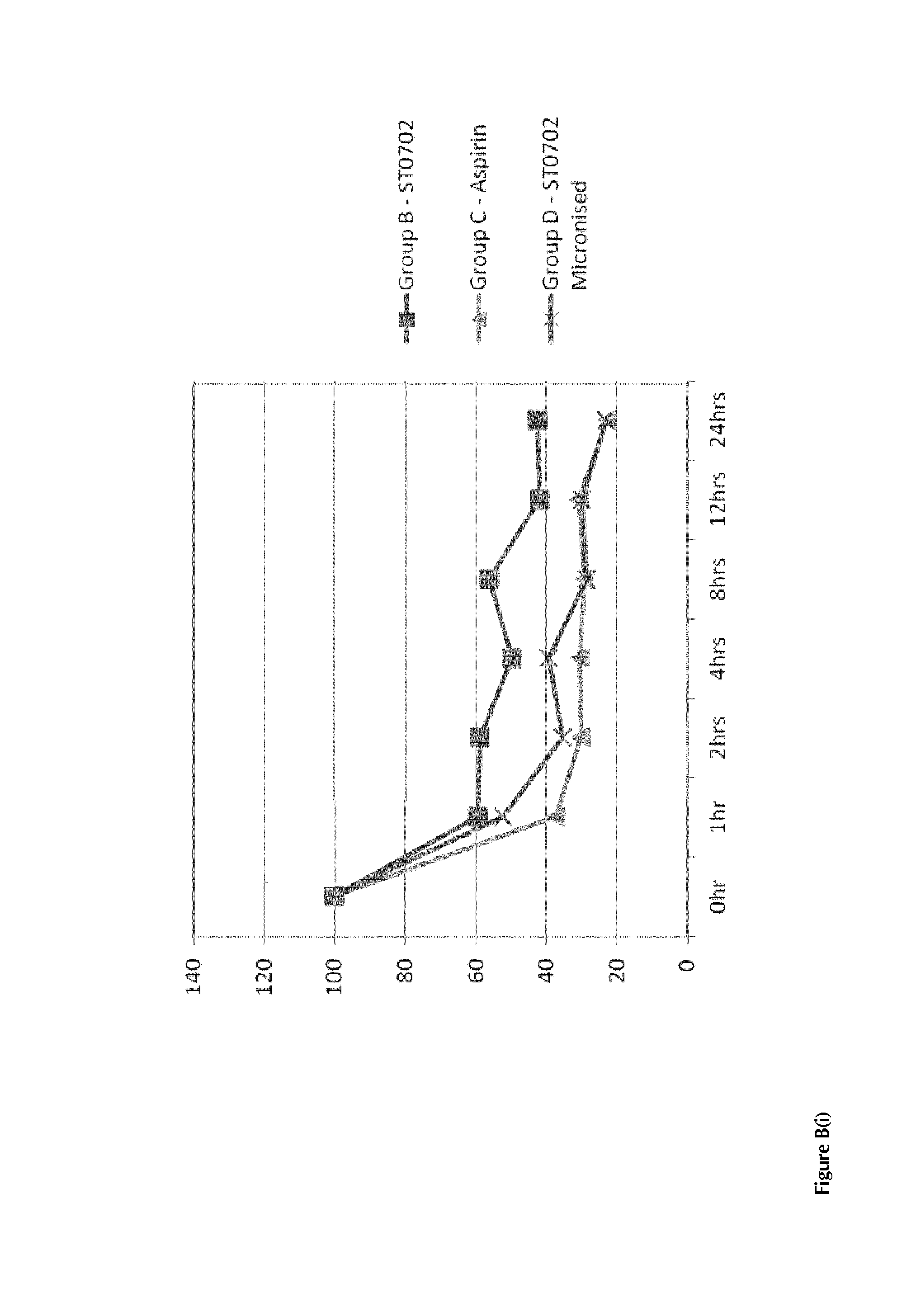
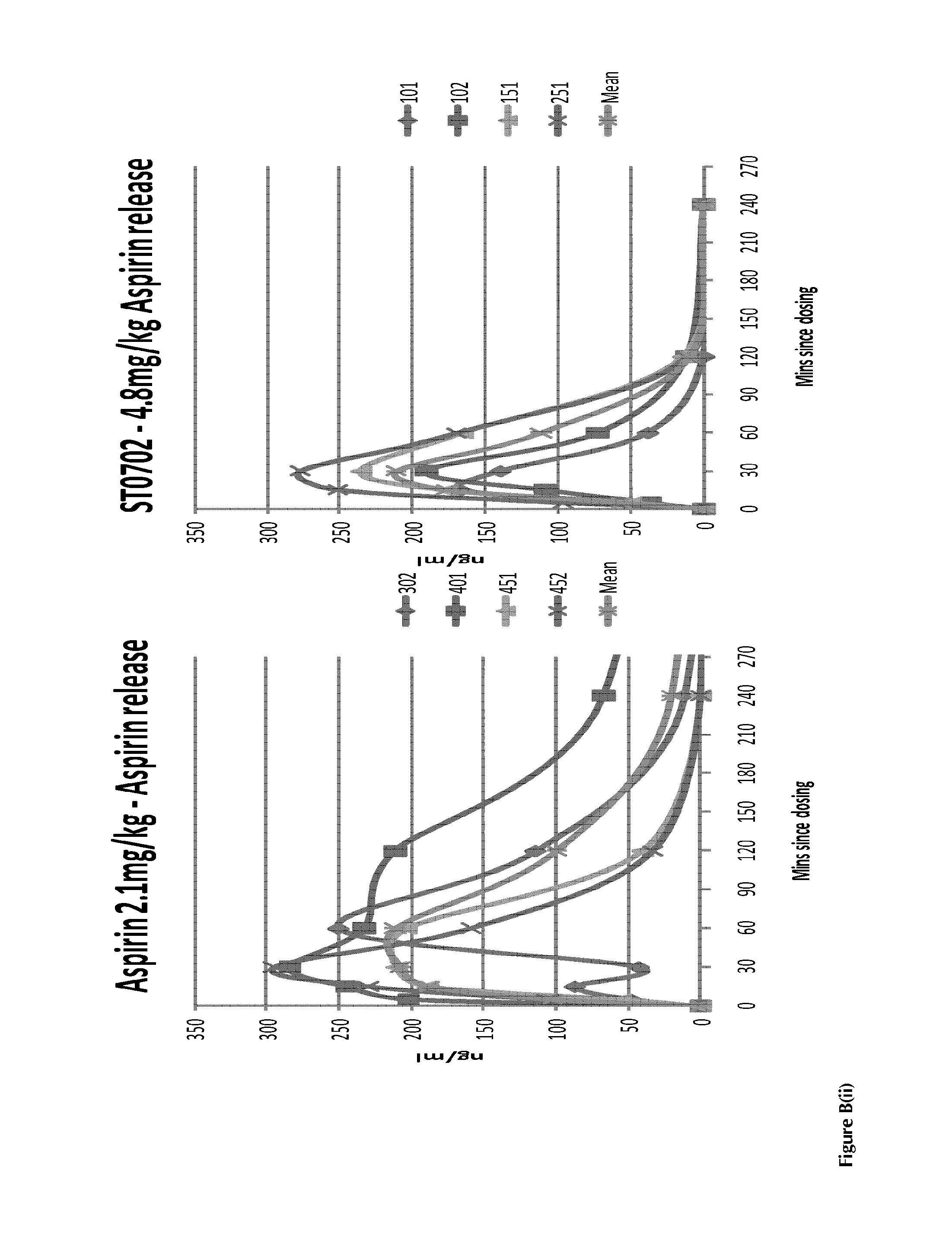
Compounds with super-aspirin effects
InactiveUS20140024681A1Prevent adhesionInhibit aggregationBiocideOrganic chemistryBenzoic acidAspirin
A compound having the structural formula (I) and pharmaceutically acceptable salt and / or hydrates thereof, (I) wherein Y is an arylester or an C1-C8 alkylaryl ester, selected from the group consisting of: benzene, toluene, xylene, benzoic acid, benzoate, nicotinate, isonicotinate and halobenzene, which can be unsubstituted or substituted with at least one nitric oxide releasing group; and / or at least one of hydroxide, —Cl, —Br, a C1-C8 alkyl, benzyl, a C1-C8 alkoxy, benzyloxy, —NHC(O)R, —NH2, —NO2—ONO2, —(CH2)nONO2, —OC(O)[(CH2)n]cyclicONO2, —OCOArONO2, —OCOAr(CH2)nONO2 or a C1-C5 haloalkyl ester, wherein R is a C1-C8 alkyl or a C1-C8 alkoxy group, n=1-8 and m=3-10, to produce a super-aspirin effect.
