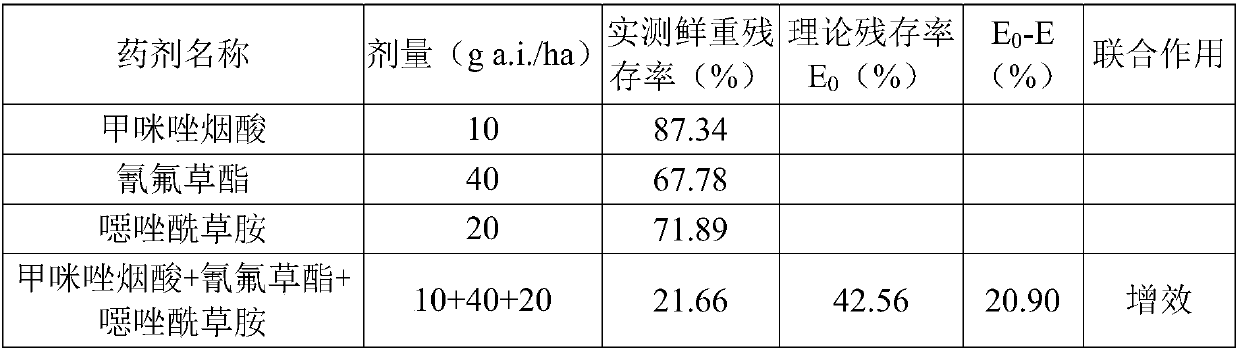
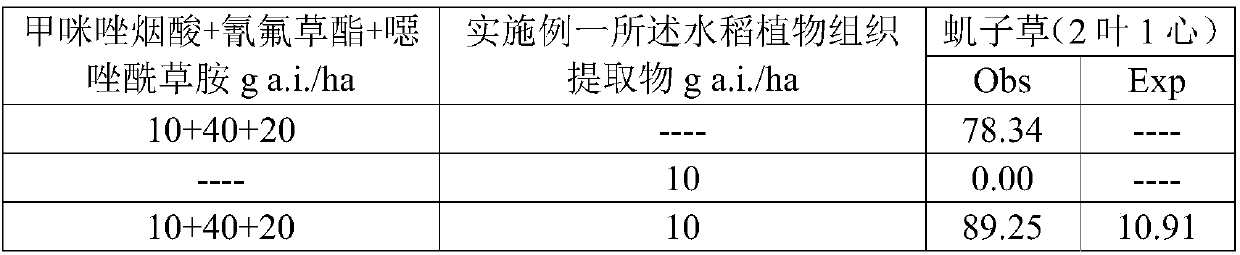
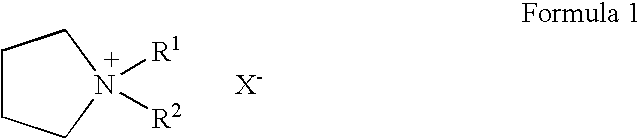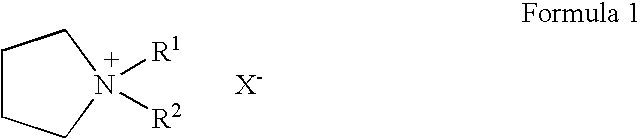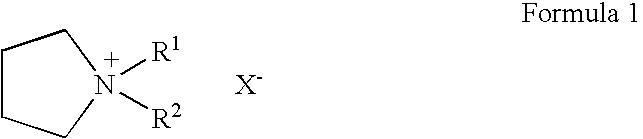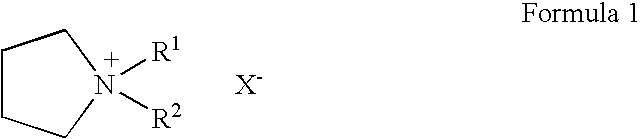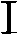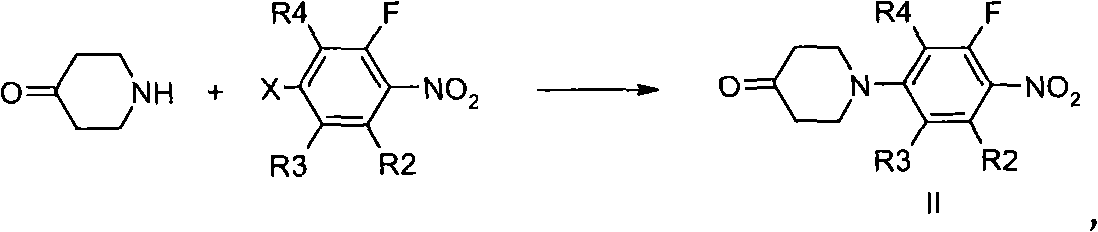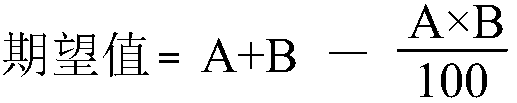Patents
Literature
223 results about "Fluorobenzenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivatives of BENZENE that contain FLUORINE.
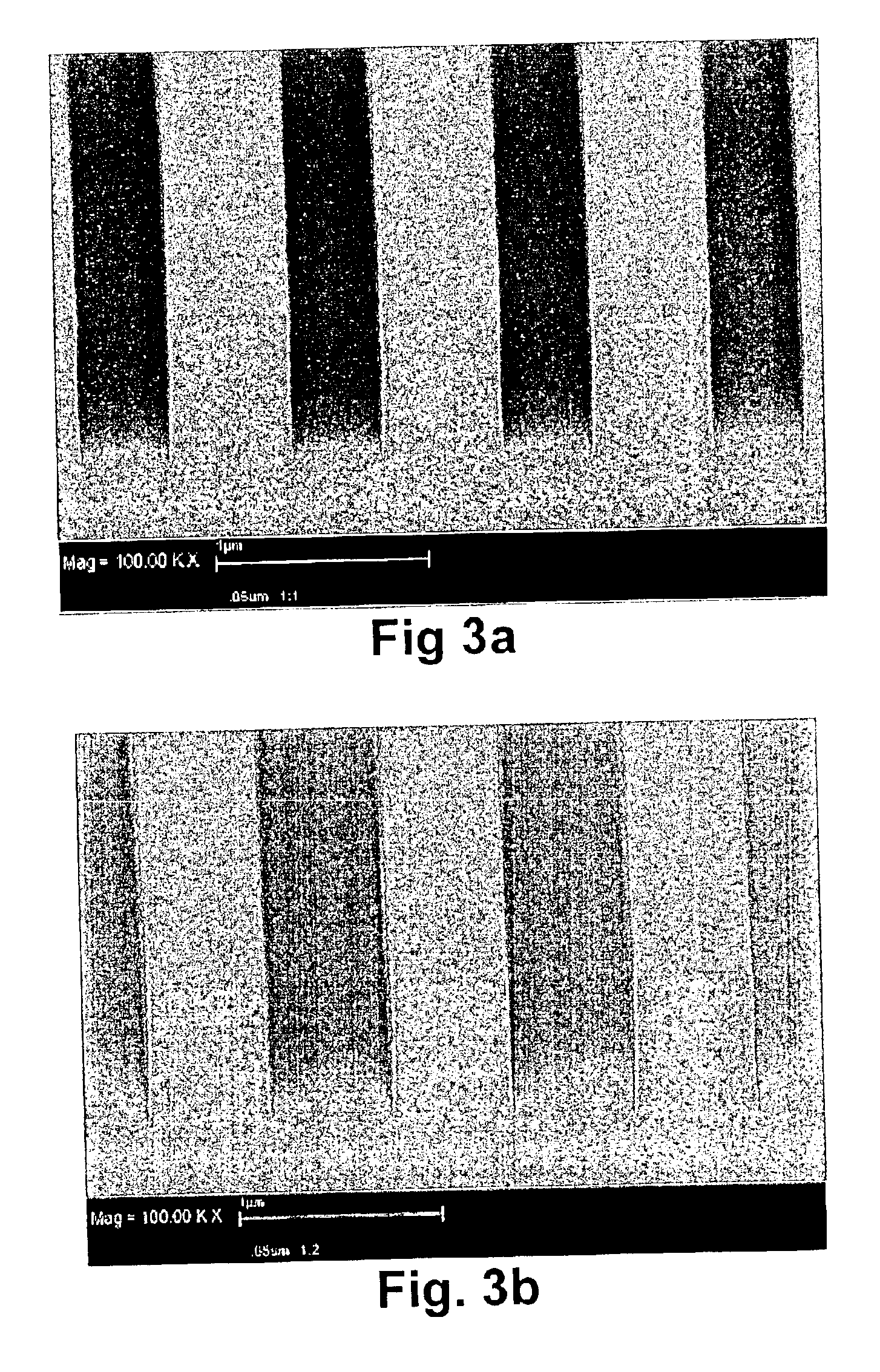
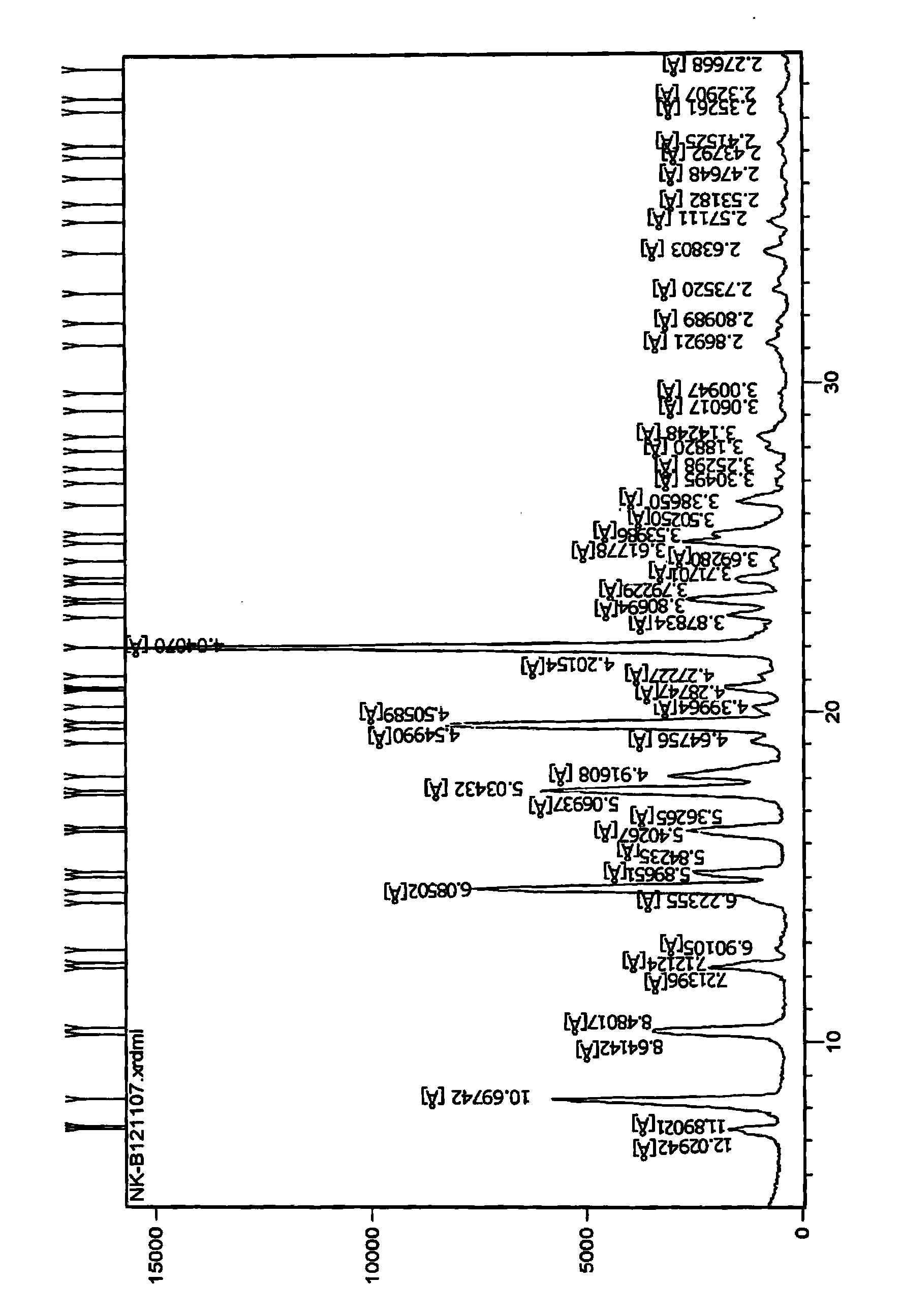
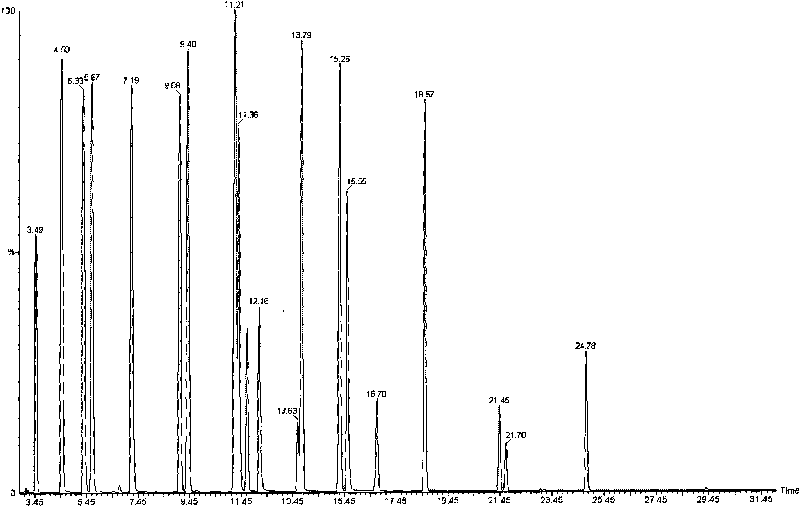
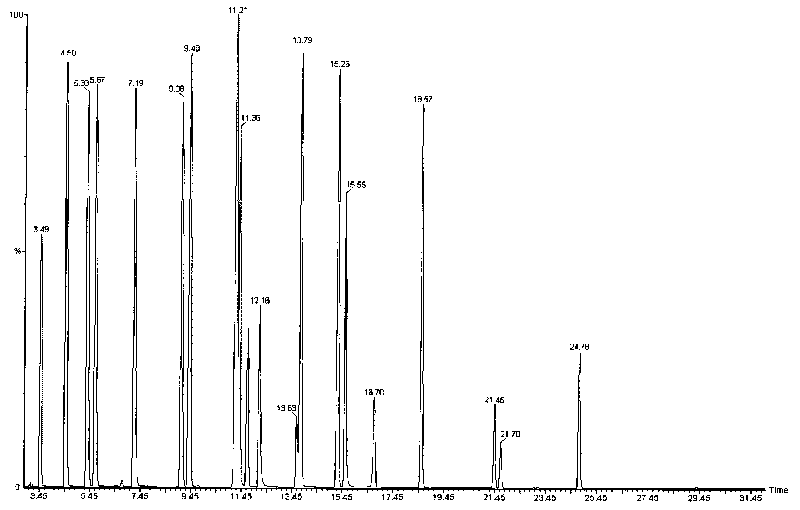
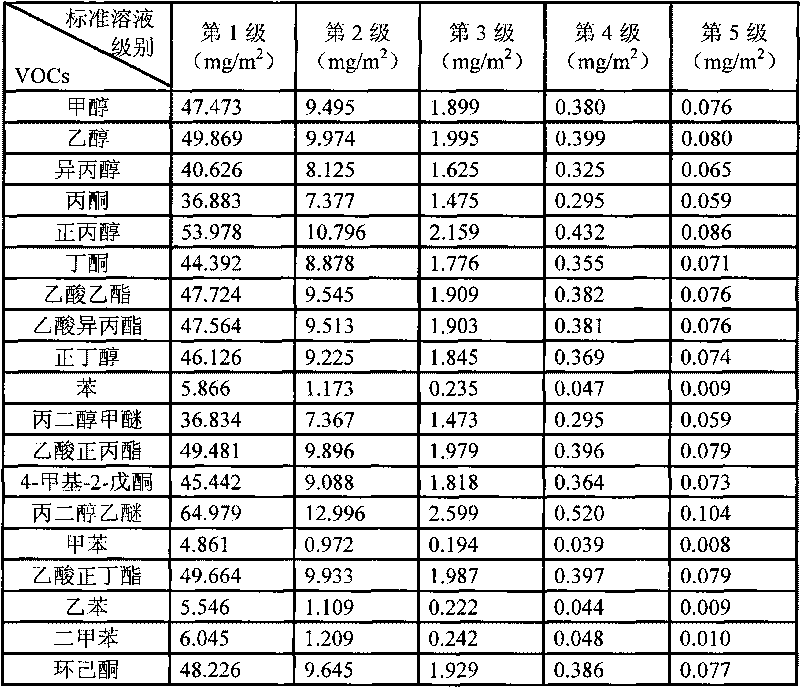
Measurement method of volatile organic compound in tobacco lining paper
ActiveCN101701949AChemically stableElimination of Assay EffectsComponent separationMass spectrometry detectorInterference factor
The invention discloses a measurement method of a volatile organic compound in tobacco lining paper with high determination accuracy and good repeatability, which is suitable for simultaneously determining dozens of volatile organic compounds. The measurement method is as follows: taking fluorobenzene as an internal standard substance; using headspace gas chromatography for measuring the content of the volatile organic compound in the tobacco lining paper; wherein a detector in the headspace gas chromatography is a mass spectrometry detector. The measurement method takes the fluorobenzene as the internal standard substance, uses the headspace gas chromatography / mass spectrometry for measuring the content of the volatile organic compound in the tobacco lining paper, and has the following advantages that the fluorobenzene has stable chemical property and are very