Organic solar cell acceptor material and preparation method thereof
A technology for solar cells and organic solar energy, applied in the field of solar cells, can solve the problems of low carrier mobility and low conversion efficiency of organic semiconductor cells, and achieve the effects of strong light absorption intensity, easy purification, and broad spectrum absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example is used to illustrate the preparation method of the intermediate 5 for the synthesis of organic solar acceptor cell materials. The synthetic steps of intermediate 5 are as follows:
[0037] The process from 9-(4-bromophenyl)-3,6-dibromocarbazole and biboronic acid pinacol ester to the synthesis of intermediate 5 is as follows:
[0038] step one:
[0039]
[0040] Get 12.00g of 9-(4-bromophenyl)-3,6-dibromocarbazole (25mmol) and 22.20g of biboronic acid pinacol ester (87.5mmol) and join in the reactor, add 0.96g of [ 1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (2.6mmol), 8.59g of methyl acetate and 75mL of dimethyl sulfoxide (DMSO), reacted at 80°C for 24h, the reaction After cooling, 375 mL of water was added to dilute the reaction solution, and extracted with dichloromethane. After being spin-dried, 13.20 g of intermediate 1 was obtained by column chromatography with a yield of 85%. The structural formula was confirmed by NMR. 1 H NMR ...
Embodiment 2
[0053] Embodiment 2: Synthesis of organic solar cell acceptor material CzTPFCN
[0054] When the terminal group is selected from A1, the reaction scheme is:
[0055]
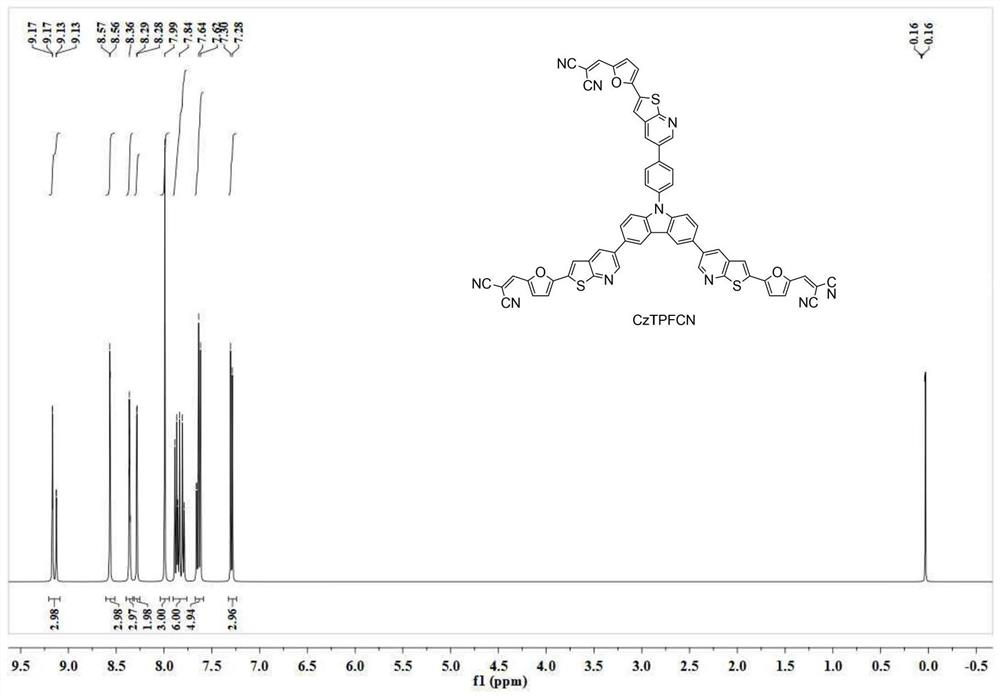
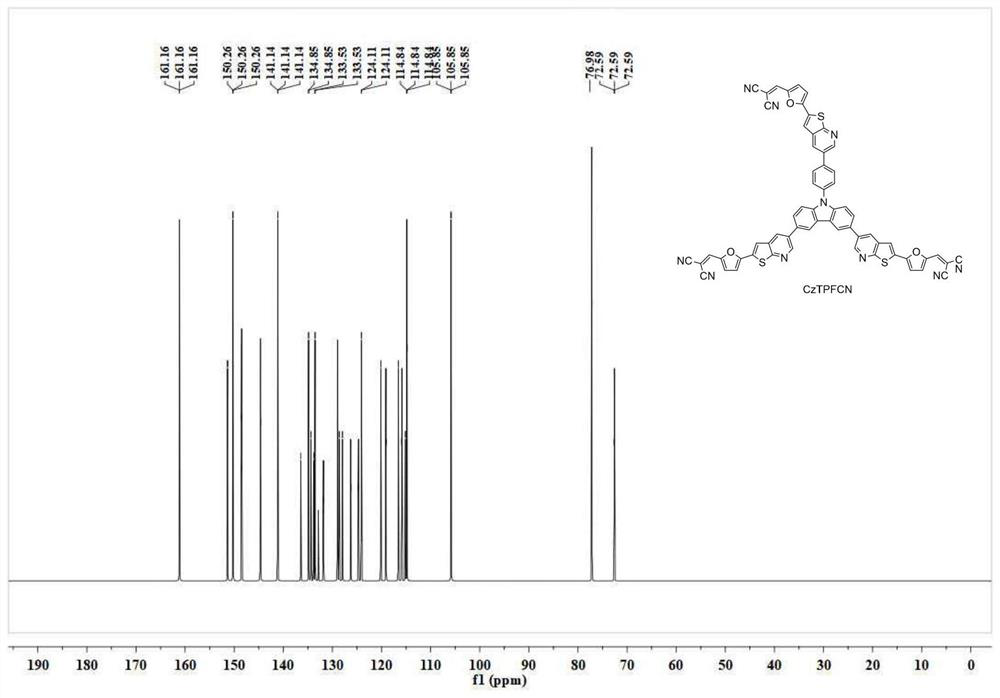