Owner:SOLVOTRIN THERAPEUTICS LTD
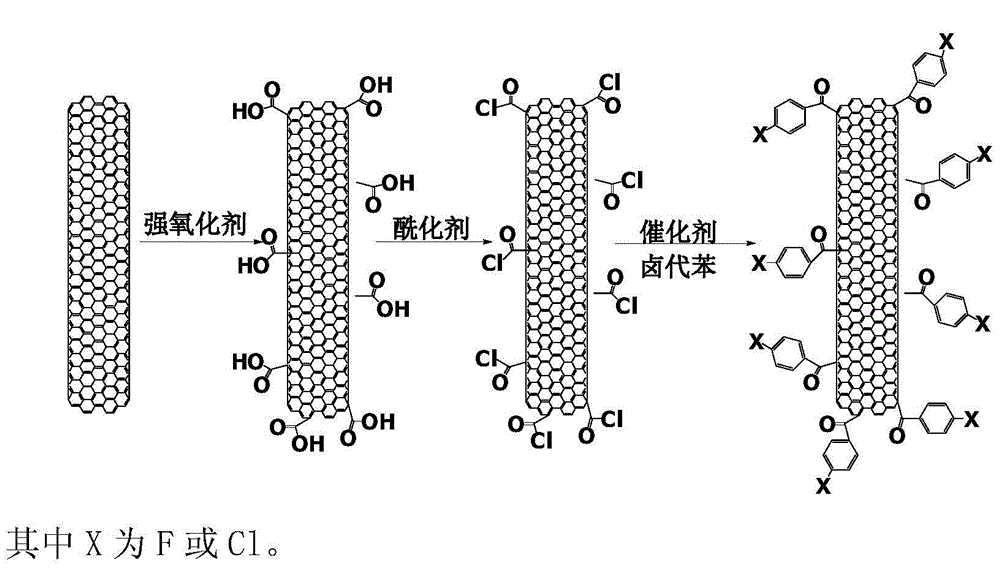
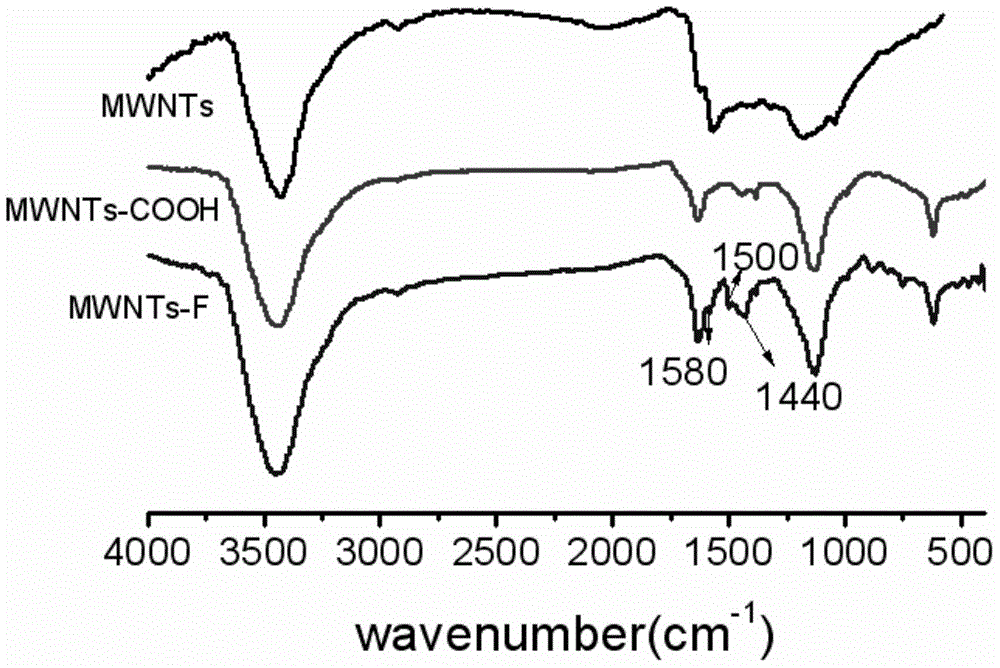
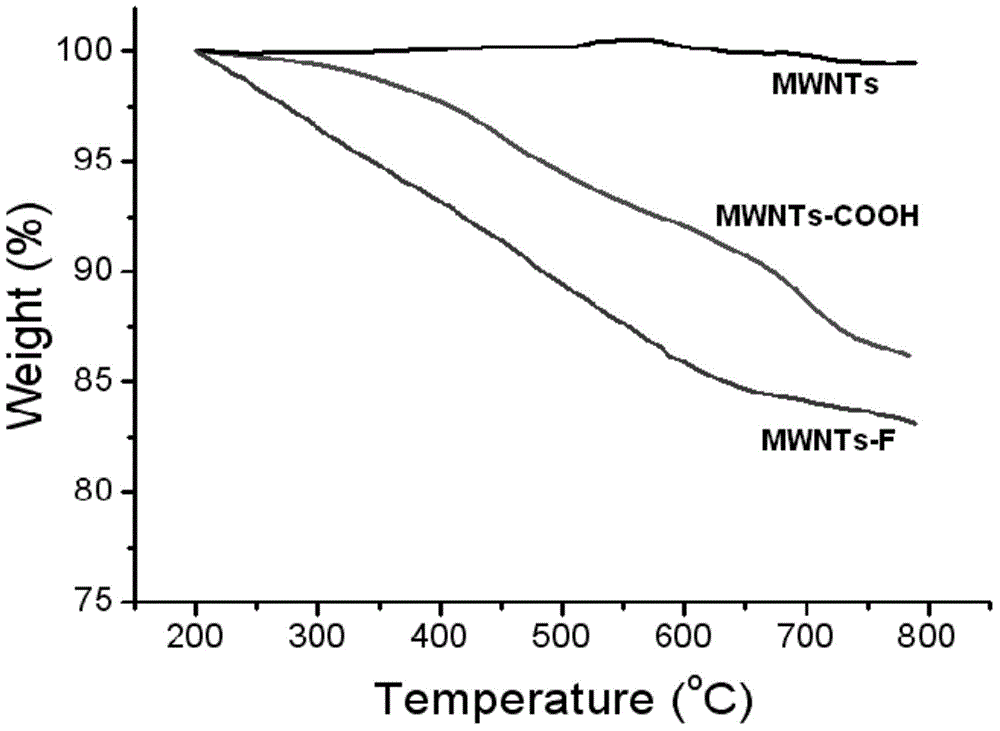
Halobenzene functionalized and modified carbon nano tube and preparation method thereof
ActiveCN104559340AEasy to controlEvenly distributedPigment treatment with non-polymer organic compoundsFiberIn situ polymerization
The invention provides a halobenzene functionalized and modified carbon nano tube and a preparation method thereof. The halobenzene functionalized and modified carbon nano tube is characterized in that after carboxy groups on the surface of a carbon nano tube are acylated, the halobenzene functionalized carbon nano tube is prepared through Friedel-crafts reaction by using a Lewis acid as a catalyst and using halobenzene as a modifying agent. The dosage of an oxidizing agent and the processing time are controlled during oxidization, so that the adoption amount of carboxy groups is controlled, and further the number of halobenzene ketone structures grafted by the carbon nano tube is controlled; the types of halobenzene are changed so as to obtain multiple kinds of halobenzene functionalized carbon nano tubes. The obtained modified carbon nano tubes have good solubleness in organic solvents, such as NMP and DMF, the carbon nano tubes can be well dispersed in a resin matrix by an in-situ polymerization and in-situ compounding method, and the obtained modified carbon nano tubes can be used for preparing high-performance nano composite materials, high-strength film materials and high-performance fibers, and can also be used for preparing superfine fibers and non-woven fabrics through electrostatic spinning. Therefore, the surface-functionalized modified carbon nano tube prepared by the method disclosed by the invention can be applied to the fields of plastic and fiber modification, and electrostatic spinning.
Owner:NO 11 INST OF NO 6 ACADEMY OF CHINA AEROSPACE SCI & TECH
New method of synthesizing 1, 2, 3-triazol 1, 3-diazacyclo compound
InactiveCN101544643AIncrease diversityThe synthesis process is simpleOrganic chemistryArylHydrogen atom