similar to determinand in every performance, and the peak time appears in the middle of 19 target VOCs to be detected; therefore, the fluorobenzene is the very suitable internal standard substance, can eliminate the influence of ground substance in the tobacco lining paper on the measurement, causes materials which can not or are difficult to peak to be measured, and has high measurement accuracy and few interference factors.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
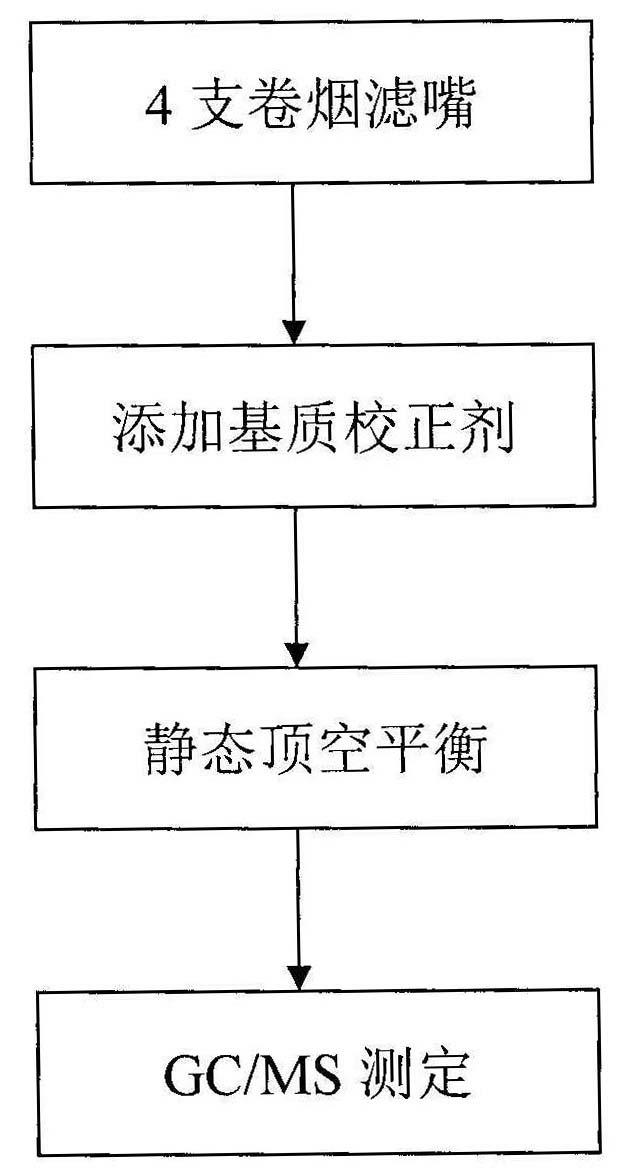
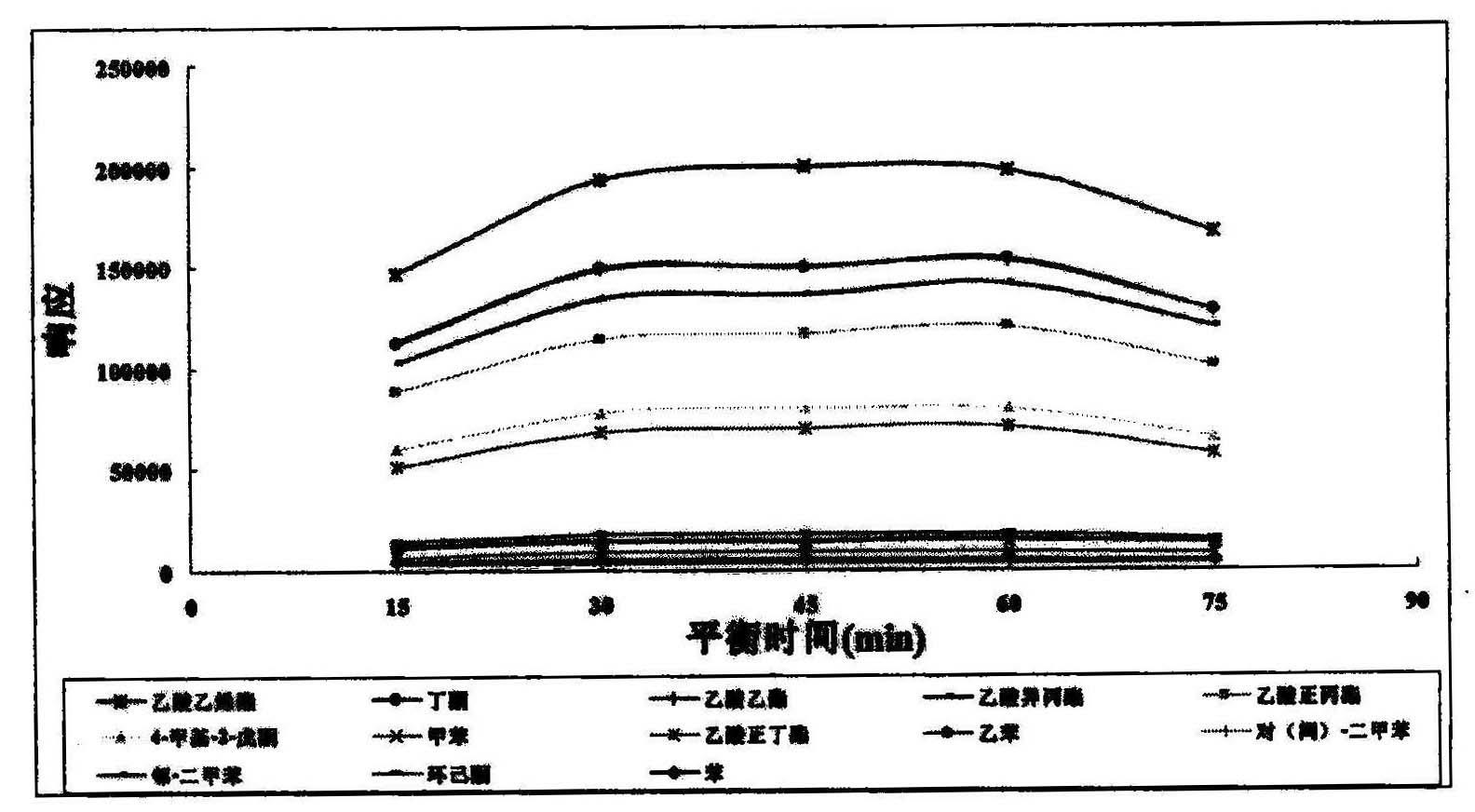
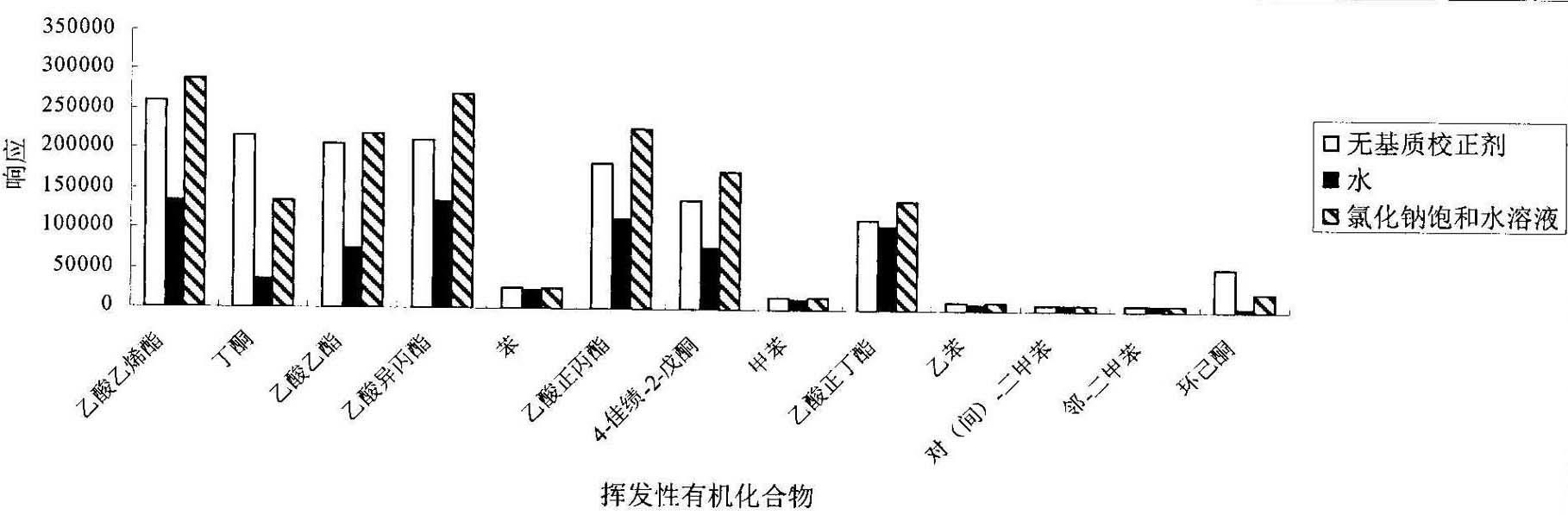
Method for detecting volatile organic compound in cigarette filter
InactiveCN102128906AEasy to handleEasy processing conditionsComponent separationInterference factorMass spectrography
The invention provides a method for detecting volatile organic compound in a cigarette filter, which comprises the following steps: a) preparing a sample; b) preparing a matrix correcting agent; c) preparing an interior label solution; d) preparing a standard sample; e) preparing a standard working solution; f) performing detection by using headspace-gas chromatography / mass-spectrography; and g) calculating a detection result of the volatile organic compound in the sample. The invention establishes the method for detecting the volatile organic compound residue in the cigarette filter for the first time. By using the saturation sodium chloride aqueous solution as the matrix correcting agent and the fluorobenzene as the interior label, the matrix effect of a solid absorption matrix (namely the cigarette filter) is efficiently reduced and the error caused by the poor repeatability of the pre-treatment and the low precision of instrument is reduced. In the whole process, no organic