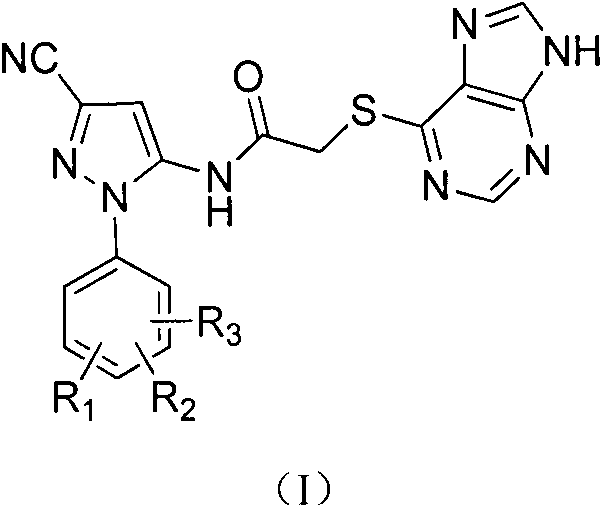
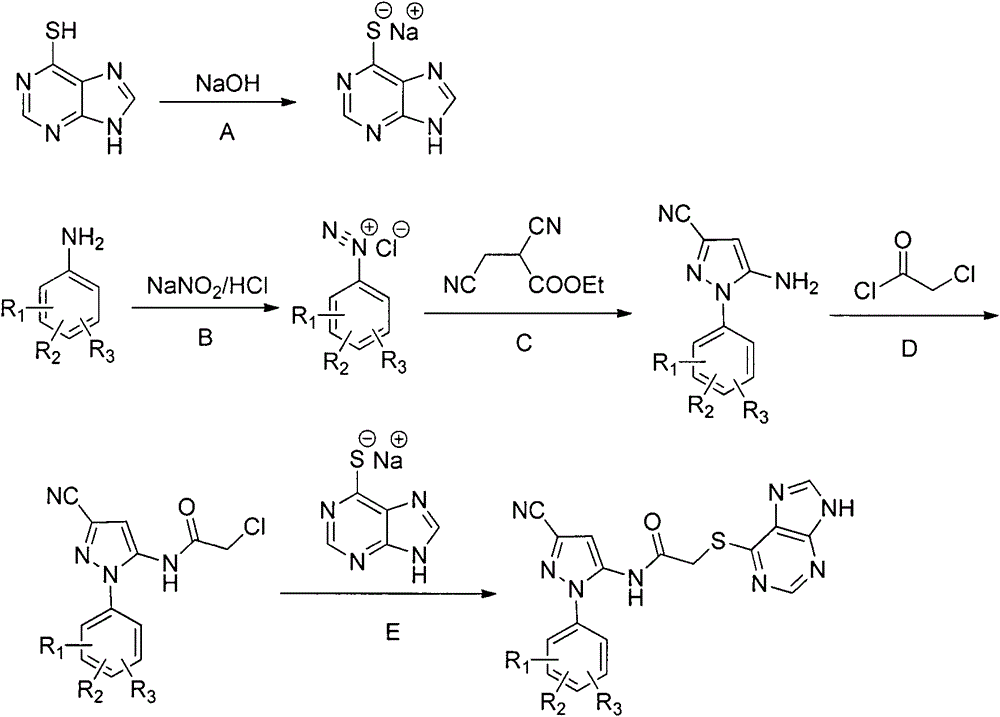
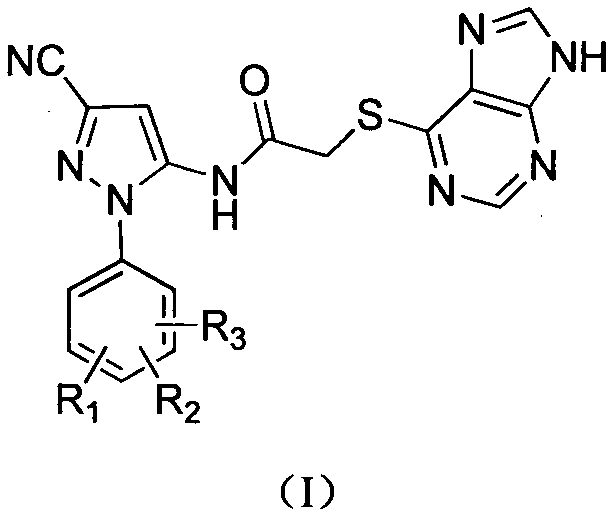
Halobenzene cyanopyrazole compound containing purine structure as well as preparation method and application
A technology of halobenzocyanopyrazoles and compounds, which is applied in the field of halobenzocyanopyrazoles and their preparation, and achieves the effects of simple synthesis process, broad application prospects, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This example illustrates the preparation of the sodium salt of 6-mercaptopurine
[0032] Add 0.01mol of 6-mercaptopurine and 20mL of methanol into a 100mL round-bottomed four-neck flask to dissolve it completely, and add methanol solution containing 0.04g (0.01mol) of NaOH into a constant pressure dropping funnel, and slowly add it dropwise. After reacting for 5-6h, the reaction was completed, and a solid was precipitated, filtered, and dried to obtain 1.70 g of a white solid, with a yield of 98%.
Embodiment 2
[0034] This example illustrates the preparation of 5-amino-1-phenyl-3-cyano-1H-pyrazole
[0035] Add 0.01 mol of aniline and a small amount of ethanol to a 250 mL round-bottomed three-necked flask, and add 3.0 mL (0.035 mol) of concentrated hydrochloric acid dropwise with stirring under ice-bath conditions. Dissolve 0.018mol of sodium nitrite in 10mL of water, slowly drop it into the flask, and react for 0.5h after the dropwise addition to obtain a yellow diazonium salt solution.
[0036] Add 0.01mol ethyl 2,3-dicyanopropionate into the three-necked flask, drop the prepared diazonium salt solution into the flask, and react for 2 hours after the dropwise addition. Add ammonia water, adjust the pH to 9-10, and react at room temperature for 2 hours. After the reaction was completed, it was extracted with 40 mL of dichloromethane, the organic layer was washed with water (2×30 mL), washed with saturated sodium chloride solution (1×40 mL), dried over anhydrous magnesium sulfate, an...
Embodiment 3
[0038] This example illustrates the preparation of 2-chloro-N-(3-cyano-1H-pyrazol-5-yl)acetamide
[0039] In a 100mL four-neck flask, add 0.01mol 5-amino-1-phenyl-3-cyano-1H-pyrazole, 40mL dichloromethane, stir to dissolve, add 0.015 chloroacetyl chloride dropwise under ice bath, and react at room temperature 2h. After the reaction was completed, filter and recrystallize the filter cake with ethanol to obtain 1.54 g of the product. Yield 82.5%. Product melting point: 182-184°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com