The invention discloses a new method of synthesizing a 1, 2, 3-triazol 1, 3-diazacyclo compound with pharmaceutical activity. The structure of the 1, 2, 3-triazol 1, 3-diazacyclo compound with pharmaceutical activity is shown in figure (1), wherein n is equal to 1, 2 or 3; z is an O atom, an S atom or NR'; R' is a hydrogen atom, methyl, nitryl, cyano, amido, C[1-8]alkyl, C[1-8]alkylamino, C[1-8]alkanoyl, aroyl, dense aroyl, aryl, dense aryl, naphthenic base, aralkyl, oxa-alkyl, oxa-acyl, thia-alkyl or thia- acyl; R is alkyl, aryl, substituted aryl or heterocyclic aryl; n is equal to 1, 2 or 3; Z is an O atom, an S atom or NH preferably; and R is methyl, phenyl, p-methylphenyl, p-chlorphenyl or p-methoxyphenyl preferably. The invention is characterized in that, substituted halobenzene, hydrazoates and heterocyclic ketene amines or derivatives thereof are used for preparing the 1, 2, 3-triazol 1, 3-diazacyclo compound which has pharmaceutical activity and has the structure shown in figure (1) at high yield by the one kettle way. The invention has the advantages of simple synthesizing technology, moderate conditions and high yield. The invention achieves the synthesis of heterocyclic compound libraries at high yield in a parallel mode by the one kettle way, and really achieves molecular multiformity. In addition, the invention has the characteristics of high yield, simple lines, moderate conditions and the like.
Owner:YUNNAN UNIV
Alkynyl carbon material, preparation method thereof and composite electrode
PendingCN113651311AHigh purityLow costNon-aqueous electrolyte accumulator electrodesLi-accumulatorsLithiumComposite electrode
The invention provides an alkynyl carbon material, a preparation method thereof and a composite electrode, and the preparation method comprises the following steps: mixing calcium carbide, hexa-halobenzene and ball milling beads according to a mass ratio of the ball milling beads to the total mass of the calcium carbide and the hexa-halobenzene being (10-150): 1, and carrying out ball milling at 400-1000r / min for 24-48h to obtain mixed powder; and seiving the mixed powder, carrying out post-treatment, and obtaining the alkynyl carbon material. According to the preparation method of the alkynyl carbon material, the calcium carbide and the hexahalobenzene are induced to be subjected to a multi-phase chemical reaction through mechanical energy generated through mechanical ball milling and heat induction, the high-purity alkynyl carbon material is obtained through post-treatment, and the prepared alkynyl carbon material is high in purity and yield; and the alkynyl carbon material has high conductivity, nanoscale pores and interlayer spacing suitable for metal ion conduction, and is beneficial to lithium ion transmission and buffering of volume strain in the electrochemical reaction process.