solvent is used. The method has the advantages of environmental friendliness, energy-saving and low consumption. The detecting method has few interference factors and good repeatability, is accurate in measuring result, is simple to operate, and can be used for obviously increasing the sensitivity of headspace analysis.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
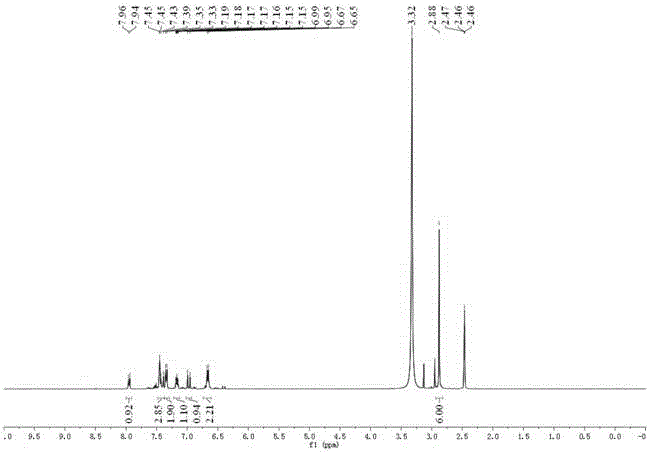
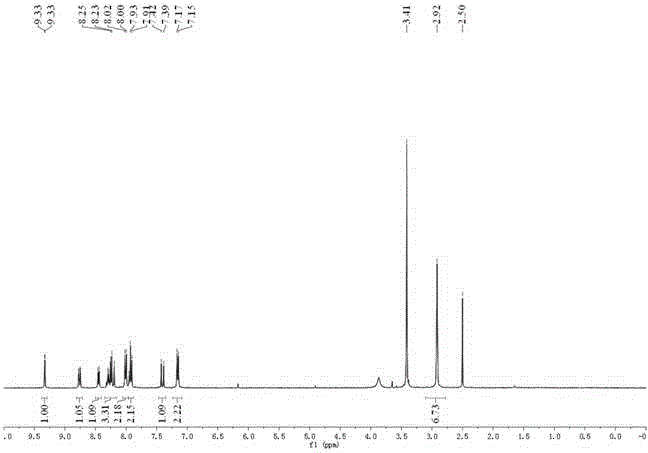
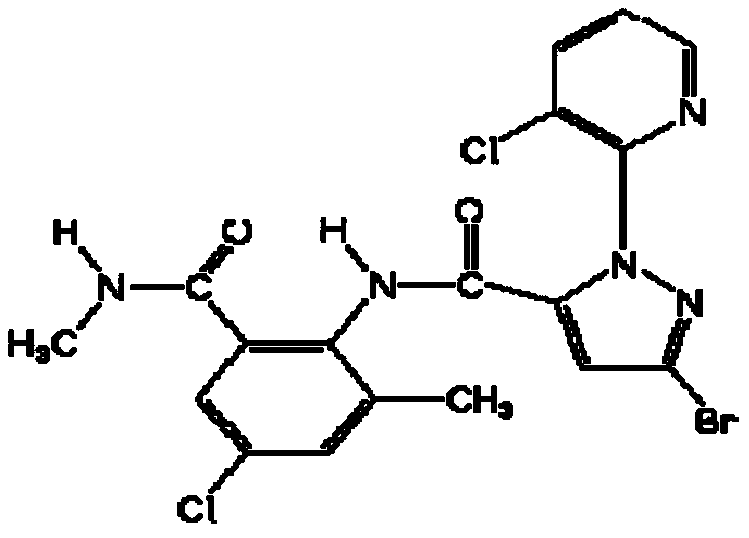
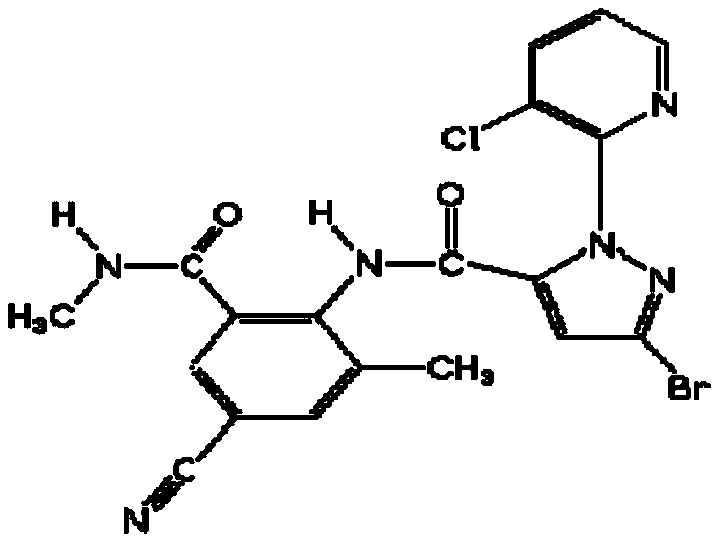
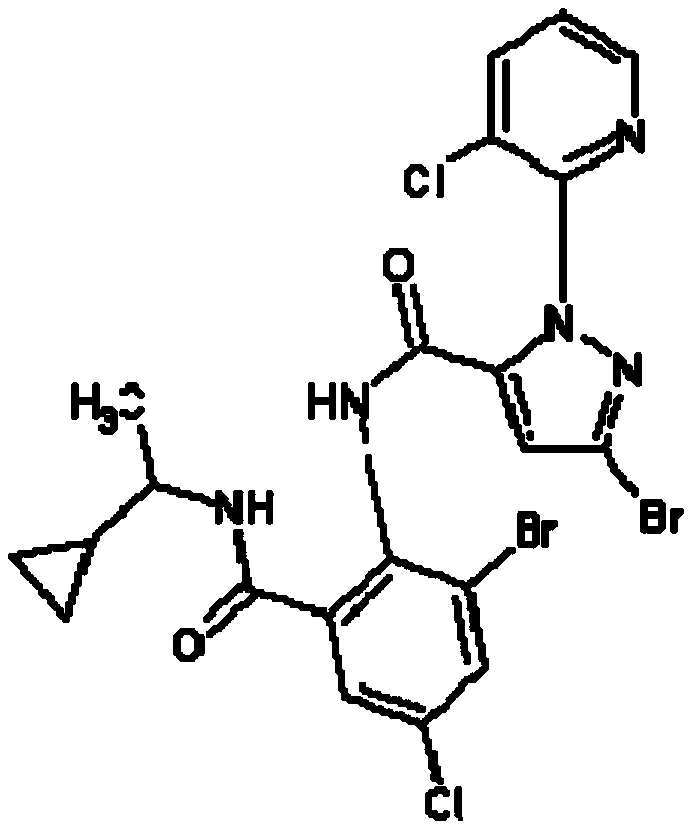
Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline
The invention discloses a method for synthesizing 4-(3-chloro-4-fluorophenylamino)-7-methoxy -6-[3-(4-morpholinyl)-propoxy] quinoline. The method comprises the following steps: 1) 3-hydroxide radical-4-methoxybenzaldehyde is used as a raw material to prepare 3-hydroxide radical-4-methoxy-benzonitrile; 2) the 3-hydroxide radical-4-methoxy-benzonitrile and 3- chloropropy morpholinehydrochloride are heated to have reflux reaction to obtain 4- methoxy-3-[3-(4- morpholinyl) propoxy] benzonitrile; 3) the 4- methoxy-3-[3-(4-morpholinyl) propoxy] benzonitrile is subjected to nitration to obtain 2- nitryl-4- methoxy -5-[3-(4-morpholinyl) propoxy] benzonitrile; 4) the 2- nitryl-4- methoxy -5-[3-(4-morpholinyl) propoxy] benzonitrile is subjected to reduction to obtain 2-amido-4- methoxy-5-[3-(4-morpholinyl) propoxy] benzonitrile; and 5) the 2-amido-4- methoxy-5-[3-(4-morpholinyl) propoxy] benzonitrile and an azomethine intermediate of 3-chloro-4-fluoroaniline have rearrangement reaction to obtain 4-(3-chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline. The method has characteristics of environmental protection and high production rate.
