Fluoro-aromatic organic tetracarboxylic dianhydride and its preparation method and use
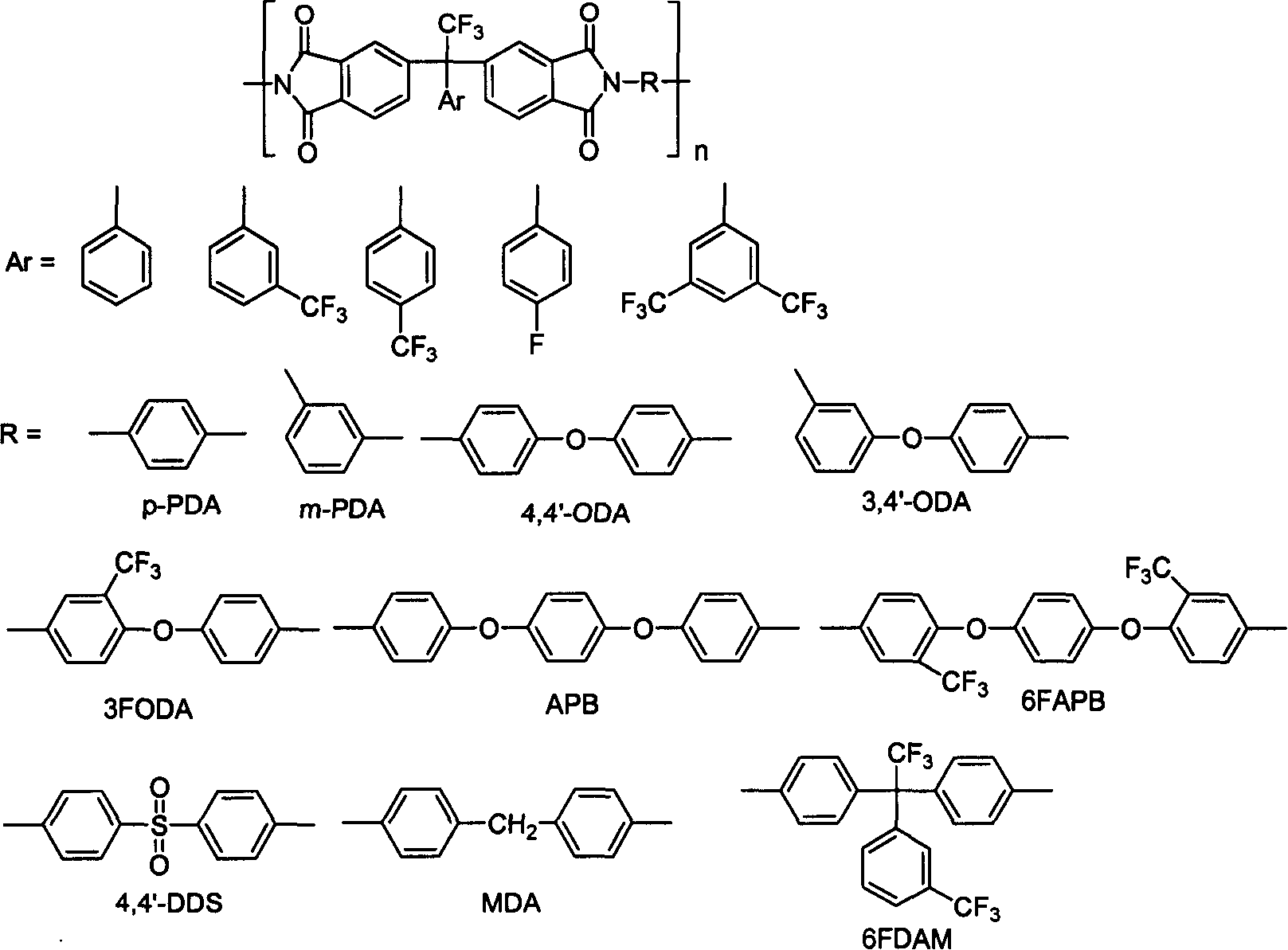
A technology of organic tetraacid dianhydride and tetraacid dianhydride, which is used in organic chemistry, chemical instruments and methods, multilayer circuit manufacturing, etc., can solve the problem of limited varieties of aromatic tetraacid dianhydrides and restrict the development of polyimide materials. , harsh chemical preparation conditions, etc., to achieve the effects of excellent storage stability, excellent solubility, and excellent comprehensive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
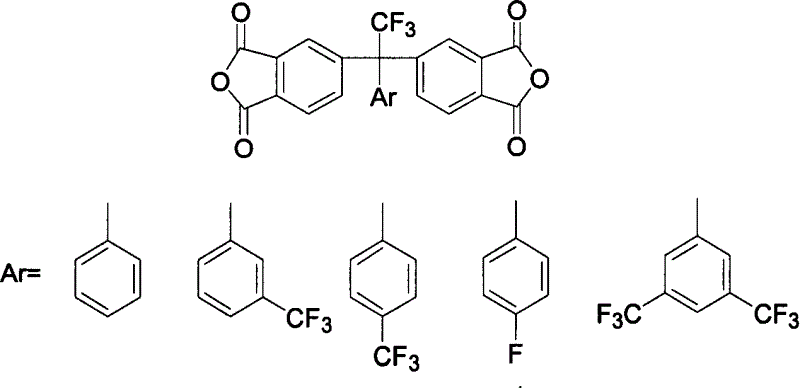
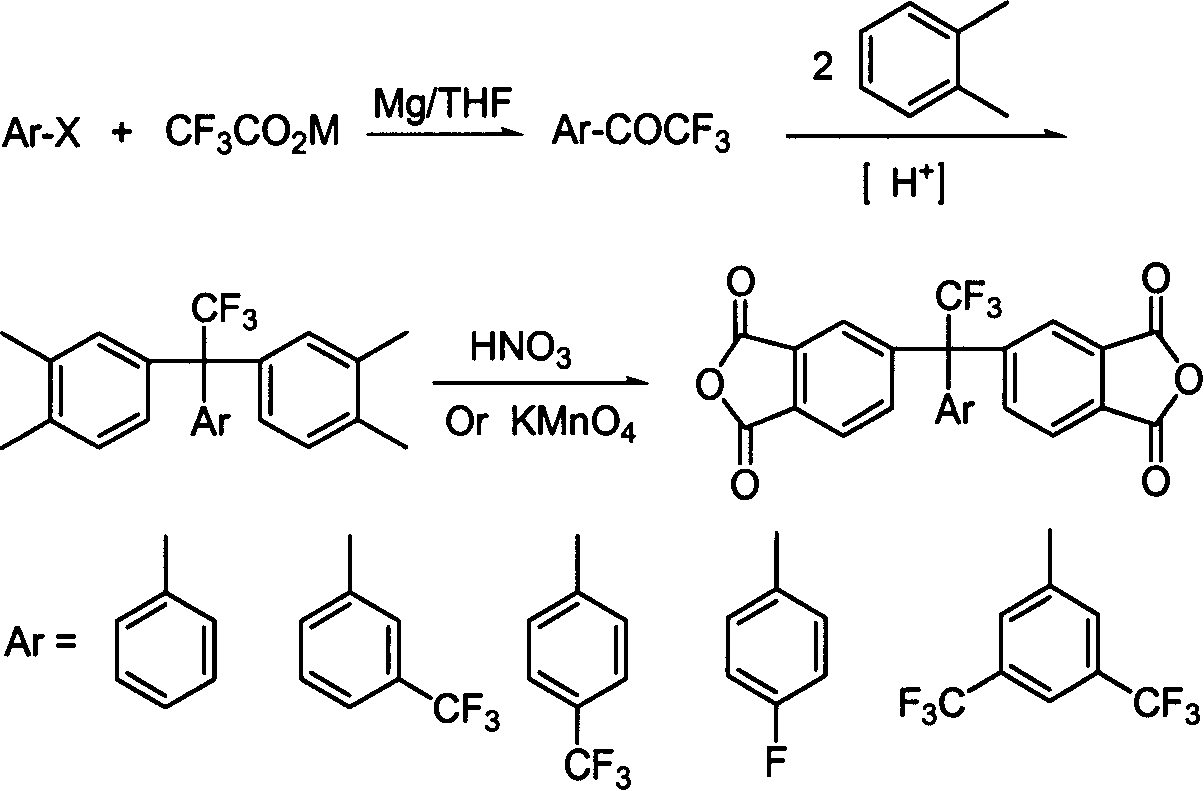
[0031] α, α, the preparation of α-trifluoromethyl acetophenone: in the there-necked flask equipped with mechanical stirring, condenser tube and nitrogen inlet and outlet, add 20 parts of magnesium powder, 60 parts of potassium trifluoroacetate, 400 parts of tetrahydrofuran, 130 parts of bromobenzene were added dropwise with stirring. The reaction mixture was refluxed for 2-3 hours under stirring to obtain a dark gray cloudy solution, which was hydrolyzed with 15% sulfuric acid solution. The organic phase was collected, the aqueous phase was extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and distilled to obtain a colorless α,α,α-trifluoromethylacetophenone liquid.