Owner:XIAN UNIV OF TECH
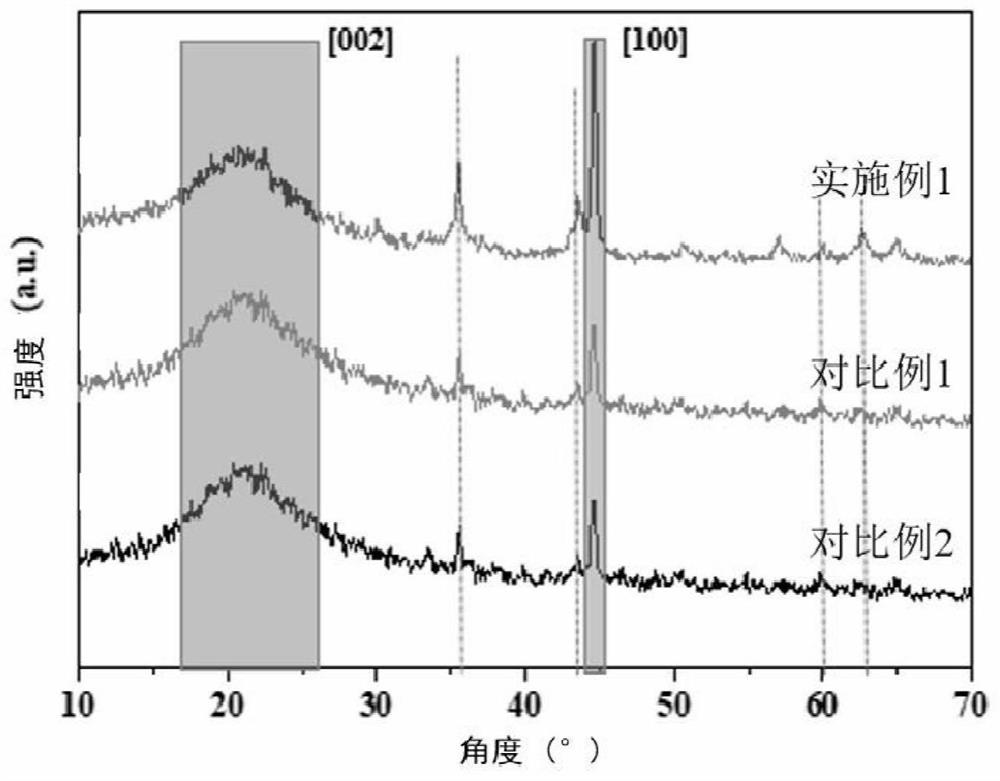
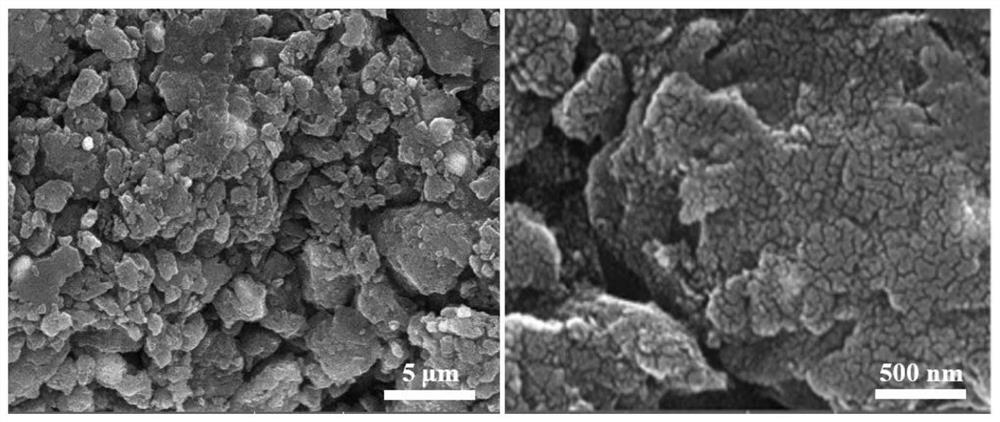
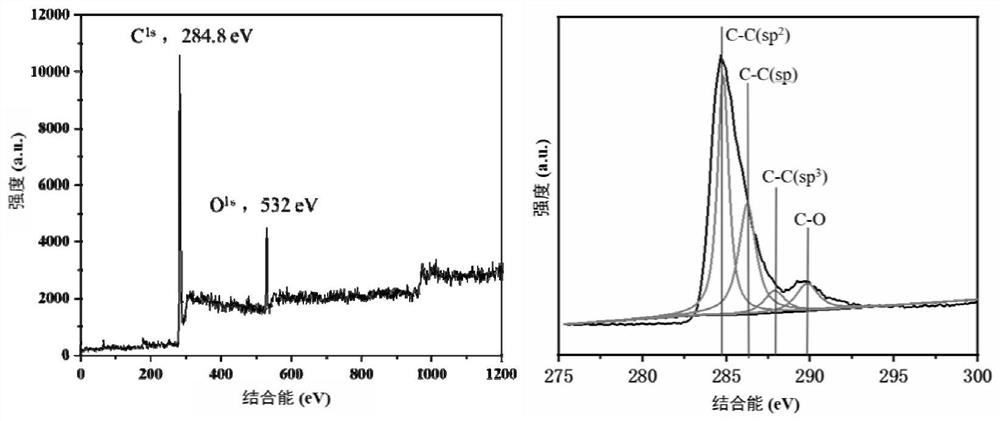
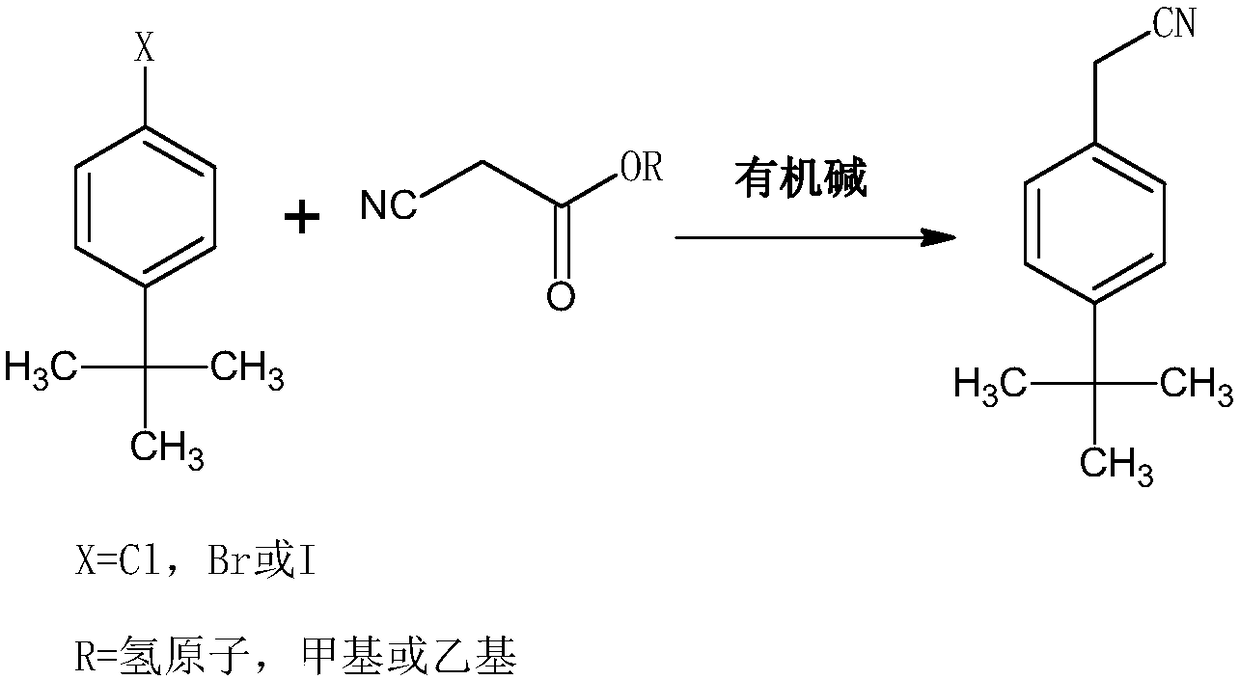
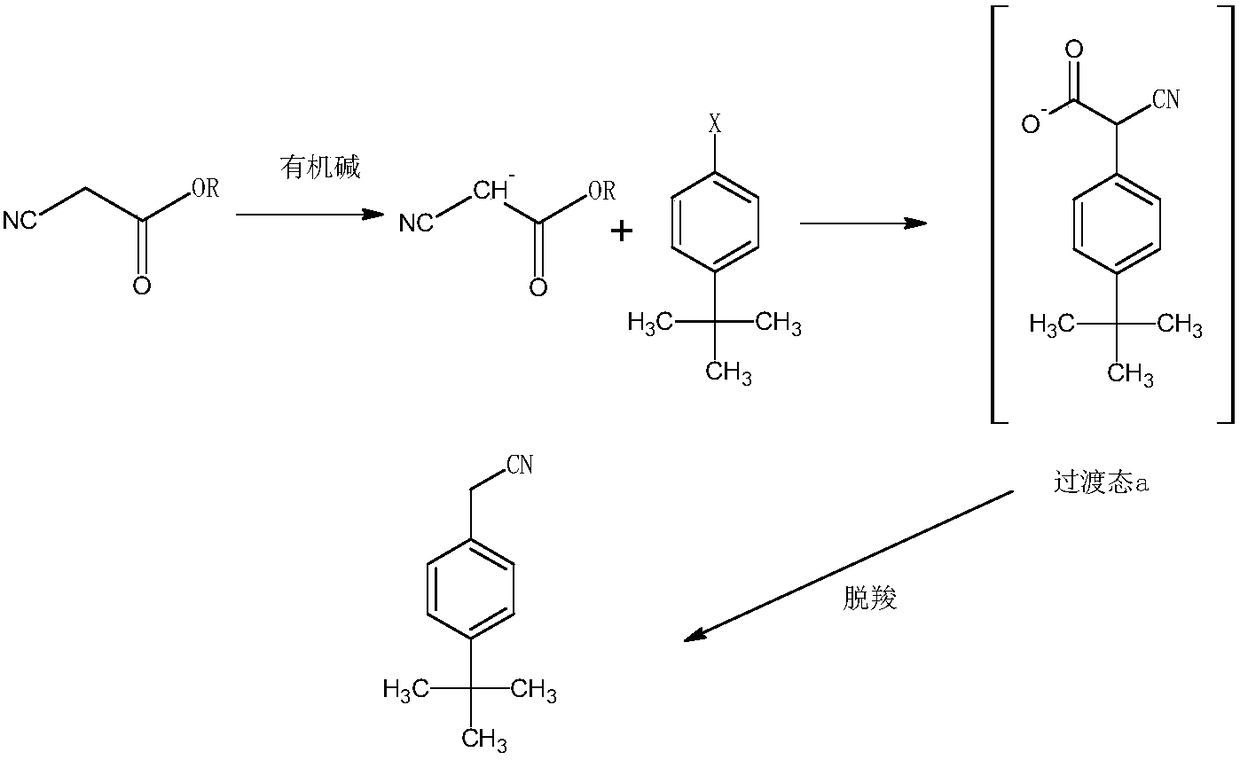
Synthesis method of acaricide cyflumetofen intermediate p-tert-butyl phenylacetonitrile
InactiveCN109053491AShort stepsLess side effectsCarboxylic acid nitrile preparationOrganic compound preparationRotary evaporatorSynthesis methods
Owner:SHANDONG ACADEMY OF PESTICIDE SCI +1
Preparation method of diphenyl disulfide compounds
ActiveCN111763163AImprove conversion rateLow costOrganic compound preparationMagnesium organic compoundsGrignard reagentDiphenyl disulfide
The invention discloses a preparation method of diphenyl disulfide compounds. The preparation method comprises the following steps: stirring an isopropyl magnesium halide Grignard reagent and a substituted halogen benzene compound in an organic solvent at -78 DEG C to -20 DEG C for 30-90 minutes to obtain a thoroughly halogen-magnesium exchanged substituted phenyl Grignard reagent; and adding dichlorodisulfide into the reaction system, slowly heating to room temperature after the reaction is finished, quenching the reaction by using a saturated ammonium chloride aqueous solution, extracting byusing ethyl acetate or diethyl ether, drying by using anhydrous magnesium sulfate, and concentrating the organic phase to obtain the diphenyl disulfide compounds. According to the method, the diphenyl disulfide compounds are prepared by taking the phenyl Grignard reagent as a raw material through a one-pot method, and has the following advantages: the synthetic route is short, the preparation process is simple, the cost is low, the operation is easy, the yield is excellent, and the industrial production is easy.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
