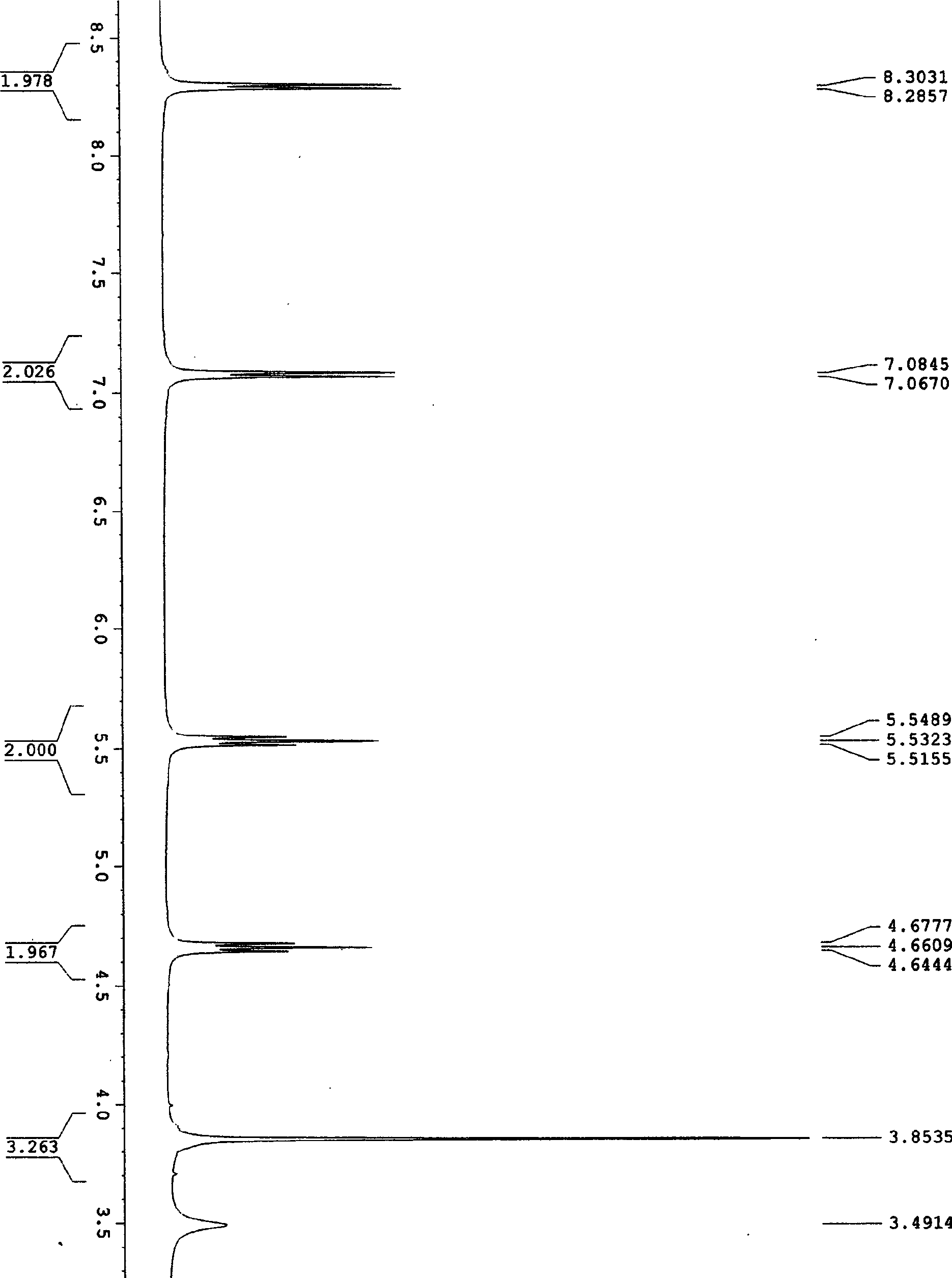New method of synthesizing 1, 2, 3-triazol 1, 3-diazacyclo compound
A technology for a diazide heterocycle and a synthesis method, which is applied in 1 field, can solve the problems of inability to meet high-throughput screening and drug research and development, and achieve the effects of achieving molecular diversity, high yield and simple route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
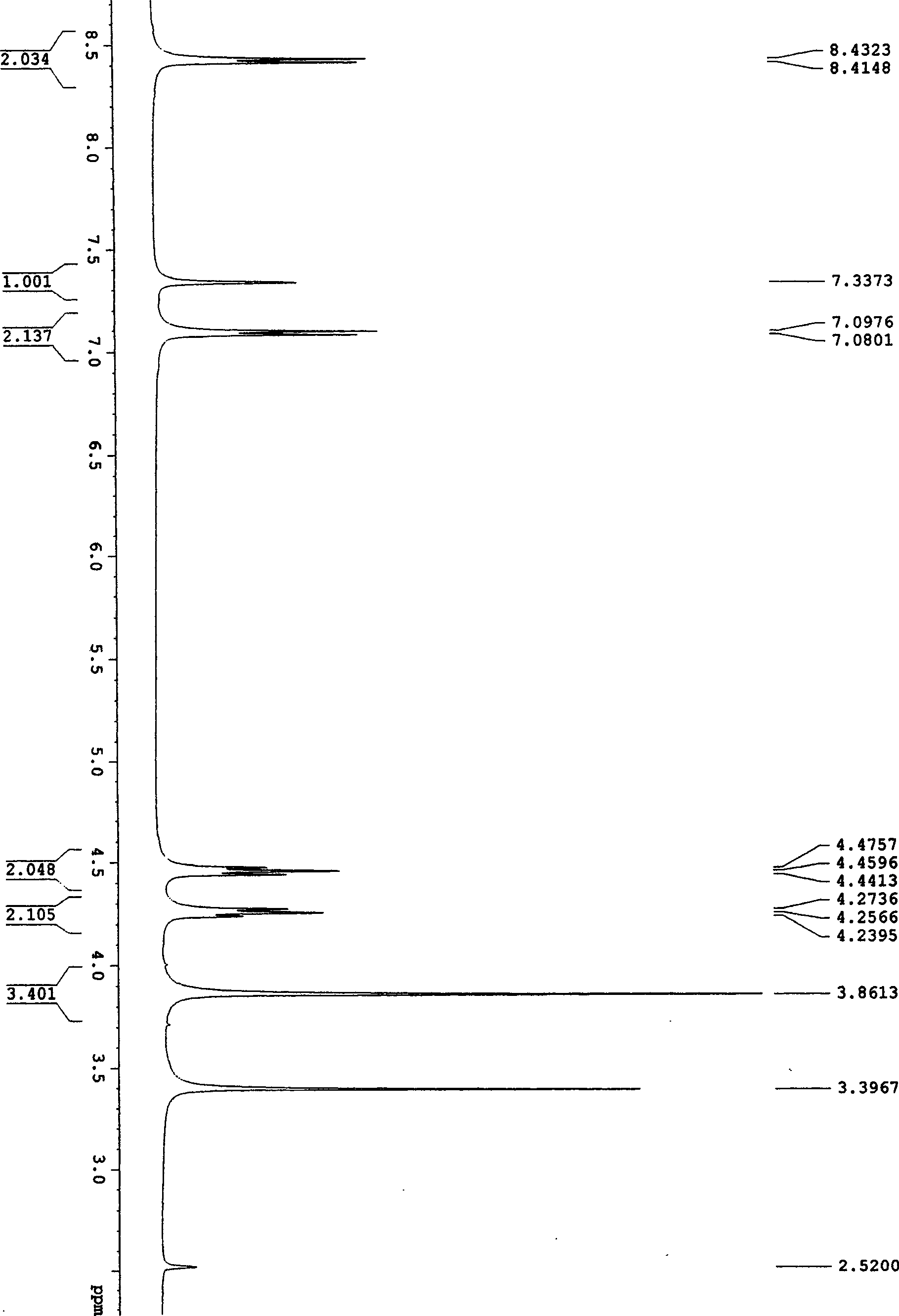
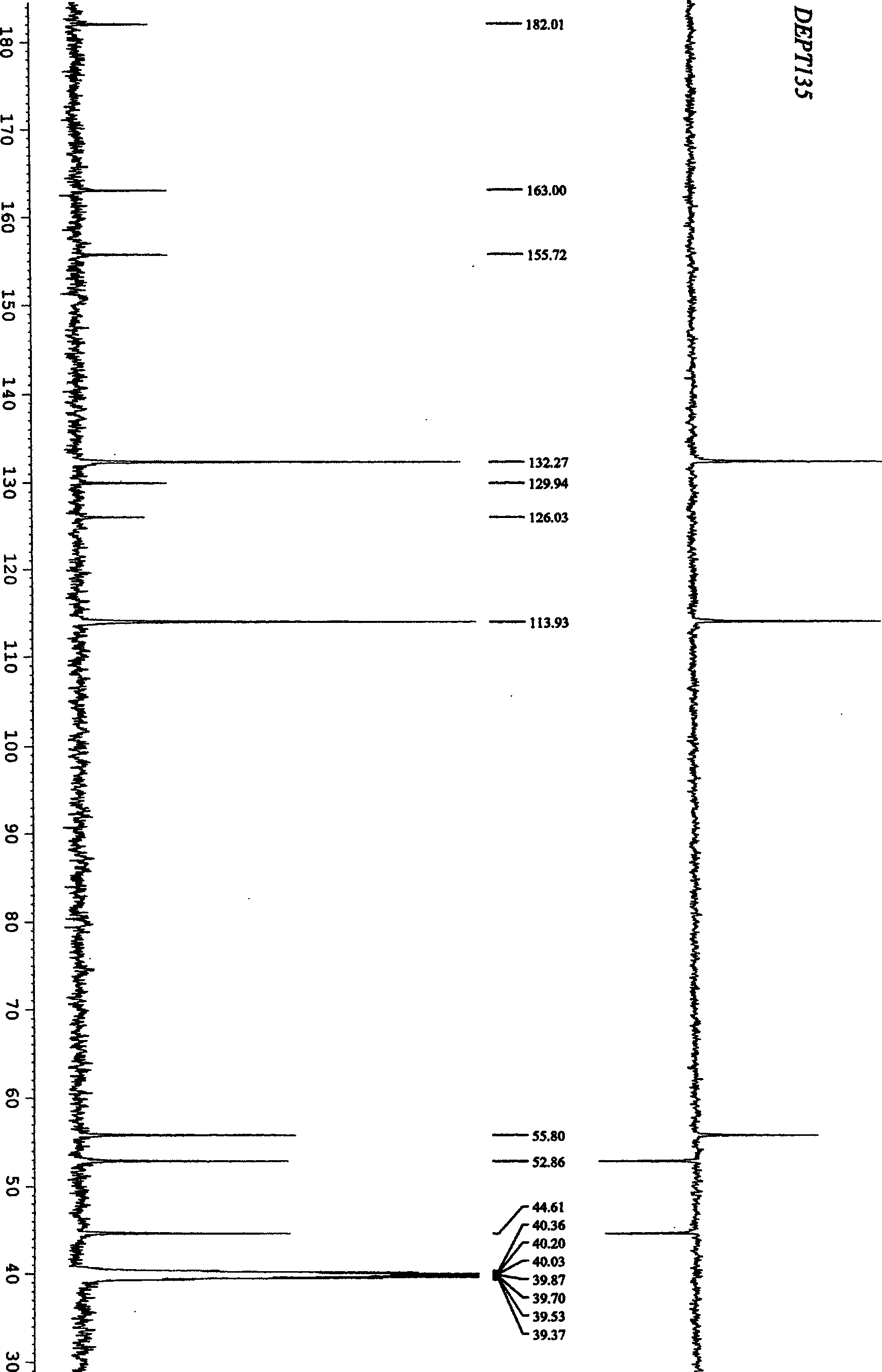
[0031] Example 1: Synthesis of 4-acetyl-1,2,3-triazol[1,5e]tetrahydroimidazole (I-A): 10 milliliters of dry DMF was added successively in a 25 milliliter round bottom flask, 2, 4, 0.53 g (2 mmol) of 5,6-tetrachloro-1,3-isophthalonitrile, 0.26 g (4 mmol) of sodium azide, stirred with a magnetic stirrer for 1 hour, and detected by TLC 2, 4, 5 , 6-1,3-isophthalonitrile reacted completely. Add 0.25 gram (2 millimoles) 2-acetylmethylenetetrahydroimidazoles in this reaction bottle again, continue to stir after 10 hours, TLC detects that 2-acetylmethylenetetrahydroimidazoles reacts completely, stop reaction, use After washing with 20 milliliters of water, extract with ethyl acetate, collect the organic layer and dry it with anhydrous sodium sulfate, remove ethyl acetate under reduced pressure, dissolve and mix silica gel with acetone, put it on the column by dry method, use eluent (ethyl acetate: petroleum Ether = 1:1) to obtain white crystals with a yield of 85-89% and a melting po...
Embodiment 2
[0034] Example 2: Synthesis of 4-acetyl-1,2,3-triazol[1,5e]tetrahydroimidazole (I-A): 10 milliliters of dry DMF, 5-chloro- 2,4,6-trifluoro-1,3-isophthalonitrile 0.43 g (2 mmol), sodium azide 0.26 g (4 mmol), magnetic stirrer for 1 hour, TLC detection 2,4 , 5,6-1,3-isophthalonitrile reacted completely. Add 0.25 gram (2 millimoles) 2-acetylmethylenetetrahydroimidazoles in this reaction bottle again, continue to stir after 10 hours, TLC detects that 2-acetylmethylenetetrahydroimidazoles reacts completely, stop reaction, use After washing with 20 ml of water, extract with ethyl acetate, collect the organic layer and dry it with anhydrous sodium sulfate, remove the ethyl acetate under reduced pressure, dissolve it with acetone and mix with silica gel, put it on the column by dry method, and use eluent (ethyl acetate: petroleum Ether = 1:1) eluted to give white crystals with a yield of 80%.
Embodiment 3
[0035] Example 3: Synthesis of 4-acetyl-1,2,3-triazol[1,5e]tetrahydroimidazole (I-A): 10 milliliters of dry DMF was added successively in a 25 milliliter round bottom flask, 2, 4, 0.40 g (2 mmol) of 5,6-tetrafluoro-1,3-isophthalonitrile, 0.26 g (4 mmol) of sodium azide, stirred with a magnetic stirrer for 1 hour, TLC detection 2, 4, 5 , 6-1,3-isophthalonitrile reacted completely. Add 0.25 gram (2 millimoles) 2-acetylmethylenetetrahydroimidazoles in this reaction bottle again, continue to stir after 10 hours, TLC detects that 2-acetylmethylenetetrahydroimidazoles reacts completely, stop reaction, use After washing with 20 ml of water, extract with ethyl acetate, collect the organic layer and dry it with anhydrous sodium sulfate, remove the ethyl acetate under reduced pressure, dissolve it with acetone and mix with silica gel, put it on the column by dry method, and use eluent (ethyl acetate: petroleum Ether = 1:1) eluted to give white crystals with a yield of 87%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com