Perfluoroalkyl styrene and application thereof
A technology of perfluoroalkyl styrene and perfluoroalkyl thiohalobenzene is applied in the field of polymer monomers and easily degradable fluorine-containing chemicals, and can solve the problem that long-carbon chain perfluoroalkyl groups are not easy to degrade and accumulate in the environment. Toxicity and other issues, to achieve the effects of excellent liquid repellency, good thermal stability, and excellent liquid repellency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
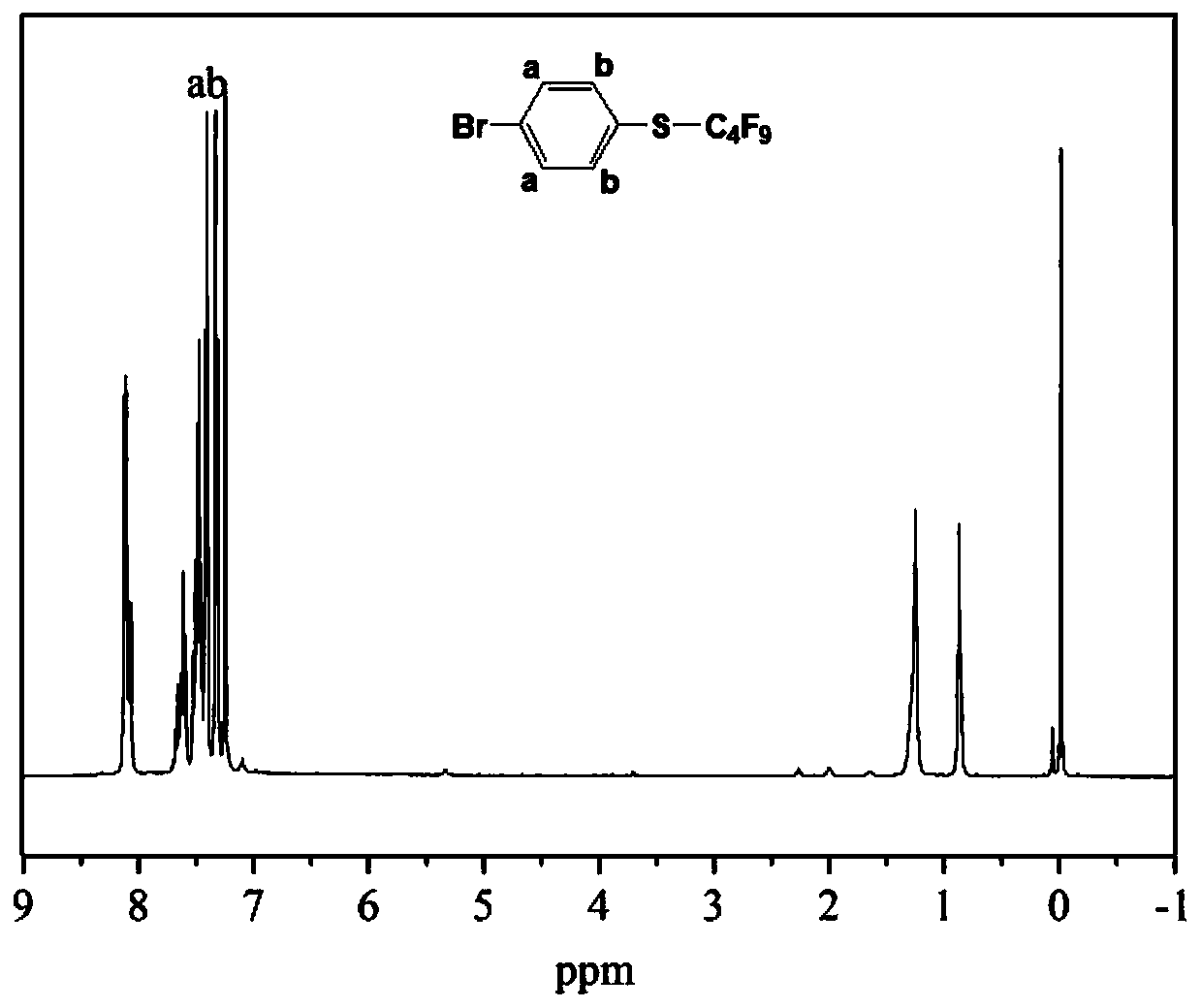
[0065] In this example, 4-(nonafluorobutyltetrafluorosulfurmethylene)styrene is synthesized, and the specific steps are as follows:
[0066] (1) Synthesis of 4-(nonafluorobutylthio)bromobenzene by addition of fluoroalkyl radicals