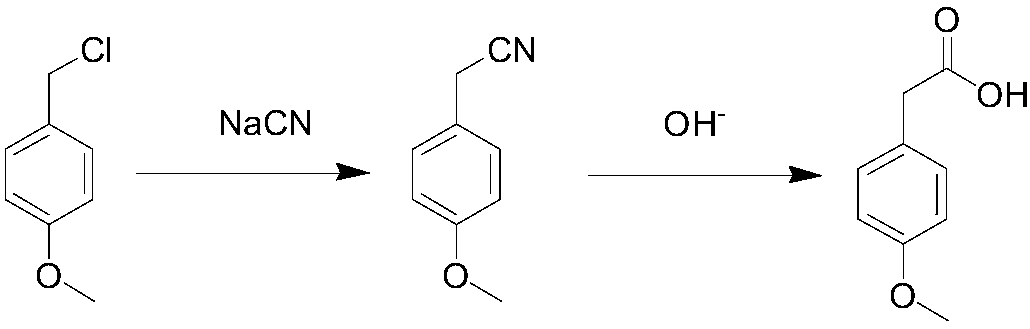
Method for synthesizing p-methoxyphenylacetic acid
InactiveCN108191633AEasy to storeLow raw material costOrganic compound preparationCarboxylic acid esters preparationEcological environmentMalonate
The invention discloses a method for synthesizing p-methoxyphenylacetic acid. The method comprises the following steps: adding malonate, alkali and p-methoxy halobenzene in a solvent, enabling the p-methoxy halobenzene and the malonate to perform alkylation reaction under the alkali condition, adding water to perform quenching reaction after the reaction is finished, directly acidizing and heatingto perform the decarboxylation hydrolysis reaction after concentrating out most solvent, and then cooling to crystallize, filter and dry, namely obtaining the p-methoxyphenylacetic acid. The method disclosed by the invention has the following advantages: 1, the raw materials used by the method of the invention are easier to obtain and convenient for storing, the raw material and operation cost are greatly reduced; 2, the method disclosed by the invention is less in reaction step, the intermediate reaction process is easy to control and easy for scale production; and 3, the three waste (wastewater, waste solid and waste gas) yield is less, the environment pollution is reduced, and the ecological environment is protected. And meanwhile, the prepared product is high in purity, and the purity can achieve 99% or more; the yield is high and can achieve 95% or more.