Owner:浙江精进药业有限公司
Method for coproducing key intermediates of quinolone medicines by using o-dichlorobenzene as raw material
ActiveCN102249881ALow production costReduce pollutionCarbonyl compound preparation by condensationDistillationNitration
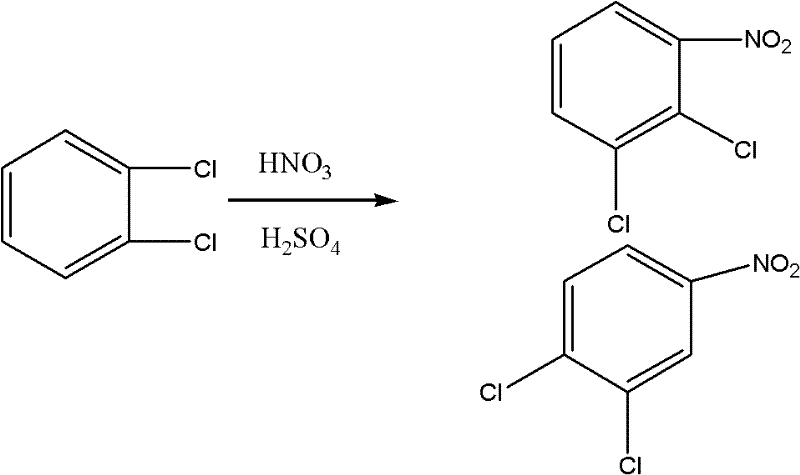
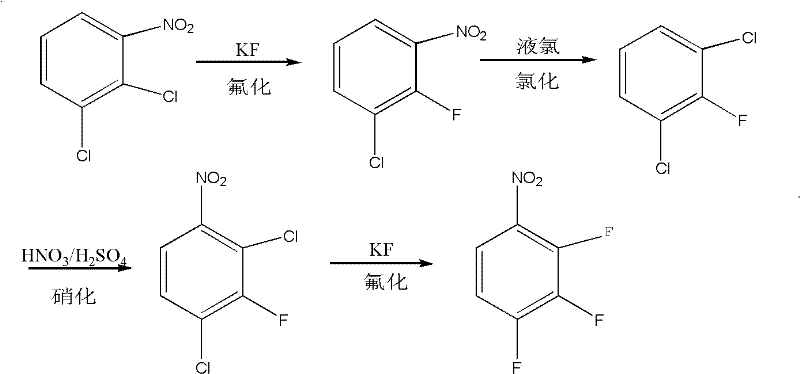
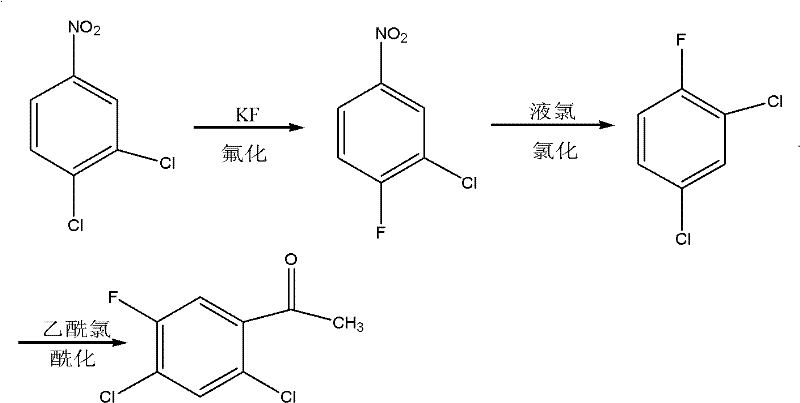
The invention relates to the field of methods for preparing medicinal intermediates, in particular to the field of methods for preparing key intermediates of quinolone medicines, and develops a method for coproducing the key intermediates of the quinolone medicines by using o-dichlorobenzene as a raw material. The method comprises the following steps of: nitrifying the o-dichlorobenzene serving as the raw material, and performing distillation, purification and stepwise crystallization to obtain 2,3-dichloronitrobenzene and 3,4-dichloronitrobenzene; performing fluoridation on the 2,3-dichloronitrobenzene to obtain 3-chloro-2-fluoronitrobenzene, performing chlorination to obtain 2,6-dichlorofluorobenzene, performing nitration to obtain 1,3-dichloro-2-fluoro-4-nitrobenzene, and finally performing fluoridation to obtain 2,3,4-trifluoronitrobenzene; and performing fluoridation on the 2,3,4-trifluoronitrobenzene to obtain 3-chloro-4-fluoronitrobenzene, performing chlorination to obtain 1,3-dichloro-4-fluorobenzene, and finally performing acylation reaction on the 1,3-dichloro-4-fluorobenzene and acetylchloride to obtain 2,4-dichloro-5-fluoroacetophenone.
Owner:内蒙古永太化学有限公司
Fluorescent probe for detecting hydrogen sulfide by virtue of fluorescence enhancement as well as synthetic method and application of fluorescent probe
The invention relates to a fluorescence enhancement type hydrogen sulfide fluorescent probe and particularly relates to a fluorescence enhancement type hydrogen sulfide fluorescent probe based on flavone derivatives. Flavone derivatives are directly reacted with 2,4-dinitrofluorobenzene, and a reaction product is subjected to column chromatography to generate a pure probe product. According to the fluorescence enhancement type hydrogen sulfide fluorescent probe, probe molecules have the maximum absorption wavelengths of 460nm, relatively good solubility in a 1.5mM CTAB water solution and stable optical performance; by adding the probe molecules together with hydrogen sulfide, the absorption wavelengths are blueshifted from 460nm to 450nm, and the intensity of a fluorescence spectrum is continuously enhanced at 612nm and is maximally enhanced by 96 times. According to the fluorescence enhancement type hydrogen sulfide fluorescent probe, the deficiencies that an existing fluorescent probe is slow in response to hydrogen sulfide and high in detection line, and detection wavelengths are in a visible light region are overcome; the probe molecules are high in sensitivity, stable in optical performance, relatively high in synthetic yield, strong in capability of identifying hydrogen sulfide, high in response speed, wide in detection range and low in detection lower limit, the response range is 0-50 mu M, and the detection limit is 0.09 mu M; therefore, the fluorescent probe has practical application values in the fields of biological chemistry, environmental science and the like.
Owner:SUZHOU ROWLAND BIOTECH
Process for preparing polyisocyanate by solid phosgene
InactiveCN1827593AIsocyanic acid derivatives preparationOrganic compound preparationPhosgeneFluorobenzenes
The invention relates to a method for preparation of 1, 2-dialkoxy-3-fluorobenzene by an intermediate of 2-fluorin-6-halogenated phenol. The said method consists of selecting 2-fluorophenol as its stock, and obtaining the intermediate of 2-fluorin-6-halogenated phenol; afterwards, performing multiple steps such as etherifing the said intermediate and etherifing again by introducing the hydroxyl group, alternatively, hydroxylating and etherifing the said intermediate; finally, obtaining the said 1, 2-dialkoxy-3-fluorobenzene. The method is of low cost, high yield rate, so it is suitable for the industrial production.
Owner:北京金源化学集团有限公司
Polymeric antireflective coatings deposited by plasma enhanced chemical vapor deposition
InactiveUS6852474B2Negative effectReduce wastePhotosensitive materialsSemiconductor/solid-state device manufacturingGas phaseChemical vapors