[0032] The preparation of 1,1-bis(3,4-dimethylphenyl)-1-phenyl-2,2,2-trifluoroethane: 70 parts of α,α,α-trifluoromethylphenyl ethyl Add ketone, 300 parts of o-xylene and 30 parts of methanesulfonic acid into a three-neck flask with a nitrogen gas i...
example 2
[0036] The preparation of 3-trifluoromethyl-α, α, α-trifluoromethyl acetophenone: In the three-neck flask equipped with mechanical stirring, condenser and nitrogen inlet and outlet, add 30 parts of magnesium powder, lithium trifluoroacetate 80 parts, 600 parts of anhydrous diethyl ether, and 150 parts of 3-trifluoromethylchlorobenzene were added dropwise under stirring. The reaction mixture was refluxed for 2-3 hours under stirring to obtain a dark gray cloudy solution, which was hydrolyzed with 20% sulfuric acid solution. The organic phase was collected, the aqueous phase was extracted three times with ether, the organic phases were combined, and the 152-154 ° C fraction was collected by distillation to obtain a colorless 3-trifluoromethyl-α, α, α-trifluoromethyl acetophenone liquid.
[0037] Preparation of 1,1-bis(3,4-dimethylphenyl)-1-(3-trifluoromethylphenyl)-2,2,2-trifluoroethane: 90 parts of 3-trifluoro Methyl-α, α, α-trifluoromethylacetophenone, 350 parts of o-xylene a...
example 3
[0041] The preparation of 4-trifluoromethyl-α, α, α-trifluoromethyl acetophenone: In the three-neck flask equipped with mechanical stirring, condenser and nitrogen inlet and outlet, add 20 parts of magnesium powder, sodium trifluoroacetate 80 parts, 450 parts of tetrahydrofuran, and 150 parts of 4-trifluoromethylbromobenzene were added dropwise with stirring. The reaction mixture was refluxed for 2-3 hours under stirring to obtain a dark gray cloudy solution, which was hydrolyzed with 10% sulfuric acid solution. The organic phase was collected, the aqueous phase was extracted three times with ether, the organic phases were combined, dried over anhydrous sodium sulfate, and distilled to obtain a colorless 4-trifluoromethyl-α,α,α-trifluoromethylacetophenone liquid.
[0042] The preparation of 1,1-bis(3,4-dimethylphenyl)-1-(4-trifluoromethylphenyl)-2,2,2-trifluoroethane: 80 parts of 4-trifluoro Methyl-α, α, α-trifluoromethylacetophenone, 400 parts of o-xylene and 40 parts of tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com