Owner:抚顺东科新能源科技有限公司
Polybenzimidazole compound with pendant group containing fluorine, and preparation method thereof
The invention relates to a polybenzimidazole compound with a pendant group containing fluorine, and a preparation method thereof. The compound is a nitrogen heterocycle polymer formed by using benzimidazole as a main chain structure and substituting H in an N-H group on an imidazole ring with a p-trifluoromethylphenyl group. The preparation method comprises the following steps: 1) dissolving polybenzimidazole in an organic solvent to obtain a PBI solution; and 2) respectively adding p-trifluoromethyl halobenzene, a catalyst and a dehydrogenating agent into the PBI solution, carrying out a condensation refluxing reaction at 110-130 DEG C for 12-24 h, and purifying and drying the obtained reaction product to obtain the polybenzimidazole compound with the pendant group containing fluorine. The polybenzimidazole compound with the pendant group containing fluorine has the advantages of good solubility, excellent chemical stability, high phosphoric acid doping rate, high high-temperature proton conductivity, and broad application prospects in the fields of proton exchange membrane fuel cells, fireproof fibers and high temperature-resistant adhesives.
Owner:TONGJI UNIV
Process for the Manufacture of Fluoroaryl Compounds and Derivatives
ActiveUS20200262770A1Efficient and energy saving processProcess environmental protectionPhysical/chemical process catalystsOrganic compound preparationHydrogen halideAryl
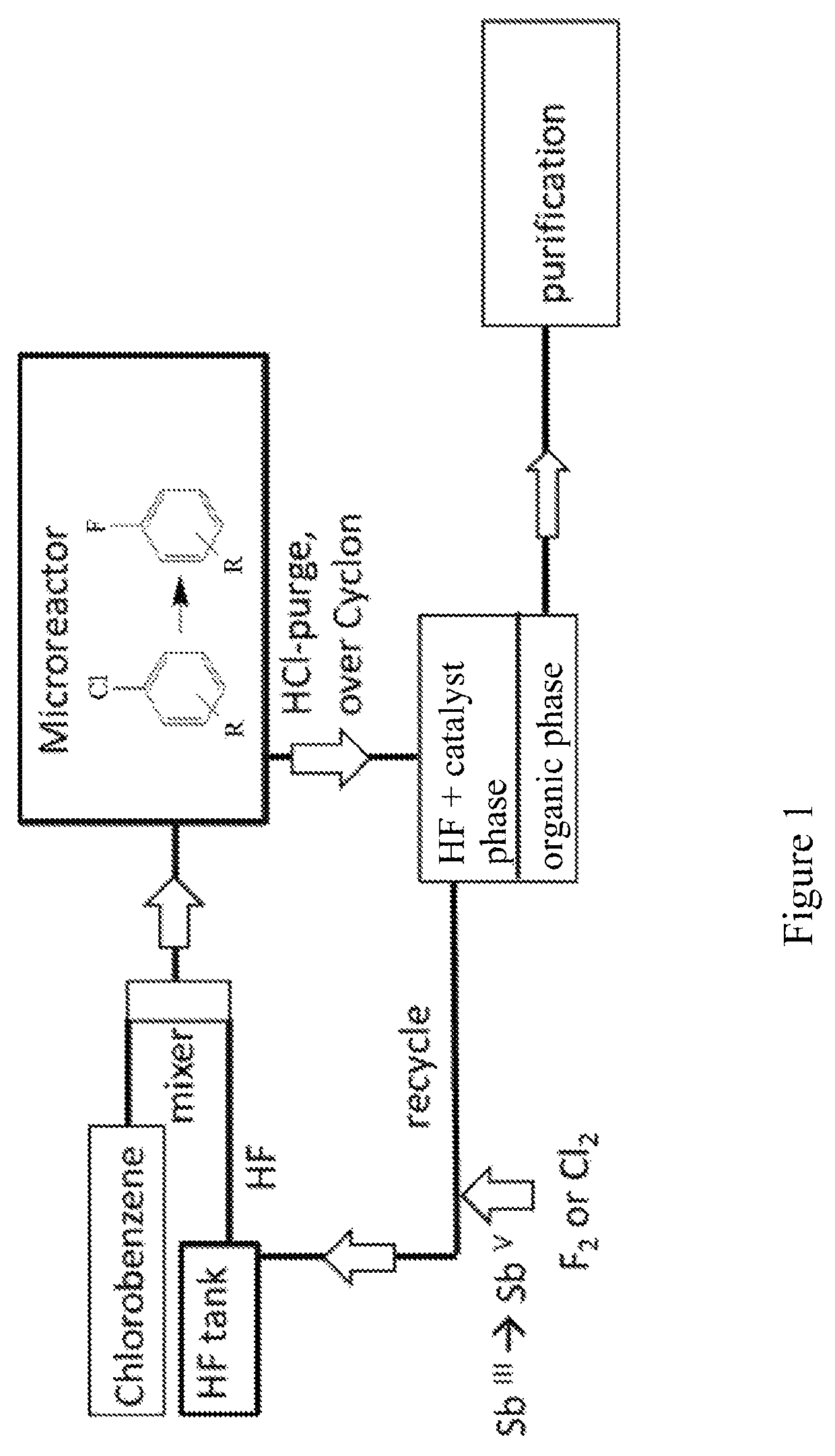
The invention relates to a new process for the manufacture of fluoroaryl compounds and derivatives thereof, in particular of fluorobenzenes and derivatives thereof, and especially wherein said manufacture relates to an environmentally friendly production of the said compounds. Thus, the present invention overcomes the disadvantages of the prior art processes, and in a surprisingly simple and beneficial manner, and as compared to the prior art processes, in particular, the invention provides a more efficient and energy saving processes, and also provides a more environmentally friendly process, for the manufacture of nuclear fluorinated aromatics, and preferably of nuclear fluorinated fluorobenzenes. Accordingly, in one aspect of the invention, an industrially beneficial process for preparing fluorobenzenes from halobenzene precursors using HF to form hydrogen halide is provided by the present invention. A beneficial and surprisingly simple use of chlorobenzene as an industrially interesting starting material in the manufacture of fluorobenzene is provided.
Owner:FUJIAN YONGJING TECH CO LTD
Preparation method of besilate compound
The invention relates to a preparation method of a besilate compound. The preparation method of the besilate compound comprises the following steps of: enabling benzenesulfonic acid, halobenzene and sodium sulphide to react in the presence of a solvent and an oxidant to obtain a target product containing besilate. The preparation method of the besilate compound is simple to operate, cheap in materials, high in product yield, high in purity and good in industrial application prospect.