An improved method for applying polymeric antireflective coatings to substrate surfaces and the resulting precursor structures are provided. Broadly, the methods comprise plasma enhanced chemical vapor depositing (PECVD) a polymer on the substrate surfaces. The PECVD processes comprise providing a quantity of a polymer generated by introducing monomer vapors into a plasma state followed by polymerization thereof, with assistance of plasma energy, onto the surface of a substrate. The most preferred starting monomers are phenylacetylene, 4-ethynyltoluene, and 1-ethynyl-2-fluorobenzene. The inventive methods are useful for providing highly conformal antireflective coatings on large surface substrates having super submicron (0.25 μm or smaller) features. The process provides a much faster deposition rate than conventional chemical vapor deposition (CVD) methods, is environmentally friendly, and is economical.
Owner:BREWER SCI
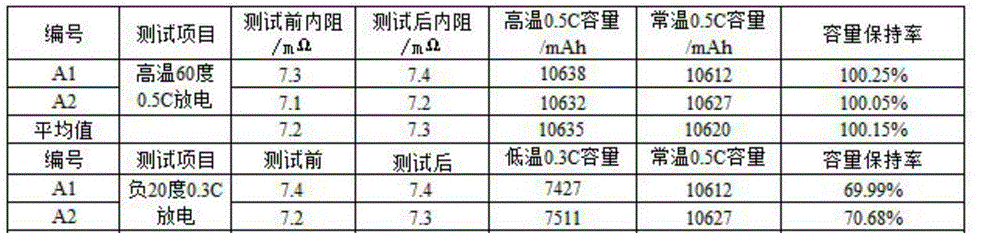
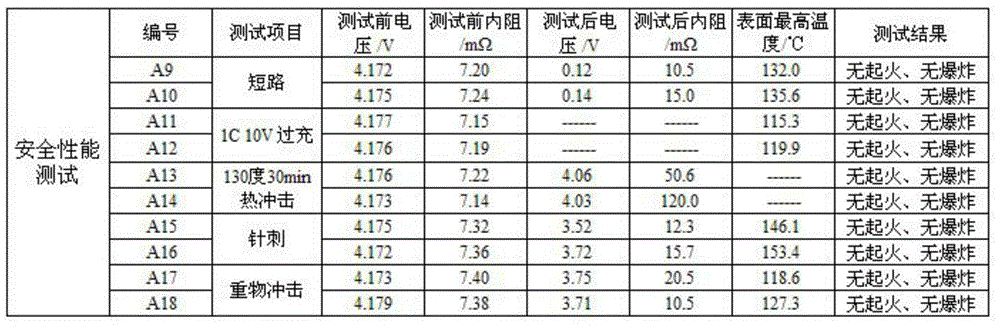
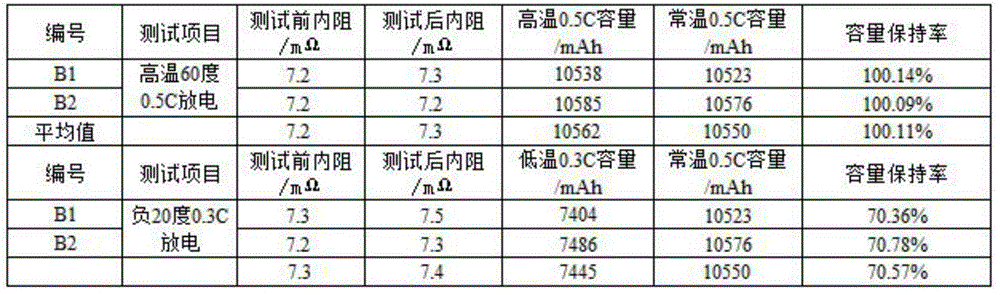
Lithium ion battery employing non-aqueous electrolyte
InactiveCN105977525ALower impedanceImprove low temperature performanceNegative electrodesSecondary cellsSodium-ion batteryLithium-ion battery
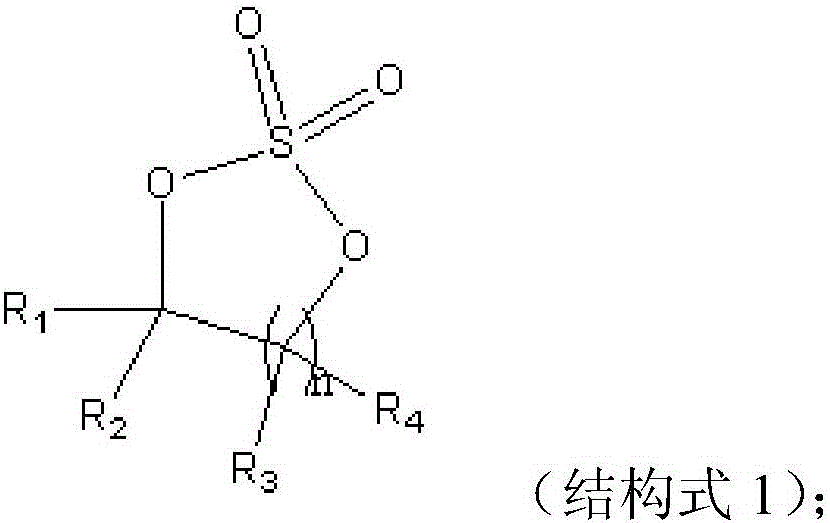
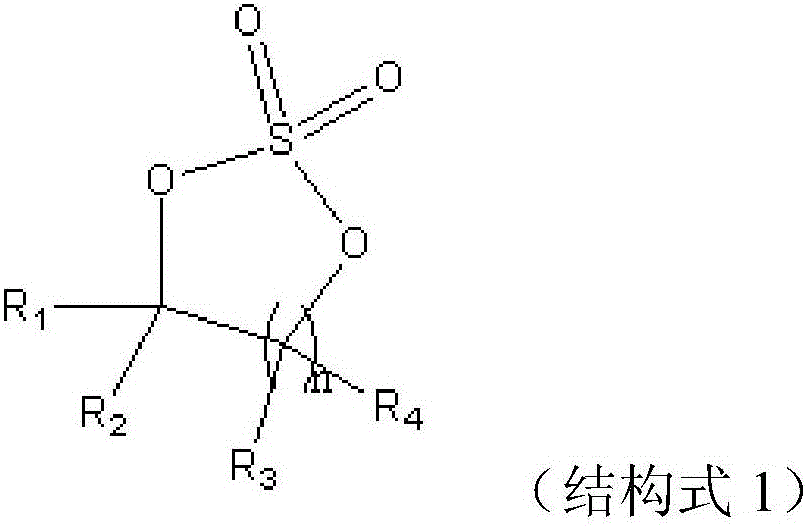
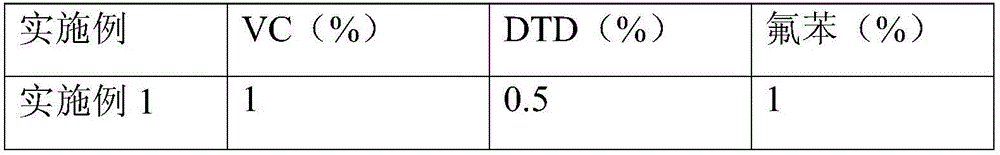
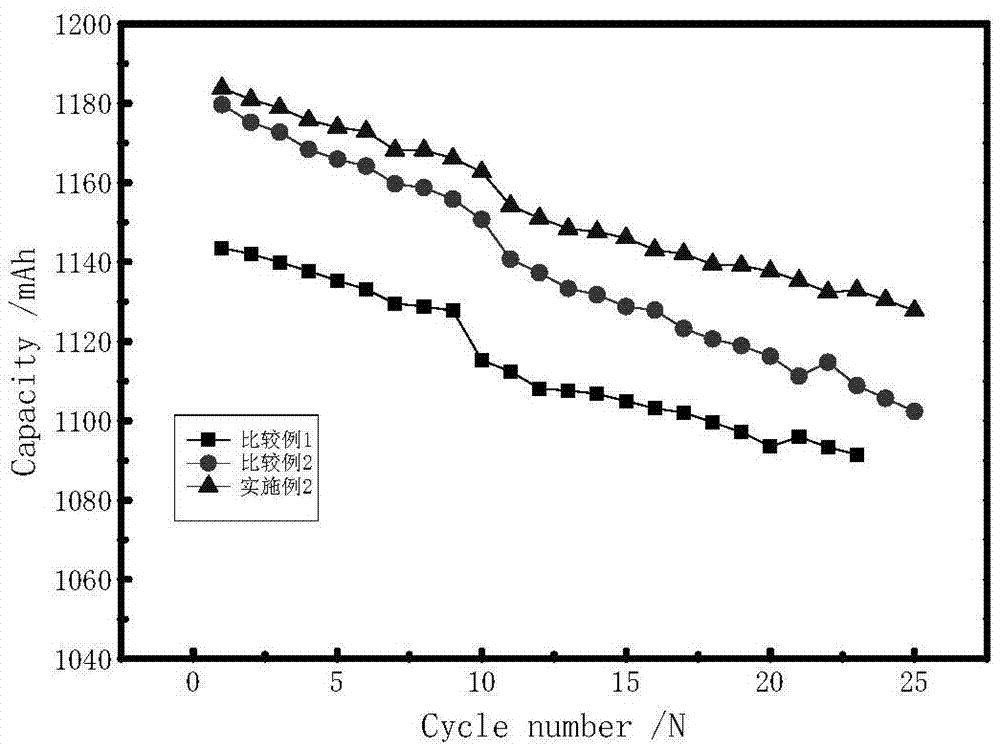
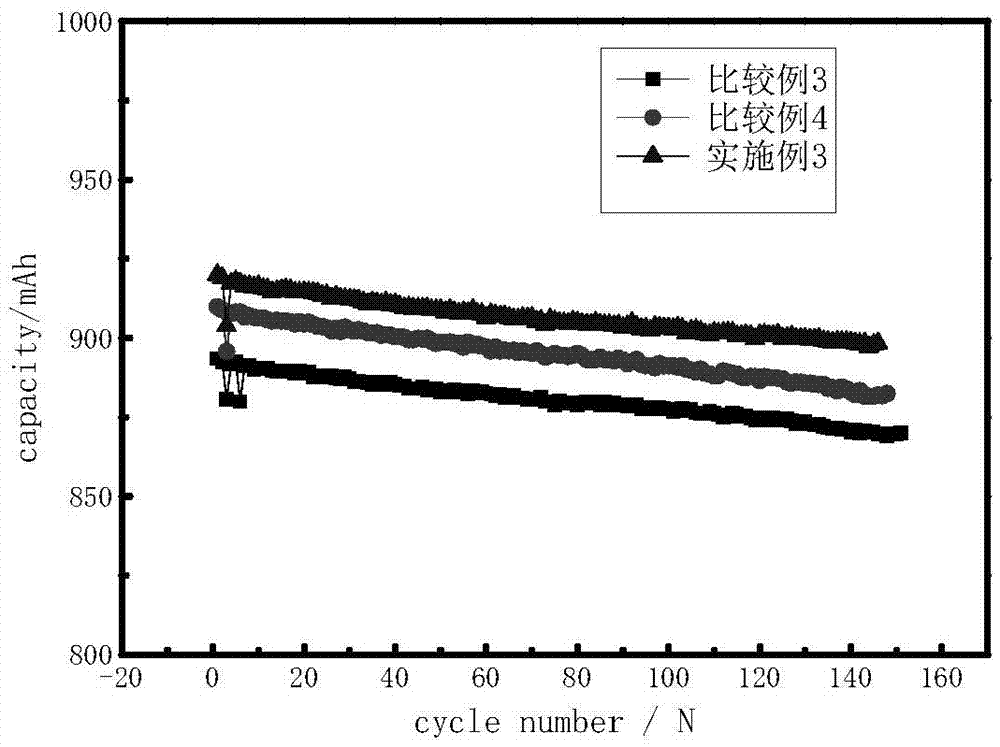
The invention discloses a lithium ion battery employing non-aqueous electrolyte. The lithium ion battery comprises a cathode, an anode, a diaphragm arranged between the cathode and the anode, and the non-aqueous electrolyte of the lithium ion battery, wherein an active substance of the cathode comprises LiFePO4; the non-aqueous electrolyte of the lithium ion battery comprises a non-aqueous organic solvent, lithium salt and an additive; the additive at least comprises (A) vinylene carbonate, and also comprises at least one of (B) a compound shown in astructural formula 1 and (C) fluorobenzene, wherein n is a natural number from 1 to 3, and R1, R2, R3 and R4 are independently selected from one of alkyl groups with 1 to 6 hydrogen atoms, fluorine atoms and carbon atoms respectively. The lithium ion battery has long cycle life and excellent high / low-temperature performance.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Externally applied formulation of cetirizine hydrochloride
InactiveCN1634063APharmacologically activeIncreased free drug concentrationOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
Pharmacological test shows that the externally-used preparation of Cetirizine has ideal allergy resistant and anti-inflammatory action to the rat passive cutaneous anaphylaxis (Rat PCA) model and dimethylbenzene caused mouse otitis model, and also prevents the adverse effect to the central system caused by whole body administration. It is also found in the test, that the externally used preparation of Cetirizine also has very fine inhibitory action to dinitrofluorobenzene caused mouse porphyria hypersensitivity (PTH), the novel pharmacological action of the Cetirizine shows that the Cetirizine external preparation has good therapeutic action to skin inflammations which mainly include porphyria hypersensitivity.
Owner:LUNAN PHARMA GROUP CORPORATION
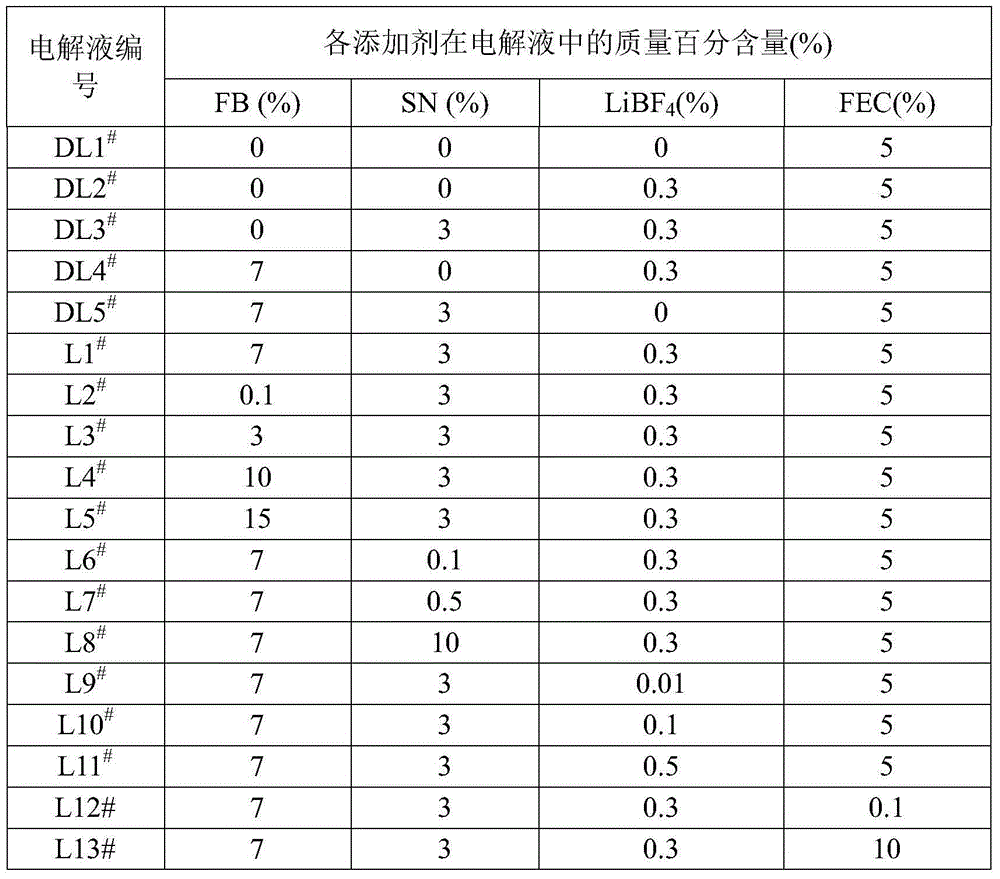
Electrolyte of lithium ion battery and lithium ion battery utilizing same
ActiveCN104466248AReduce oxidationIncrease loopSecondary cellsOrganic electrolytesOrganic solventInternal resistance
The invention discloses electrolyte of a lithium ion battery. The electrolyte comprises an organic solvent, electrolyte lithium salt and additives, wherein the additives include butanedinitrile, fluorobenzene and lithium tetrafluoroborate; the mass percentage of the fluorobenzene in the electrolyte is 0.1 to 15 percent; the mass percentage of the butanedinitrile in the electrolyte is 0.1 to 10 percent; the mass percentage of the lithium tetrafluoroborate in the electrolyte is 0.01 to 1 percent. By adopting the electrolyte, while the charging upper limit voltage and high temperature intermittent cycling performance of the lithium ion battery are improved, simultaneously the expansion rate of the battery is reduced, the internal resistance is reduced, and the stability and the safety of the lithium ion battery can be improved.
