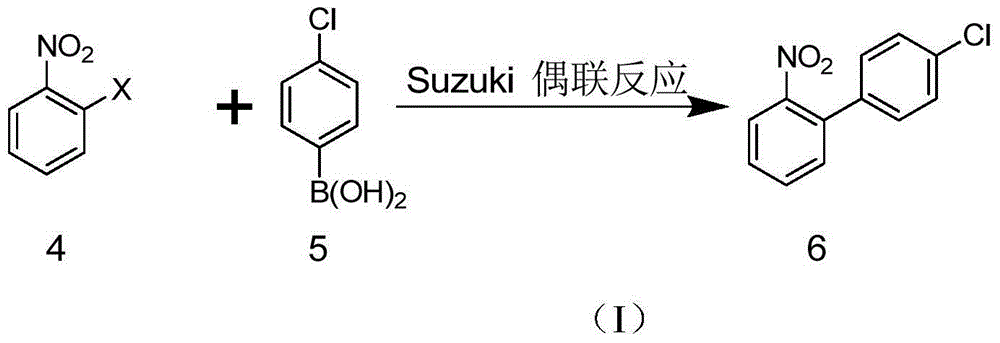
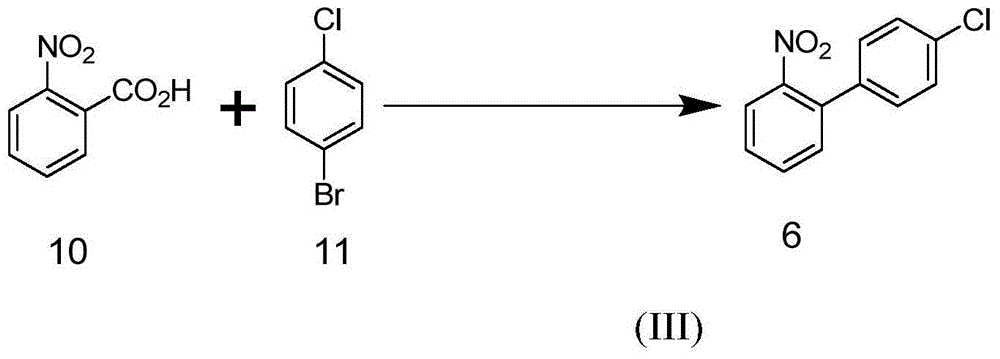
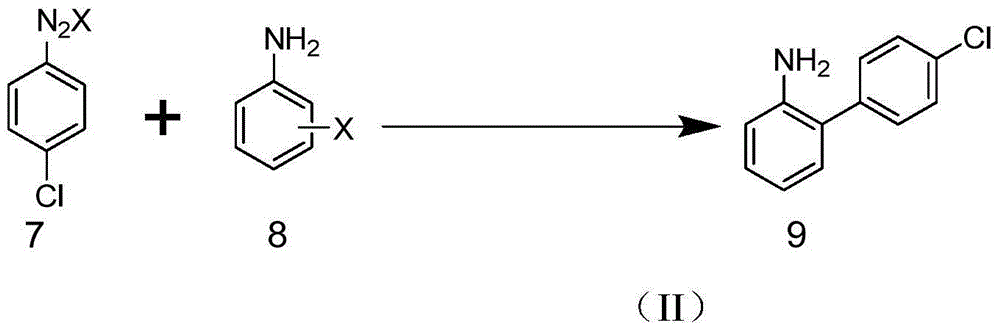Method for synthesizing amino biaromatic compound
A technology for synthesizing amino groups and compounds, which is applied in the preparation of amino compounds, the preparation of amino compounds from amines, the preparation of organic compounds, etc., can solve the problems of high cost, long route and high manufacturing cost, and achieve the effect of breaking through novelty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Add 6.18 g of potassium o-nitrobenzoate, 0.11 g of cuprous iodide, 0.06 g of palladium acetylacetonate, 0.18 g of o-phenanthroline, 3.80 g of p-chloro Bromobenzene and 40 grams of anhydrous polyethylene glycol PEG-400, then nitrogen vacuum replacement three times, under the protection of nitrogen, stirring
[0052] Raise the temperature to 190 degrees Celsius and react for 24 hours. After detecting the reaction with a gas chromatograph or a liquid chromatograph, the reaction solution was cooled to room temperature, and the reaction solution was extracted 10 times with 40 milliliters of ether. After the extracts were combined, they were washed twice with 200 milliliters of water, and the organic phase was concentrated with a rotary evaporator. The obtained oily mixture was recrystallized with petroleum ether, and after filtration, 3.87 g of yellow-brown 4'-chloro-2-aminobiphenyl was obtained, with a yield of 95% and a purity of 99%. The target compound was characterized...
Embodiment 2
[0054] Add 6.18 g of potassium o-nitrobenzoate, 0.11 g of cuprous iodide, 0.06 g of palladium acetylacetonate, 0.18 g of o-phenanthroline, 3.80 g of p-chloro Bromobenzene and 3A molecular sieves were replaced by nitrogen vacuum three times. Under the protection of nitrogen, 40 grams of anhydrous polyethylene glycol PEG-400 was added, and then replaced by nitrogen vacuum three times. Under the protection of nitrogen, the temperature was raised to 190 degrees Celsius with stirring. Reaction 24 Hour. After detecting the reaction with a gas chromatograph or a liquid chromatograph, the reaction solution was cooled to room temperature, and the reaction solution was extracted 10 times with 40 milliliters of ether. After the extracts were combined, they were washed twice with 200 milliliters of water, and the organic phase was concentrated with a rotary evaporator. The obtained oily mixture was recrystallized with petroleum ether, and after filtration, 3.7 g of yellow-brown 4'-chloro-...
Embodiment 3
[0056] Add 6.18 g of potassium o-nitrobenzoate, 0.11 g of cuprous iodide, 0.06 g of palladium acetylacetonate, 0.18 g of o-phenanthroline, 3.80 g of p-chloro Bromobenzene and 3A molecular sieves were replaced by nitrogen vacuum three times. Under the protection of nitrogen, 40 grams of anhydrous trimethylbenzene was added, and then replaced by nitrogen vacuum three times. Under the protection of nitrogen, the mixture was stirred and heated to 160 degrees Celsius, and reacted for 24 hours. After detecting the end of the reaction with a gas chromatograph or a liquid chromatograph, the reaction solution was cooled to room temperature, added 5 grams of diatomaceous earth and stirred for 5 minutes, then filtered under reduced pressure through a Buchner funnel covered with 3 cm of diatomaceous earth, and washed with ethyl acetate The ester was washed with diatomaceous earth until the filtrate flowing down was colorless and clear. The filtrate was concentrated with a rotary evaporator...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com