Owner:心邀(深圳)生物科技有限公司
Method for preparing p-methoxycinnamic acid
ActiveCN110540501AReduce manufacturing costReduce usageOrganic compound preparationCarboxylic acid salt preparationInorganic saltsCoupling reaction
The invention relates to a method for preparing p-methoxycinnamic acid. The method comprises the following steps: a salt forming reaction: reacting acrylic acid with an alkaline inorganic salt to obtain acrylate; and a coupling reaction: reacting 4-methoxyhalobenzene and acrylate, and carrying out acidifying to obtain p-methoxycinnamic acid. According to the method, an organic phase reaction system is changed into a water phase reaction system; a brand-new, efficient and low-cost catalyst is adopted for catalysis; so high-quality p-methoxycinnamic acid is prepared at low cost, and the preparedp-methoxycinnamic acid has HPLC content of no less than 99.5%, single impurity content of no more than 0.2% and total impurity content of no more than 0.5%.
Owner:ANHUI SHENGNUOBEI CHEM TECH
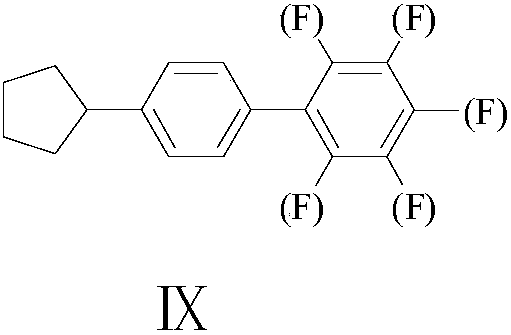
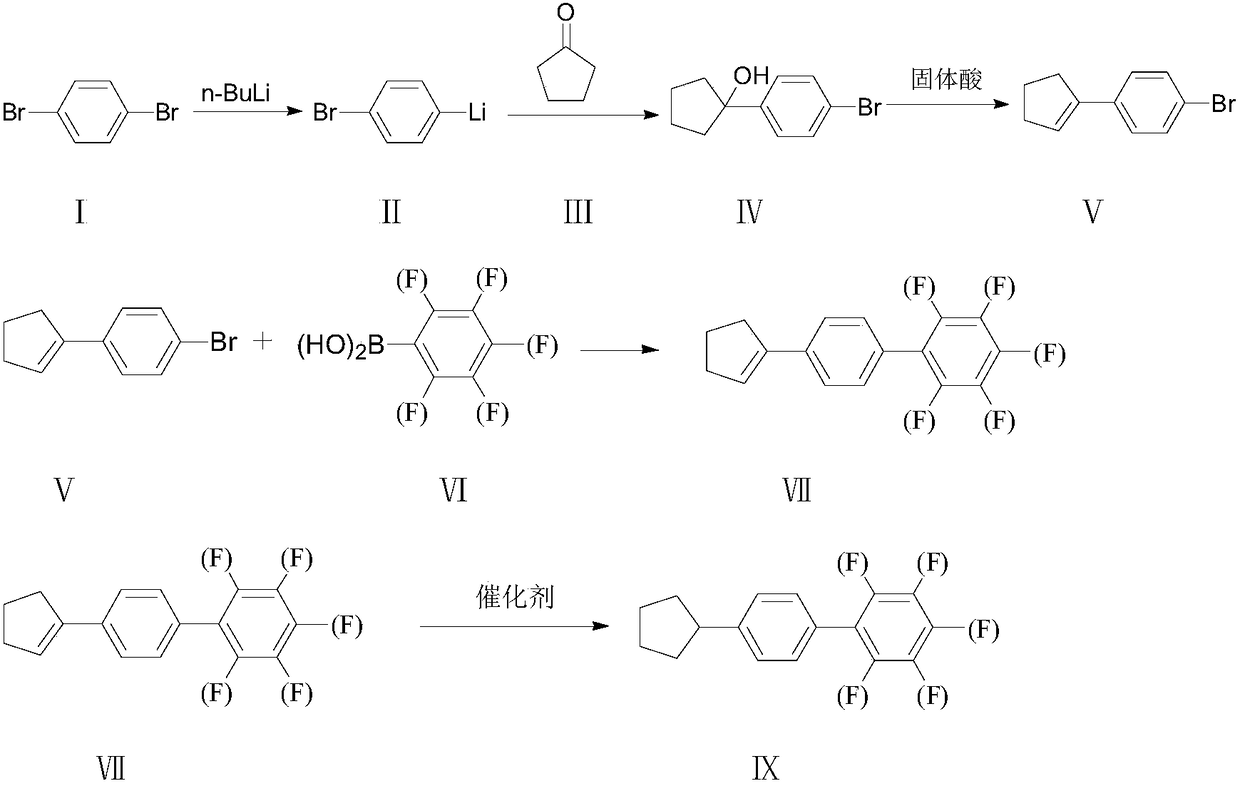
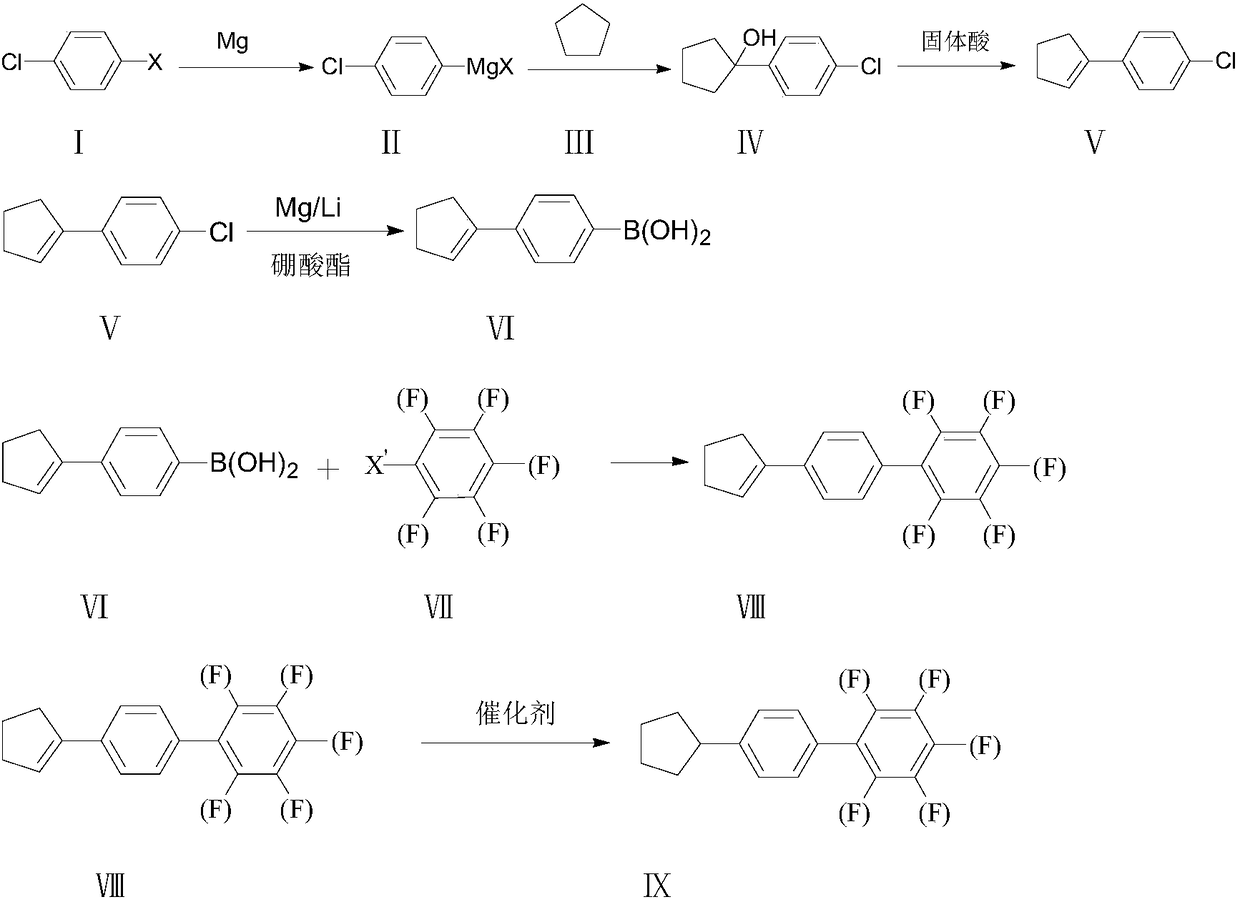
Synthesis method of 4-cyclopentyl biphenyl fluorinated compound
ActiveCN108191601ALow priceEasy to buyPreparation by OH and halogen introductionGroup 3/13 element organic compoundsChlorobenzeneSynthesis methods
Owner:山东盛华新材料科技股份有限公司 +1
Ketoprofen intermediate, preparation method and applications thereof
InactiveCN110857269AExpand industrial applicationsImprove economyCarboxylic acid nitrile preparationOrganic compound preparationOrtho positionBenzyl cyanide
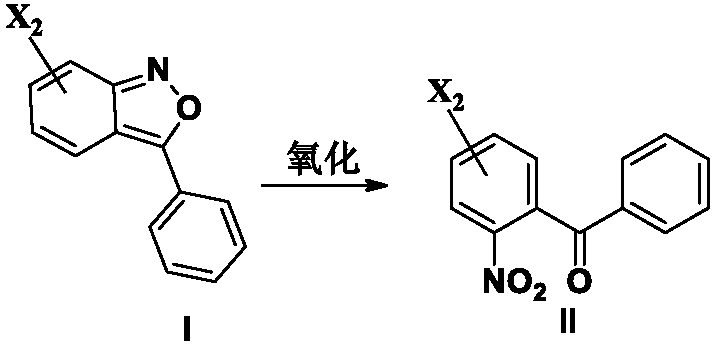
The invention relates to the field of medicine synthesis, particular to a ketoprofen intermediate, a preparation method and applications thereof. The preparation method comprises the following steps:carrying out a Diels-Alder reaction on p-nitrohalobenzene or o-nitrohalobenzene or a mixture of p-nitrohalobenzene and o-nitrohalobenzene as a raw material and phenylacetonitrile to form an isoxazolecompound, and sequentially carrying out an oxidation reaction, a substitution reaction, a reduction reaction, deamination, ester group removal and an acidic hydrolysis reaction to prepare ketoprofen,wherein the reaction formula is defined in the specification, X2 or the group defined in the specification is located at the ortho-position or para-position of nitro or amino, R1 is -CONR4R5, -COX1, -COOR2 or -CN, R2, R3, R4, R5 and R6 are the same or different and are H or C1-C6 alkyl, and X1 and X2 are the same or different and are F, Cl, Br or I.
Owner:JIANGSU RUIKE MEDICAL SCI & TECH CO LTD
Process for halogenation of benzene and benzene derivatives
InactiveUS20050177010A1High selectivityHigh yieldMolecular sieve catalystsCatalyst activation/preparationBenzeneArame