Owner:DONGGUAN AMPEREX TECH +1
Lithium battery electrolyte with wettability improved, and lithium battery
InactiveCN106920991AGood high temperature output characteristicsRaise the ratioSecondary cellsOrganic solventHydrogen
The invention relates to a lithium battery electrolyte with wettability improved. The lithium battery electrolyte comprises a lithium salt, an organic solvent and an additive, wherein the additive comprises an additive A; the additive A is selected from one or a combination of the components as the structural formula as follows: a formula is as shown in the specification, wherein R1, R2, R3, R4 and R5 are one of hydrogen, hydroxyl group, alkyl group, alkoxyl group, alkenyl group, ketone group, phenyl group, phenoxyl group, fluoroalkyl group, fluoroalkoxy group, fluoroalkenyl group, fluoroketone group, fluorobenzene group and fluorophenoxyl group independently, wherein fluoro refers to partial substitution or complete substitution. By adding the additive A to the lithium battery electrolyte, the wettability of the electrolyte is obviously improved; and the lithium battery adopting the electrolyte has high high-temperature output characteristic, rate capability and cycling performance.
Owner:ZHANGJIAGANG GUOTAI HUARONG NEW CHEM MATERIALS CO LTD
Electric double layer capacitor
InactiveUS7173807B2Improve featuresImprove pressure resistanceHybrid capacitor electrolytesElectrolytic capacitorsSulfolaneCarbonate
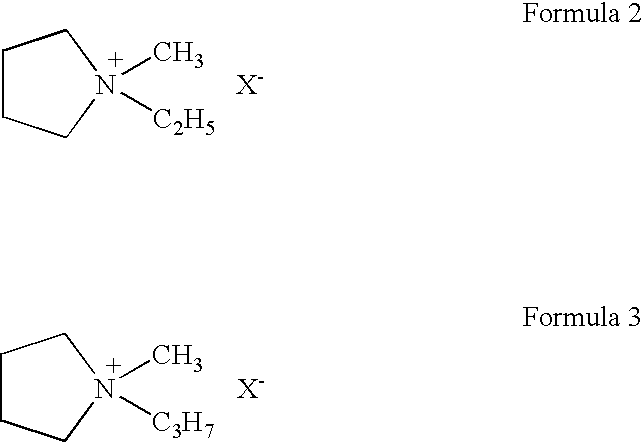
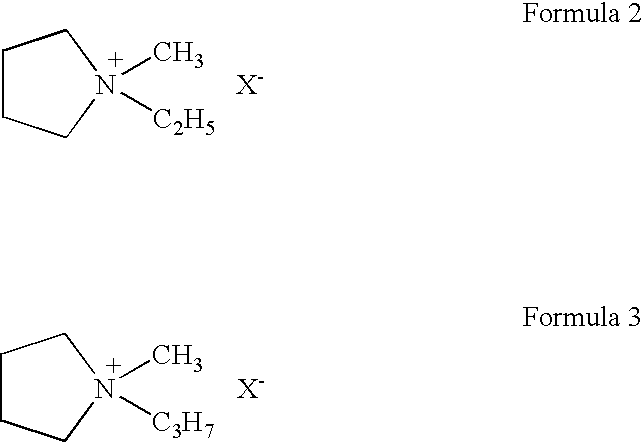
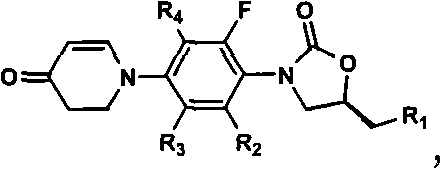
To provide an electric double layer capacitor having a low resistance, a high withstanding voltage and an excellent low-temperature characteristic. This object is achieved by employing, for an electric double layer capacitor having a pair of polarized electrodes and an electrolytic solution capable of forming an electric double layer at the interface with the polarized electrodes, an electrolytic solution containing as an electrolyte a salt represented by the formula 1, and as solvents at least (1) a chain carbonate having at most 5 carbon atoms, (2) sulfolane or its derivative and (3) a fluorobenzene:wherein each of R1 and R2 which are independent of each other, is a C1-4 alkyl group, and X− is an anion.
Owner:ASAHI GLASS CO LTD
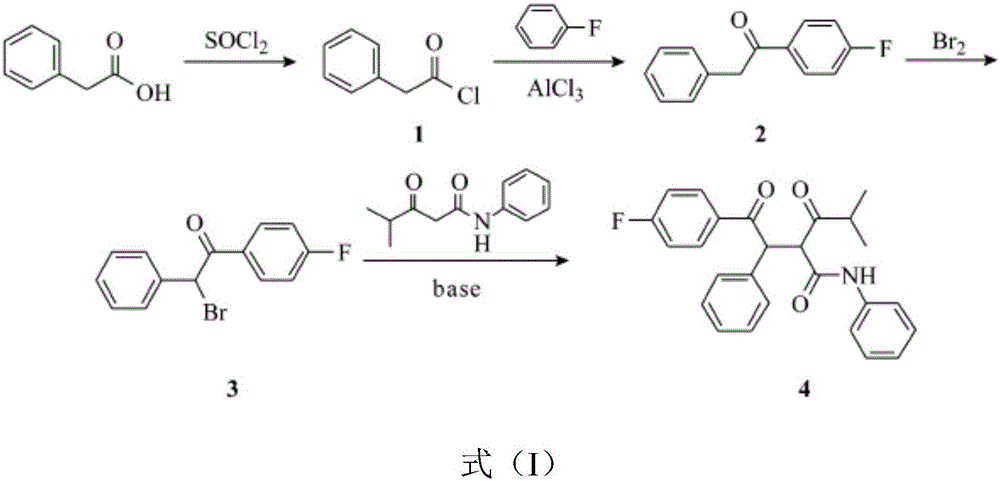
Preparation technology of atorvastatin
The invention discloses preparation technology of atorvastatin. The preparation technology comprises the following steps: a first step, the reaction of phenylacetic acid and thionyl chloride is carried out in order to obtain phenylacetyl chloride; a second step, the Friedel-Crafts acylation reaction of phenylacetyl chloride and fluorobenzene is carried out under the action of catalyst, in order to obtain 4-fluorophenyl acetophenone; a third step, 4-fluorophenyl acetophenone is brominated and the brominated 4-fluorophenyl acetophenone is reacted with N-phenyl-isobutyloylacetamide in order to obtain M-4; a fourth step, a reaction is carried out for M-4 and ATS-9 in a cyclohexane, toluene or a mixed solvent of cyclohexane and toluene, pivalic acid is used for catalysis, and a condensation product is obtained. Phenylacetyl chloride and fluorobenzene are reacted in a catalytic action of zeolite molecular sieve, a complexation reaction of the catalyst and products is avoided, reaction yield is improved, and side reactions are few in order to facilitate purification; post-treatment can be carried out for excess M-4 for recycling and reusing, reaction yield is improved, mole proportion of M-4 to ATS-9 and the addition amount of pivalic acid can be adjusted, and final yield of the reaction is improved.