[0067] In a 250 ml three-neck flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, add 5.1 g of p-bromothiophenol, 10.4 g of nonafluoro-1-iodobutane, 0.5 g of copper acetate and 40 g of 1,4-dioxane, stirred and heated to 70°C, the reaction solution turned yellow. Dissolve 7.2 g of benzoyl peroxide in 45 g of 1,4-dioxane, slowly add it dropwise through a constant pressure dropping funnel, and keep it warm for 4 h after the drop is complete. After the reaction was completed, 1,4-dioxane was removed by rotary evaporation under reduced pressure, the resulting precipitate was filtered off, washed with water, dried over anhydrous magnesium sulfate for 8 h, and then rotary evaporated under red...
Embodiment 2
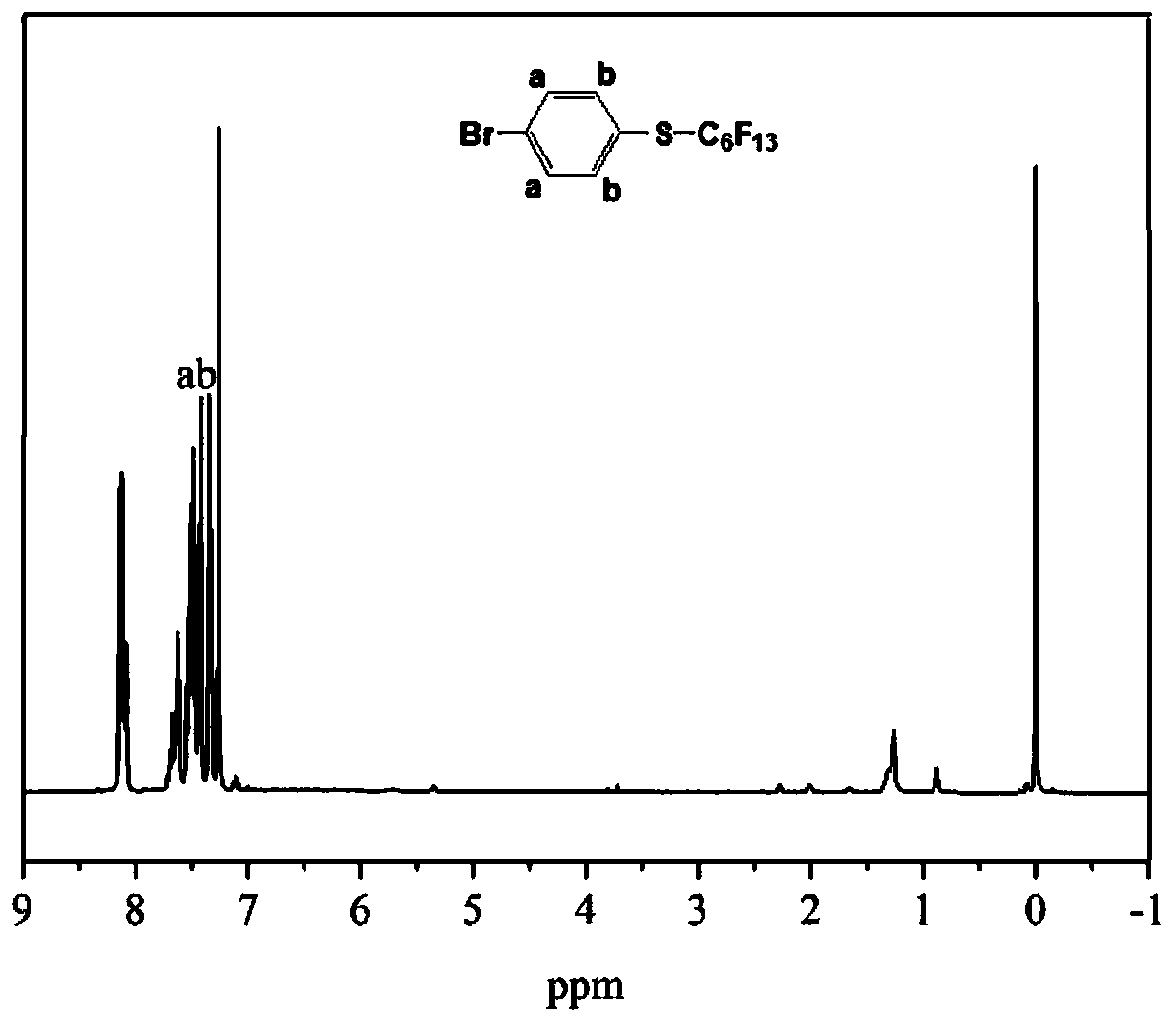
[0073] In this embodiment, 4-(tridecafluorohexyltetrafluorosulfurmethylene)styrene is synthesized, and the specific steps are as follows:
[0074] (1) Synthesis of 4-(tridecafluorohexylthio)bromobenzene by addition of fluoroalkyl radicals
