A kind of organic solar cell electron acceptor material and its preparation method and application
A technology for electron acceptor materials and solar cells, applied in organic chemistry, electrical solid-state devices, semiconductor/solid-state device manufacturing, etc., to achieve the effects of simple regulation, easy purification, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
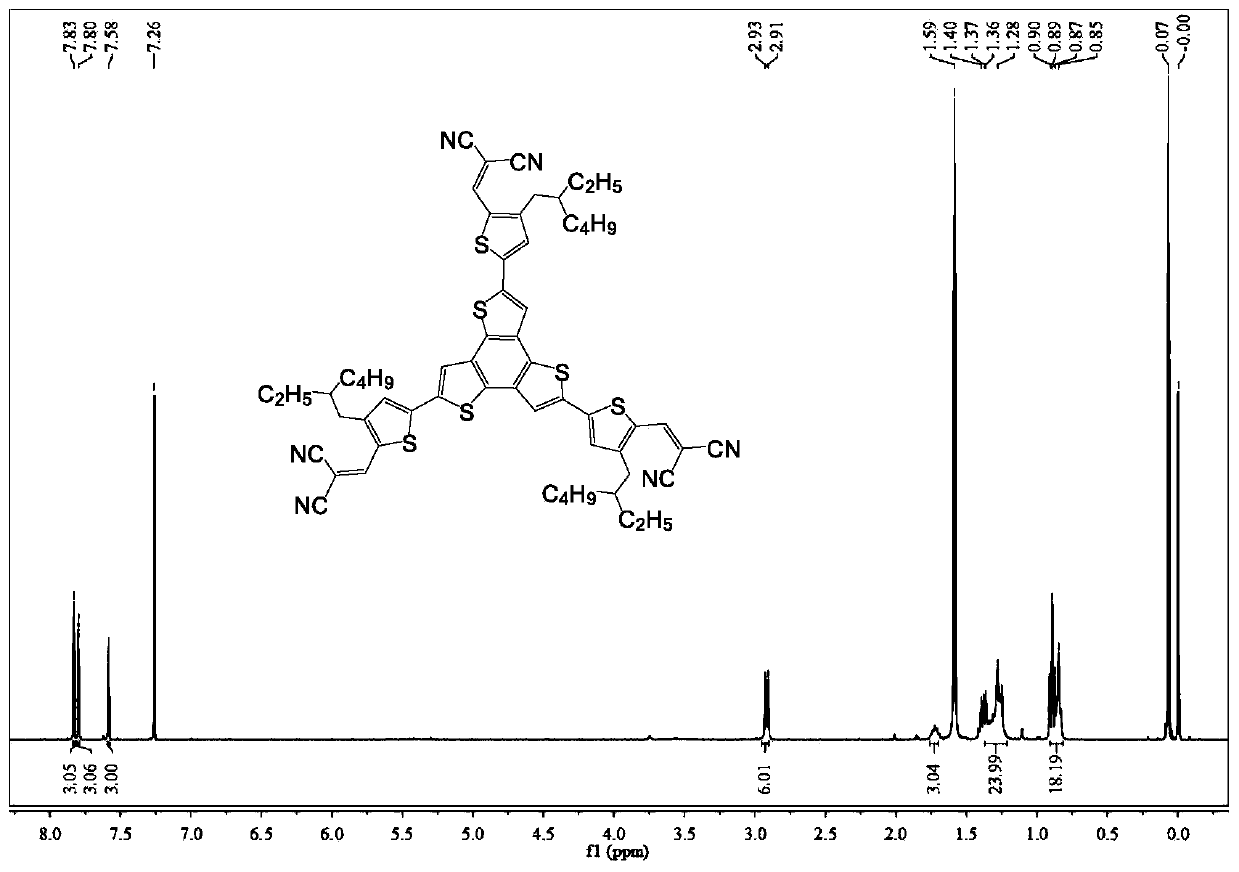
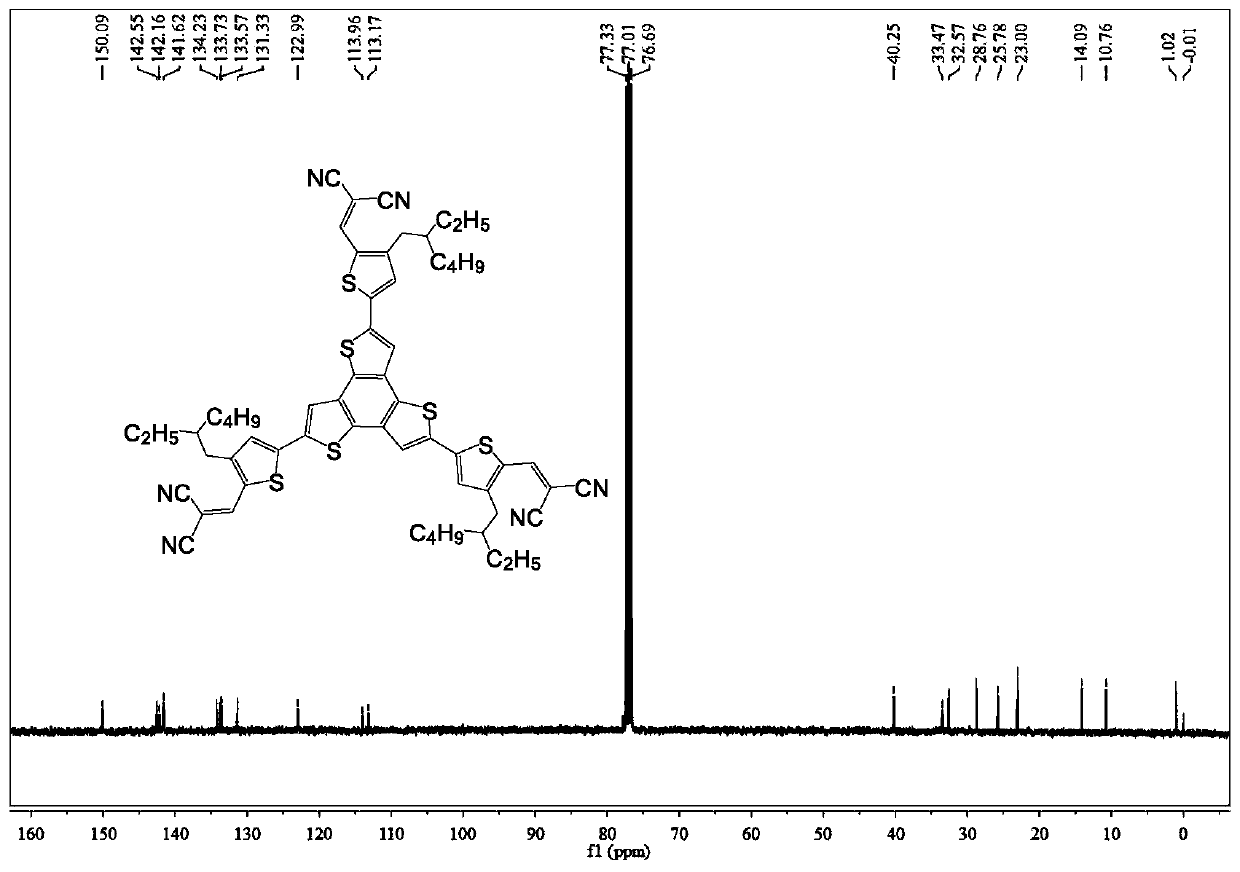
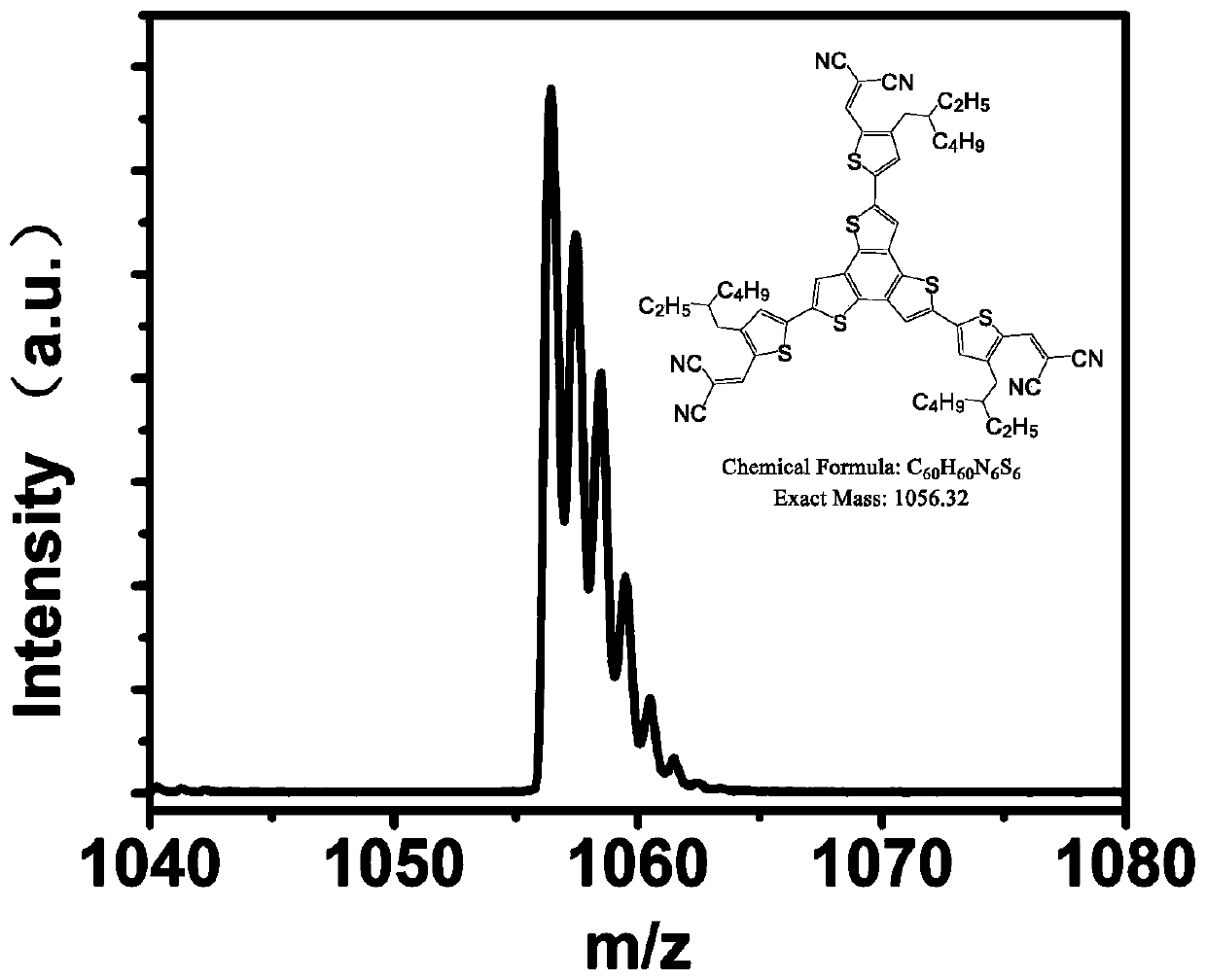
[0040] Embodiment 1: the synthesis of BTTCN
[0041] When terminal group A is selected from group A1, reaction scheme 1:
[0042]
[0043]Synthetic procedure for preparing BTTCN intermediate BrRTCN: Add malononitrile (52.3 mg, 0.79 mmol) and BrRTCHO (200 mg, 0.66 mmol) into 20 mL of chloroform. Then 0.1 mL of triethylamine was added and stirred at room temperature for 10 minutes. The reaction was quenched with ice water, then extracted and washed three times with dichloromethane and water respectively, dried over anhydrous magnesium sulfate, filtered and concentrated. Purify by column chromatography using a mixed solvent of petroleum ether and dichloromethane to obtain BrRTCN (211.5 mg, 91.3%). 