Owner:JIANGSU ALPHA PHARM CO LTD
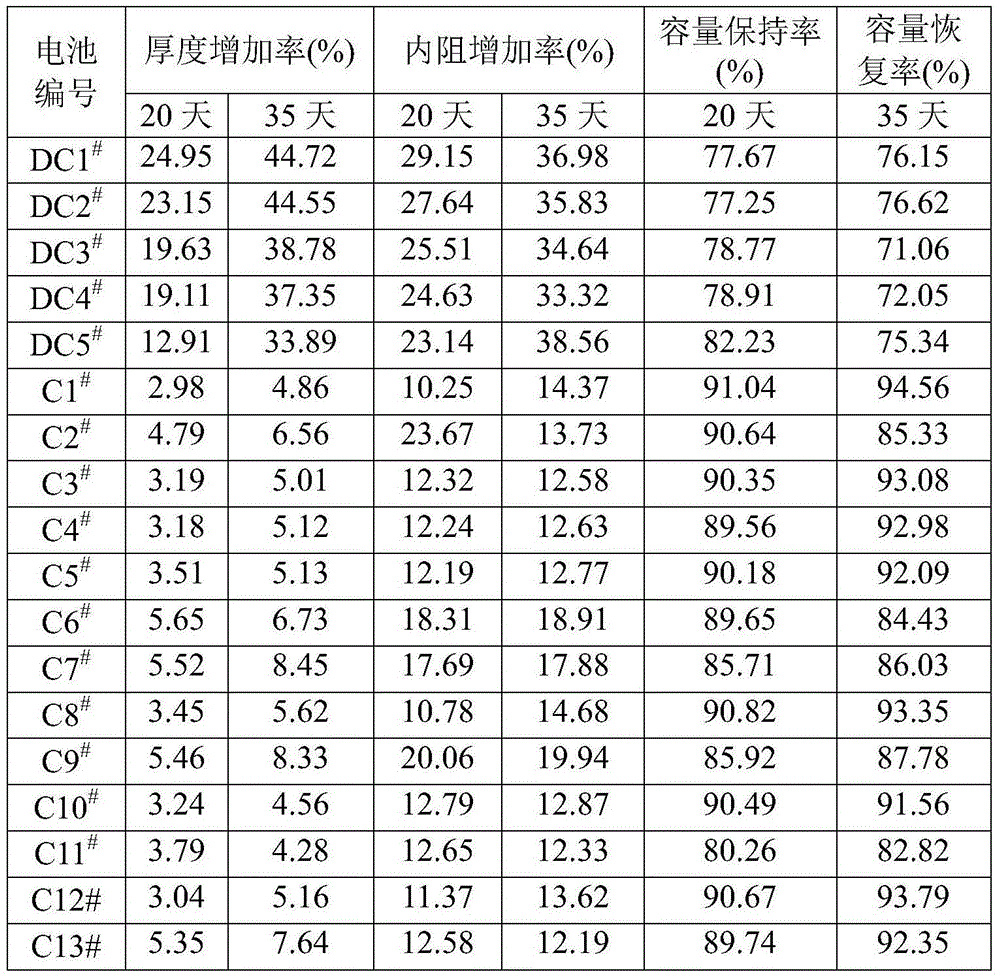
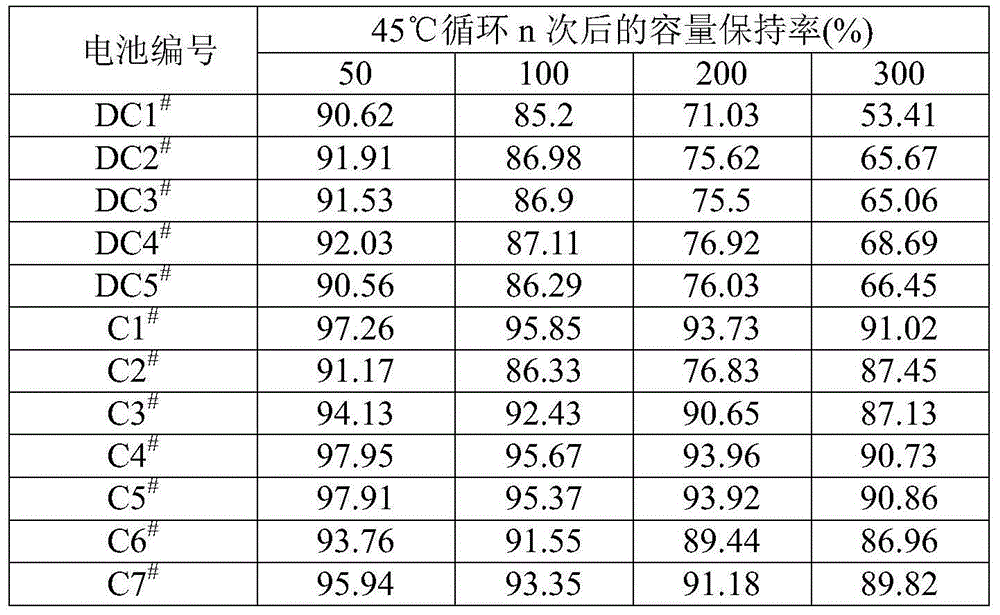
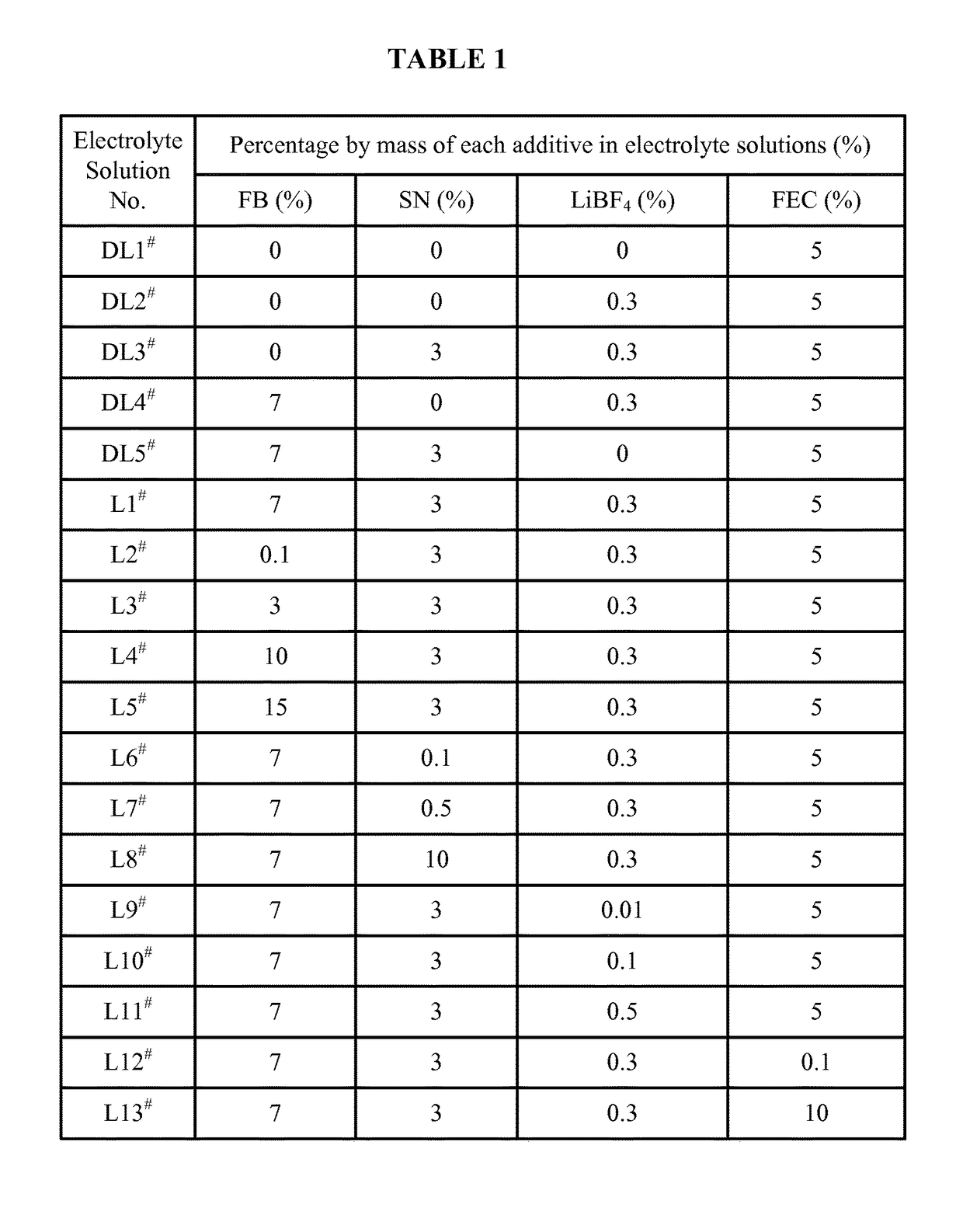
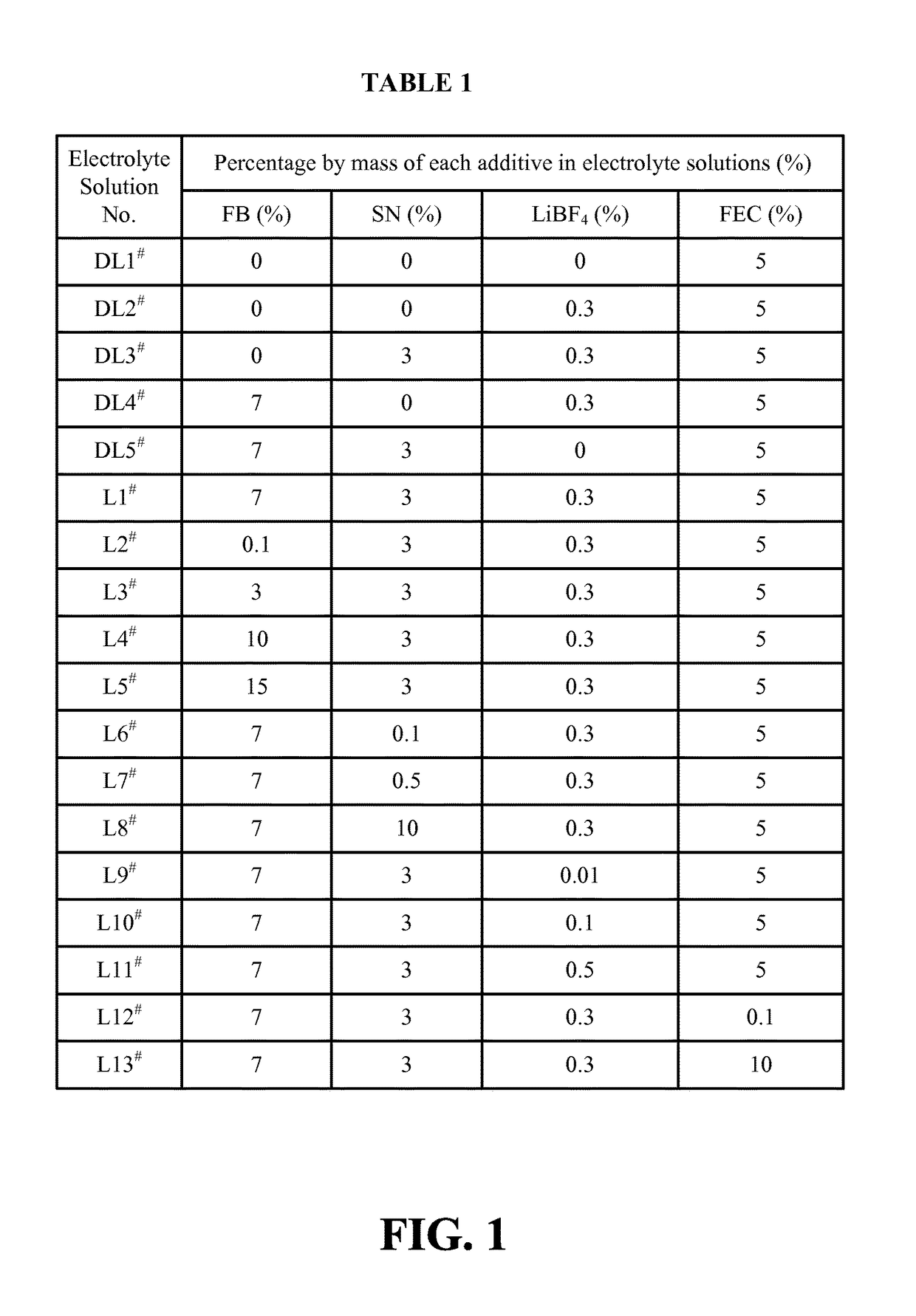
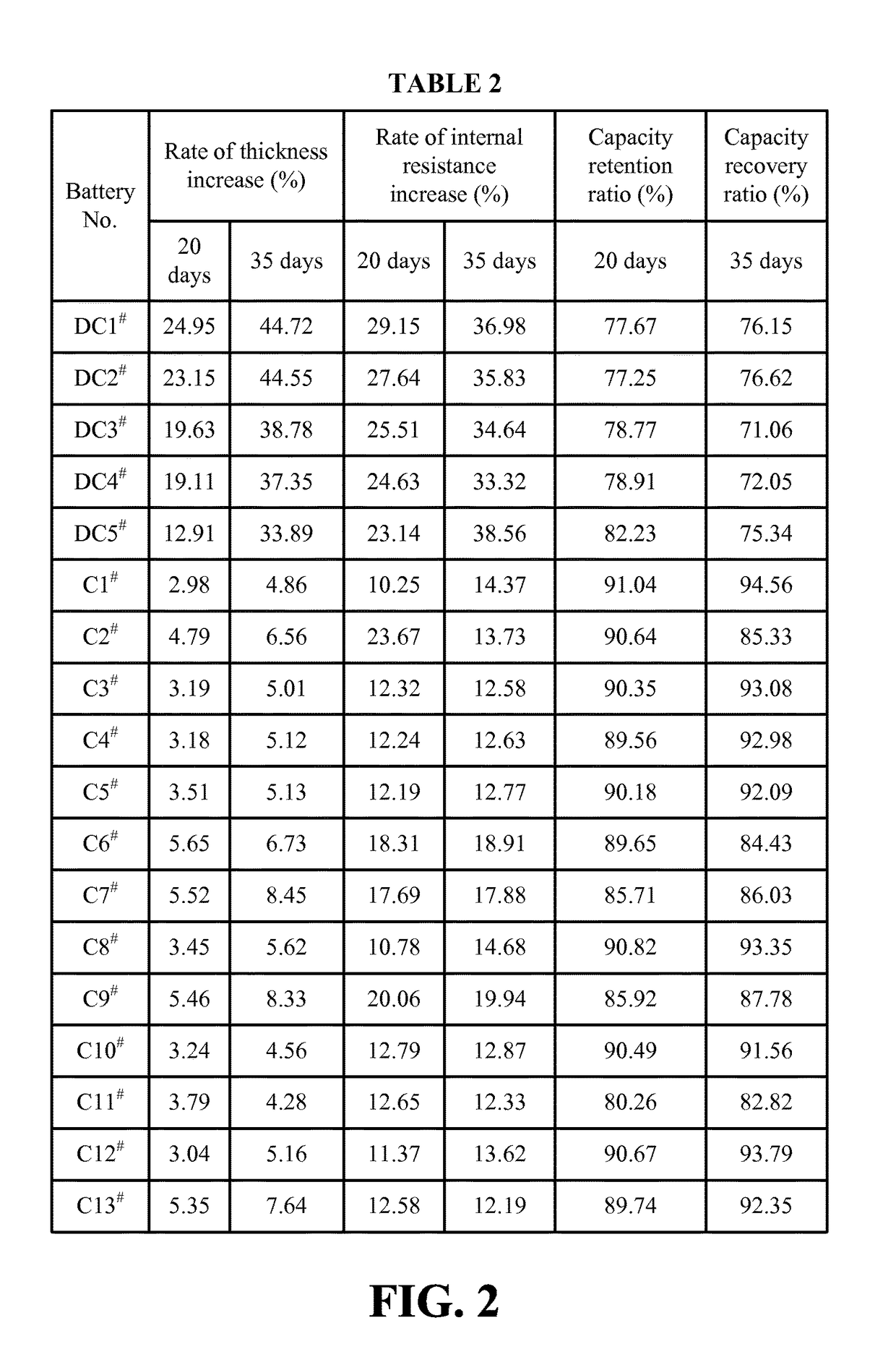
Lithium ion battery and electrolyte solution thereof
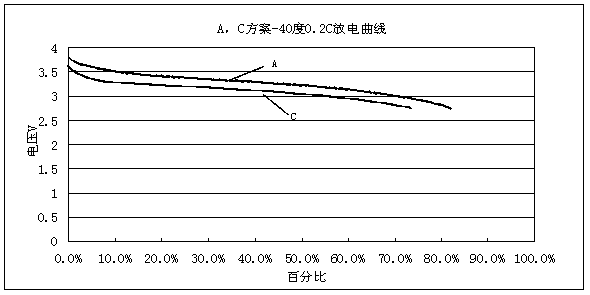
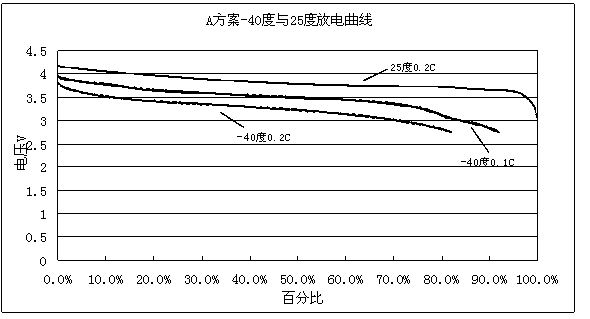
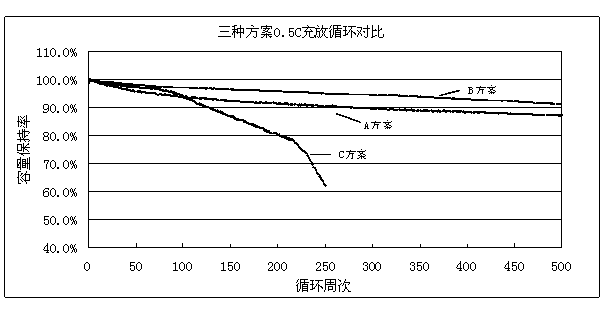
ActiveCN103531846AImprove high temperature storage gas productionImprove long-term storage capacity recovery rate is lowSecondary cellsAluminium-ion batteryElectric discharge
The invention discloses a lithium ion battery and an electrolyte solution thereof. The electrolyte solution comprises an electrolyte solution solvent, an electrolyte and an additive, wherein the electrolyte solution solvent comprises EC (ethylene carbonate), DEC (Diethyl Carbonate), DMC (Dimethyl Carbonate), EMC (ethyl methyl carbonate) and EP (ethyl propionate); the electrolyte is LiPF6; the additive comprises a LiBF4, PS (propane sultone) and FB (fluorobenzene) which are added for improving the high-temperature performance, and FEC (fluoroethylene carbonate) which is added for improving the low-temperature performance and the long-cycling performance. The lithium ion battery comprising the electrolyte solution can meet requirements that the electric discharge capacity of the lithium ion battery under 0.2C (Capacity) at a lower temperature of -40 DEG C is greater than the electric discharge capacity at a normal temperature by more than 75%; the electric discharge capacity of the lithium ion battery under 0.1C is greater than the electric discharge capacity at the normal temperature by more than 85%. Meanwhile, the capacity retention ratio of the lithium ion battery can be retained by more than 80% after the lithium ion battery is cycled for 500 circles. After the lithium ion battery with full charge is stored for 7 days at 60 DEG C, the thickness swelling rate of the lithium ion battery is less than or equal to 5.0%; the capacity recovery rate of the lithium ion battery is greater than 90%. After the lithium ion battery is stored for 90 days, the capacity recovery rate of the lithium ion battery is greater than 95%.
Owner:EVE HYPERPOWER BATTERIES INC
Electric double layer capacitor
InactiveUS20060171101A1Improve featuresImprove pressure resistanceHybrid capacitor electrolytesElectrolytic capacitorsSulfolaneCarbonate
To provide an electric double layer capacitor having a low resistance, a high withstanding voltage and an excellent low-temperature characteristic. This object is achieved by employing, for an electric double layer capacitor having a pair of polarized electrodes and an electrolytic solution capable of forming an electric double layer at the interface with the polarized electrodes, an electrolytic solution containing as an electrolyte a salt represented by the formula 1, and as solvents at least (1) a chain carbonate having at most 5 carbon atoms, (2) sulfolane or its derivative and (3) a fluorobenzene: herein each of R1 and R2 which are independent of each other, is a C1-4 alkyl group, and X− is an anion.