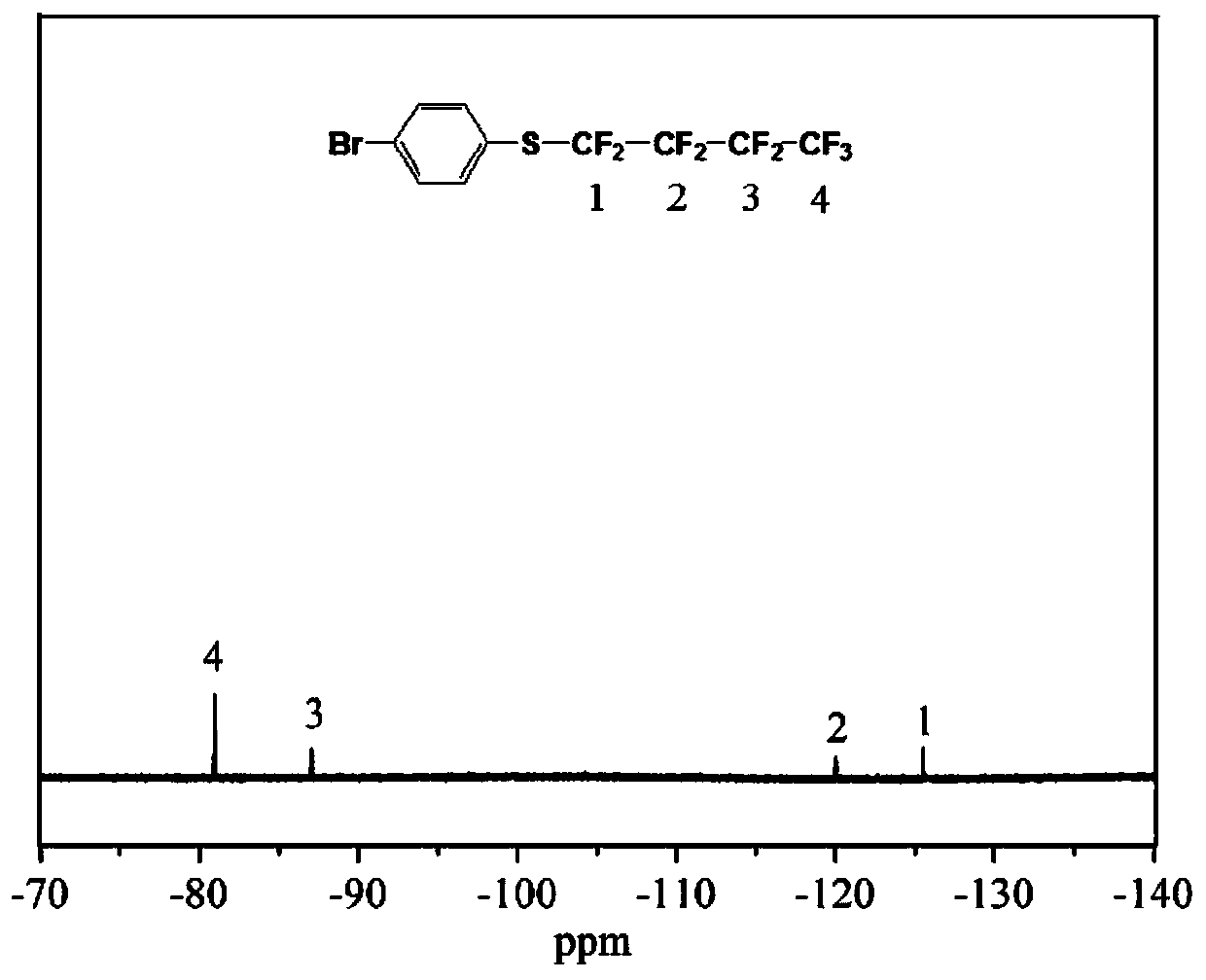
[0075] The synthesis steps and feed ratio of 4-(tridecafluorohexylthio)bromobenzene refer to the synthesis steps of 4-(nonafluorobutylthio)bromobenzene in Example 1, and 13.4 g of perfluoroiodohexane is added when feeding, The same amount of other materials was used to obtain 8.5 g of the product 4-(tridecafluorohexylthio)bromobenzene, yield: 62.0%. image 3 and Figure 4 Respectively the 4-tridecafluorohexylthiobromobenzene prepared above 1 HNMR and 19 F NMR spectrum: product 1 H NMR (400 MHz, CDCl 3 ): δ 7.41 (d, J = 8.4 Hz, 2H, o -H),7.32 (d, J = 8.4 Hz, 2H, m -H). 19 F NMR (564 MHz, CDCl 3 ): δ -80.78 (3F, CF 3 CF 2 CF 2 CF 2 CF 2 CF 2 S), -86.88 (2F, CF 3 CF 2 CF 2 CF 2 CF 2 CF 2 S), -119.30 (2F, CF 3 CF ...
Embodiment 3
[0081] In this example, 4-(nonafluorobutyltetrafluorosulfurmethylene)styrene is synthesized, and the specific steps are as follows:
[0082] (1) Synthesis of 4-(nonafluorobutylthio)chlorobenzene by addition of fluoroalkyl radicals
[0083] In a 250 ml three-necked flask equipped with a magnetic stirrer, a thermometer, a constant pressure dropping funnel, and a reflux condenser, add 4.7 g of p-chlorothiophenol, 10.5 g of nonafluoro-1-iodobutane, 0.5 g of copper acetate and 45 g of 1,4-dioxane, stirred and heated to 72°C, the reaction solution turned yellow. Dissolve 7.3 g of benzoyl peroxide in 50 g of 1,4-dioxane, slowly add it dropwise through a constant pressure dropping funnel, and keep it warm for 4 h after the drop is complete. After the reaction was completed, 1,4-dioxane was removed by rotary evaporation under reduced pressure, the resulting precipitate was filtered off, washed with water, dried over anhydrous magnesium sulfate for 8 h, and then rotary evaporated under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com