1 H NMR (400MHz, CDCl 3 ):δ7.71(s,1H),7.38(s,1H),2.54-2.53(d,2H),1.61-1.58(m,1H),1.32-1.24(m,8H),0.90-0.86(m ,6H). 13 C NMR (100MHz, CDCl 3 ): δ150.17, 143.93, 139.56, 134.75, 124.64, 113.78, 113.03, 39.85, 33.37, 32.38, 28.71, 25.58, 22.98, 14.10, 10.75, 0.97. MALDI-TOF-MS(m / z...
Embodiment 2
[0046] Embodiment 2: the synthesis of BTTIC:
[0047] When terminal group A is selected from group A8, reaction scheme 2:
[0048]
[0049] Intermediates for the preparation of BTTIC:
[0050] Synthetic steps of BrRTIC: BrRTCHO (200mg, 0.66mmol) and (3-oxo-2,3-dihydro-1H-inden-1-ylidene) malononitrile (154.2mg, 0.79mmol) were added to 20mL chloroform middle. Then add 0.1 mL of triethylamine and stir at room temperature for one hour. The reaction was quenched with ice water, then extracted and washed three times with dichloromethane and water respectively, dried over anhydrous magnesium sulfate, filtered and concentrated. Purify by column chromatography using a mixed solvent of petroleum ether and dichloromethane to obtain BrRTIC (256.9 mg, 81.2%). 1 H NMR (400MHz, CDCl 3 ):δ7.71(s,1H),7.69(s,3H),7.68(t,3H),7.52(s,3H),7.33(t,3H),7.38(s,1H),2.54-2.53( d,2H),1.61-1.58(m,1H),1.32-1.24(m,8H),0.90-0.86(m,6H). 13 C NMR (100MHz, CDCl 3 ):δ161.61,161.01,159.53,158.31, 157.66...
Embodiment 3
[0053] Embodiment 3: Apply the product BTTCN in Embodiment 1 to an organic photovoltaic device, and its device construction method and its performance parameters are as follows:
[0054] Device 1: The target product BTTCN in Example 1 was blended with the commercial polymer donor material PTB7-Th, using chloroform as the solvent, and the device structure was prepared as ITO / ZnO / PTB7-th:BTTCN(1:1.5) / MoO 3 / Ag organic photovoltaic devices, the thickness of the active layer is about 90nm.
[0055] Device 2: Combine the target product BTTCN in Example 1 with commercialized PTB7-Th and fullerene electron acceptor material PC 71 BM blending, with chlorobenzene / 1,8-diiodooctane as solvent, the device structure is ITO / ZnO / PTB7-th:PC 71 BM:BTTCN(1:1.5:0.03) / MoO 3 / Ag organic photovoltaic devices, the thickness of the active layer is about 100nm.
[0056] Reference device preparation: the commercial polymer donor material PTB7-Th and PC 71 BM blending, with chlorobenzene / 1,8-diiod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com