[0056] Take 4.6g of intermediate 5 (5mmol) prepared in Example 1 and add it to the reactor, add 1.1g of malononitrile (16mmol) and 50mL of chloroform, stir and react at room temperature for 0.5h, then slowly add 1.6g of triethylamine The reaction was continued for 20 min. After the reaction was completed, it was quenched by adding ice water, extracted with dichloromethane, and then separated by column chromatography to obtain 4.6 g of CzTPFCN as a yellow solid with a yield of 86%. Spectrum see figure 1 and figure 2 : 1 H NMR (400MHz, CDCl 3 )δ9.17-9.13(m,3H),8.57-8.56(m,3H),8.37-8.35(m,3H),8.29-8.28(m,2H),7.99(s,3H),7.89-7.81( m,6H),7.66-7.62(m,5H),7.30-7.28(m,3H). 13 C NMR (100MHz, CDCl 3 )δ161.2, 161.2, 161.2, 151.5, 151.5, 151.5, 142.5, 142.3, 141.1, 141.1, 141.1, 134.9, 134.9, 133.5, 133.5, 124.1,...
Embodiment 3
[0058] Embodiment 3: the synthesis of organic solar cell acceptor material CzTPFTC
[0059] When the terminal group is selected from A4, the reaction scheme is as follows:
[0060]
[0061] Get the intermediate 5 (5mmol) prepared by 4.6g embodiment 1 and join in the reactor, add 3.2g of 1,3-diethyl-2-thiobarbituric acid (16mmol) and 50mL chloroform, at room temperature After stirring and reacting for 0.5h, slowly add 1.6g triethylamine to continue the reaction for 20min. After the reaction, add ice water to quench, and extract with dichloromethane, then carry out column chromatography to obtain 6.2g CzTPFCN of yellow solid. The yield is 84%. 1 H NMR (400MHz, CDCl 3 )δ9.16(d,J=1.4Hz,2H),9.09(d,J=1.4Hz,1H),8.34–8.24(m,5H),7.94–7.77(m,9H),7.64(dd,J =7.5,1.5Hz,2H),7.56(s,3H),7.40–7.30(m,6H),5.26(q,J=6.3Hz,6H),4.16(q,J=6.3Hz,6H),1.17 (t,J=6.3Hz,18H). 13 C NMR (100MHz, CDCl 3 )δ175.9,161.2,126.2,158.0,150.3,148.5,144.6,137.0,136.5,134.9,133.8,133.5,132.9,131.8,129.0,128.6,1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com