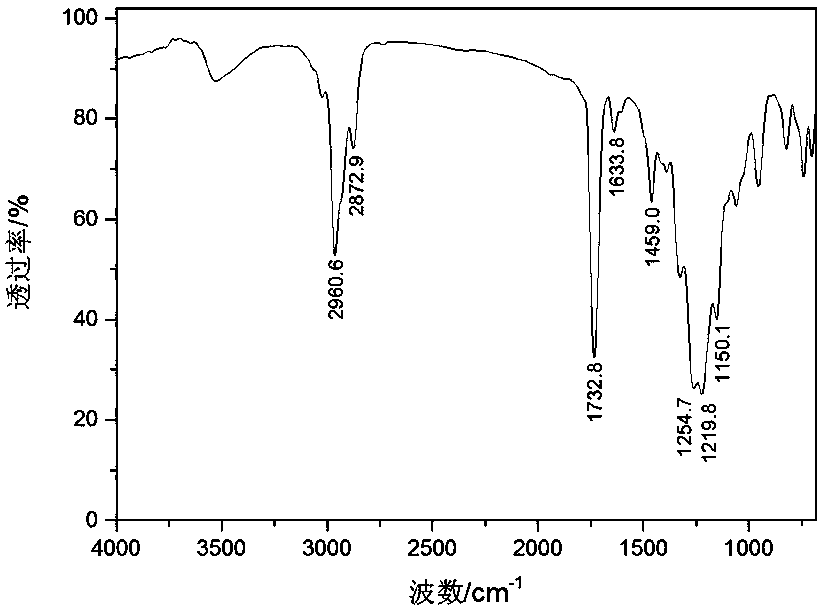
A sulfur-containing long heterochain perfluoroalkyl enoate and its preparation method
A technology of perfluoroalkyl acrylate and perfluoroalkyl acrylate, which is applied in the field of polymer monomer and fine chemical synthesis, can solve the problem of not being able to provide anti-repellent function, short carbon chain perfluoroalkyl is not rigid and crystallinity, to achieve excellent liquid repellency, easy to degrade, and overcome the effect of not easy to degrade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In this example, 1H, 1H, 2H, 2H-7-thia-perfluoroundecyl methacrylate is synthesized, and the specific steps are as follows:
[0042] 1. Telomerization reaction
[0043] Add 46.9g of nonafluorobutyltetrafluorosulfur bromide, 0.40g of di-tert-butyl peroxide, 0.4g of calcium carbonate and 48g of 1,3-bis(trifluoromethyl)benzene into the reaction kettle, seal it, and pump After vacuuming, fill in nitrogen to replace the air in the reactor, and then vacuumize and replace. A total of 4 replacements were performed.
[0044]After raising the temperature to 45°C, feed tetrafluoroethylene into the reactor to keep warm and stir for reaction. The stirring rate is controlled to be 55 rpm, the feeding speed of tetrafluoroethylene is 12g / hour, and the pressure is 0.48MPa. Stop after 2 hours. Tetrafluoroethylene was introduced, and the reaction was continued for 2 hours. After cooling, the reaction mixture was distilled off under reduced pressure to remove 1,3-bis(trifluoromethyl)ben...
Embodiment 2
[0054] In this example, 1H, 1H, 2H, 2H-7-thia-perfluoroundecyl acrylate is synthesized, and the specific steps are as follows:
[0055] 1. Telomerization reaction
[0056] Add 40g of nonafluorobutyl tetrafluorosulfur chloride, 0.1g of dibenzoyl peroxide, 0.15g of calcium carbonate and 15g of trifluorotoluene solvent into the reaction kettle in turn, seal it, and after vacuuming, fill the reaction kettle with nitrogen replacement The air in the chamber is then vacuumed and replaced. A total of 5 replacements were performed.
[0057] After raising the temperature to 50°C, feed tetrafluoroethylene into the reactor to keep warm and stir for reaction. The stirring rate is controlled to be 55 rpm, the feeding speed of tetrafluoroethylene is 10g / hour, and the pressure is 0.45MPa. Stop after 2 hours. Tetrafluoroethylene was introduced, and the reaction was continued for 3 hours. After cooling, the reaction mixture was distilled off under reduced pressure to remove benzotrifluoride....
Embodiment 3
[0065] In this example, 1H, 1H, 2H, 2H-7-thia-perfluoroundecyl acrylate is synthesized, and the specific steps are as follows:
[0066] 1. Telomerization reaction
[0067] Add 36.3g of nonafluorobutyl tetrafluorosulfur chloride, 0.36g of di-tert-butyl peroxide, 0.4g of calcium carbonate and 45g of trifluorotoluene solvent into the reaction kettle in turn, seal it, vacuumize it, and fill it with nitrogen for replacement reaction The air in the kettle was evacuated and replaced. A total of 5 replacements were performed.
[0068] After raising the temperature to 50°C, feed tetrafluoroethylene into the reactor to keep warm and stir for reaction. The stirring rate is controlled to be 55 rpm, the feeding speed of tetrafluoroethylene is 11g / hour, and the pressure is 0.45MPa. Stop after 2 hours. Tetrafluoroethylene was introduced, and the reaction was continued for 2 hours. After cooling, the reaction mixture was distilled off under reduced pressure to remove benzotrifluoride. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com