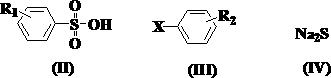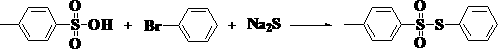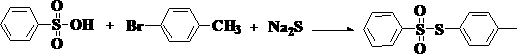Preparation method of besilate compound
A technology of benzenesulfonate and compounds, applied in the field of organic synthesis, can solve the problems of increasing operation complexity and high requirements of equipment, avoiding the use of sealing equipment and protective tools, facilitating operation and control, product yield and The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the synthesis of S-p-tolylbenzenesulfonate
[0030]
[0031] In terms of molar ratio, the ratio of benzenesulfonic acid, p-bromotoluene, sodium sulfide and oxidant is 1:1:1:0.5. Wherein oxidizing agent is hydrogen peroxide, and its mass percent concentration is 30%, and used solvent is benzene, and its quality is 5 times of benzenesulfonic acid quality, and concrete reaction process is as follows:
[0032] Slowly add benzenesulfonic acid into the solvent, stir evenly, then add p-bromotoluene dropwise, keep stirring during the dropwise addition, after the dropwise addition, continue stirring for 5-10 minutes to make the two evenly mixed, and then Add sodium sulfide and hydrogen peroxide respectively from two different feeding ports under continuous stirring, and after all the addition is completed, keep the reaction at this temperature for 1 hour. After the reaction was completed, saturated brine was added, and the organic layer was fully extracted. T...
Embodiment 2
[0034] Embodiment 2: the synthesis of S-p-tolylbenzenesulfonate
[0035] In terms of molar ratio, the ratio of benzenesulfonic acid, p-iodotoluene, sodium sulfide and oxidant is 1:2:2:1. Wherein oxidant is sodium hypochlorite, and used solvent is tetrahydrofuran, and its quality is 10 times of benzenesulfonic acid quality, and concrete reaction process is as follows:
[0036]Slowly add benzenesulfonic acid into the solvent, stir evenly, then add p-iodotoluene dropwise, keep stirring during the dropwise addition, after the dropwise addition, continue stirring for 5-10 minutes to make the two evenly mixed, and then Add sodium sulfide and sodium hypochlorite respectively from two different feeding ports under continuous stirring, and after all the addition is completed, keep the temperature for 2 hours for reaction. After the reaction was completed, saturated brine was added, and the organic layer was fully extracted. The organic layer was dried with anhydrous magnesium sulfat...
Embodiment 3
[0037] Embodiment 3: the synthesis of S-p-tolylbenzenesulfonate
[0038] In terms of molar ratio, the ratio of benzenesulfonic acid, p-chlorotoluene, sodium sulfide and oxidant is 1:3:3:1.5. Wherein oxidant is ammonium persulfate, and used solvent is ethanol, and its quality is 15 times of benzenesulfonic acid quality, and concrete reaction process is as follows:
[0039] Slowly add benzenesulfonic acid into the solvent, stir evenly, then add p-chlorotoluene dropwise, keep stirring during the dropwise addition, after the dropwise addition, continue stirring for 5-10 minutes to make the two evenly mixed, and then Add sodium sulfide and ammonium persulfate respectively from two different feeding ports under continuous stirring, and after all the addition is completed, keep the temperature for 3 hours for reaction. After the reaction was completed, saturated brine was added, and the organic layer was fully extracted. The organic layer was dried with anhydrous magnesium sulfate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com