In a process of halogenation of benzene or benzene derivatives, di-substituted halobenzene derivatives having para-aromatic compounds or tri-substituted halobenzene derivatives having 1,2,4-substituted aromatic compounds are selectively produced. In halogenation of benzene or benzene derivatives, a fluorine-containing zeolite catalyst such as L-type zeolite, or a zeolite catalyst having the crystal size of at most 100 nm is used. The reaction is preferably effected in the presence of a solvent, and the solvent is preferably a halogenated compound.
Owner:TORAY IND INC
Process for making organic photosensitive pigment
Processes for making photosensitive organic pigments for use in imaging members, specifically processes for making a photosensitive phthalocyanine pigments having a specific crystal form, comprising dissolving the pigment in a mixture of a haloacetic acid and alkylene halide to form a solution, precipitating the pigment by adding the solution to a non-solvent system, the solution comprised of one or more organic solvents and a small amount of water, wherein the amount of water controls the crystal form of the pigment, followed by a treatment with a halobenzene to obtain a highly photosensitive second crystal form of the pigment.
Owner:XEROX CORP
Perfluoroalkyl styrene and application thereof
ActiveCN110294699AExcellent liquid repellencySusceptible to UV degradationOrganic chemistryCoatingsUltraviolet lightsElectron
The invention discloses a perfluoroalkyl styrene monomer and application thereof. The perfluoroalkyl styrene monomer is prepared by the following steps of using monohalogenated thiophenol and perfluoro-iodoalkane as the raw materials, so as to obtain an intermediate, namely perfluoroalkyl thiohalobenzene; further fluorinating, so as to obtain perfluoroalkyl tetrafluorothio methylene halobenzene; finally, reacting with vinyl magnesium bromide, so as to obtain perfluoroalkyl tetrafluorothio methylene styrene. The prepared tetrafluorothio methylene-containing perfluoroalkyl styrene has the advantages that the reaction property is realized, and the tetrafluorothio methylene-containing perfluoroalkyl styrene can be used for preparing fluorine-containing surface treatment materials and liquid-repelling surfaces; because the perfluoroalkyl is bonded with rigid benzene rings, the whole structure of the fluorine-compound has larger crystallizing property, and the excellent liquid-repelling property is obtained; the tetrafluorothio methylene is used as a bridge group, and is directly bonded with the benzene ring, and the good heat-resistant stability is provided by the electron stable reaction of large phi bond of the benzene ring corresponding to the sulfur tetrafluoride; the tetrafluorothio methylene-containing perfluoroalkyl styrene is easy to degrade under the radiation by ultraviolet light, and the obtained fluorine-containing material is environment-friendly.
Owner:东莞市德伦新材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com