Owner:ASAHI GLASS CO LTD
Method and technology for synthesizing and producing antibiotic medicament namely 1-(o-fluorophenyl) dihydropyridone
ActiveCN101798302AAntibacterial agentsGroup 4/14 element organic compoundsFormic Acid EstersOrtho position
The invention relates to a method and technology for synthesizing and producing an antibiotic medicament namely 1-(o-fluorophenyl) dihydropyridone. The type of compound can be used for treating infections of mammals. An entire preparation process comprises the following steps: i) transforming O-silanized N-(o-fluorophenyl)-4-piperidone into substituted 4-[4-(2,3-alkene) pyridine-1-]-radical-2-fluoronitrobenzene, wherein the latter can be reduced and acylated to generate carbamic acid ester; and ii) performing a reaction of the carbamic acid ester and an epoxy compound or chlorohydrin to generate corresponding 5-hydroxymethyl or substituted 5-aminomethyl oxazolidinone, wherein 5-aminomethyl oxazolidinone can be further transformed into antibiotic oxazolidinone with a structure I, and the definition of each group is as shown by the specification.
Owner:SHANGHAI MICURX LTD
Nickel-cobalt-manganese-lithium power battery and manufacturing method thereof
InactiveCN104393332AIncrease energy densityImprove high temperature storage performanceCell electrodesSecondary cellsAlkaline earth metalCobalt
The invention discloses a nickel-cobalt-manganese-lithium power battery and a manufacturing method thereof. A positive electrode active substance with the diameter 50 being 8-10 microns adopts nickel-cobalt-manganese-lithium, and a negative electrode adopts one or two of a middle-phase carbon microsphere, artificial graphite and composite graphite; a diaphragm adopts a three-layer composite diaphragm of which the surface is coated with Al<2-x>BxO3 (x=0.02-0.1), namely an Al<2-x>BxO3 / PP / PE / PP high-security ceramic diaphragm, wherein B is one or several of alkaline-earth metal ions, rare earth metal ions and transitional metal ions; electrolyte consists of 25-30 percent of acid-containing vinyl ester, 5-10 percent of propylene carbonate (PC), 25-30 percent of fluorobenzene (FB) and 10-15 percent of diethyl carbonate (DEC); lithium salt is lithium hexafluorophate (LiPF6) with the concentration being 10-12 percent; an additive is vinylethylene carbonate (VEC), sulfuric acid and vinyl resin, fluorinated hydrocarbon, biphenyl, maleate and maleimide and accounts for 10 percent of the total amount of the electrolyte; positive and negative plates of a profile aluminum shell lithium ion battery are subjected to winding, core lapping, cover plate welding, feeding into a shell, baking, laser welding, secondary baking, liquid injection, formation, gas extraction, steel ball pressing, partial containing, high-temperature aging under 45-75 DEG C and high-temperature heating for 120 hours to 24 hours for film forming.
Owner:YUNNAN TIN GROUP HLDG
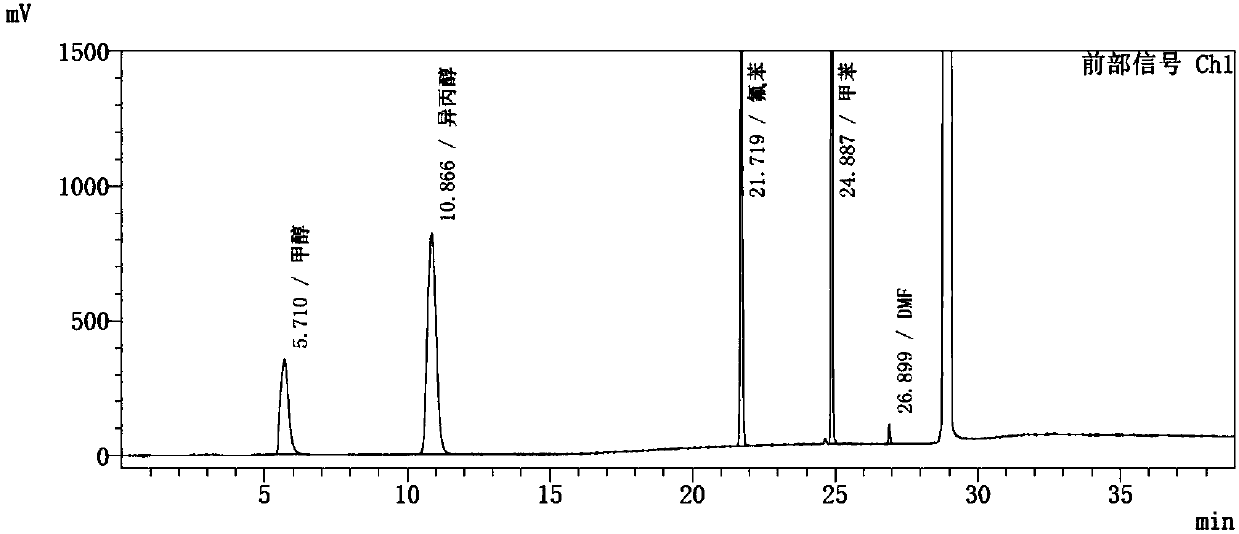
Method for determining solvents residual in ezetimibe bulk drug through headspace gas chromatography
ActiveCN107860826AAvoid interferenceQuality improvementComponent separationGas liquid chromatographicNitrogen gas
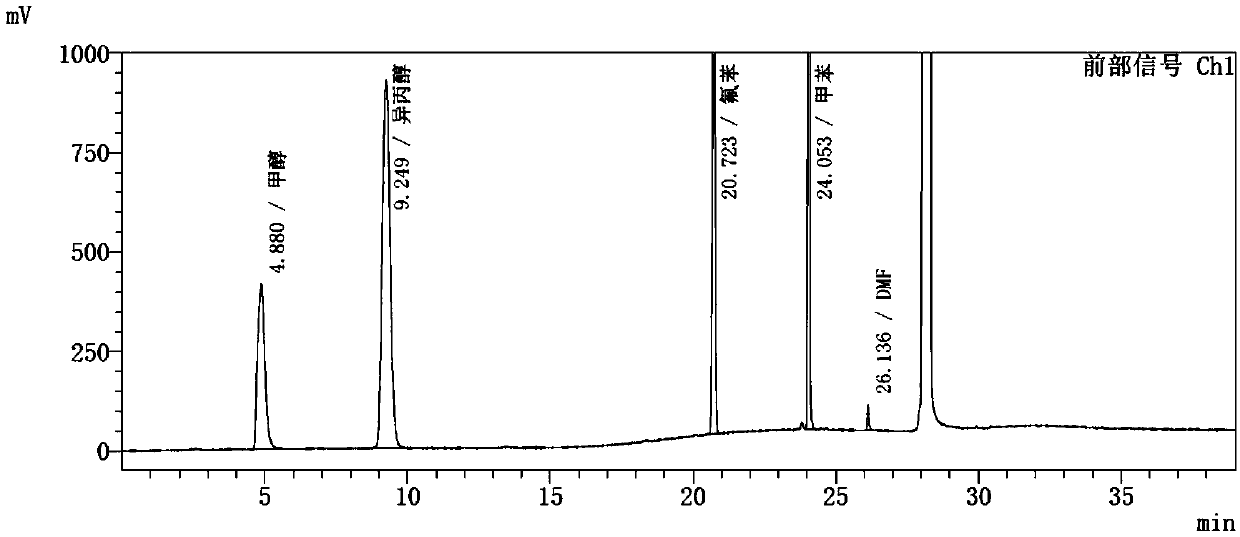
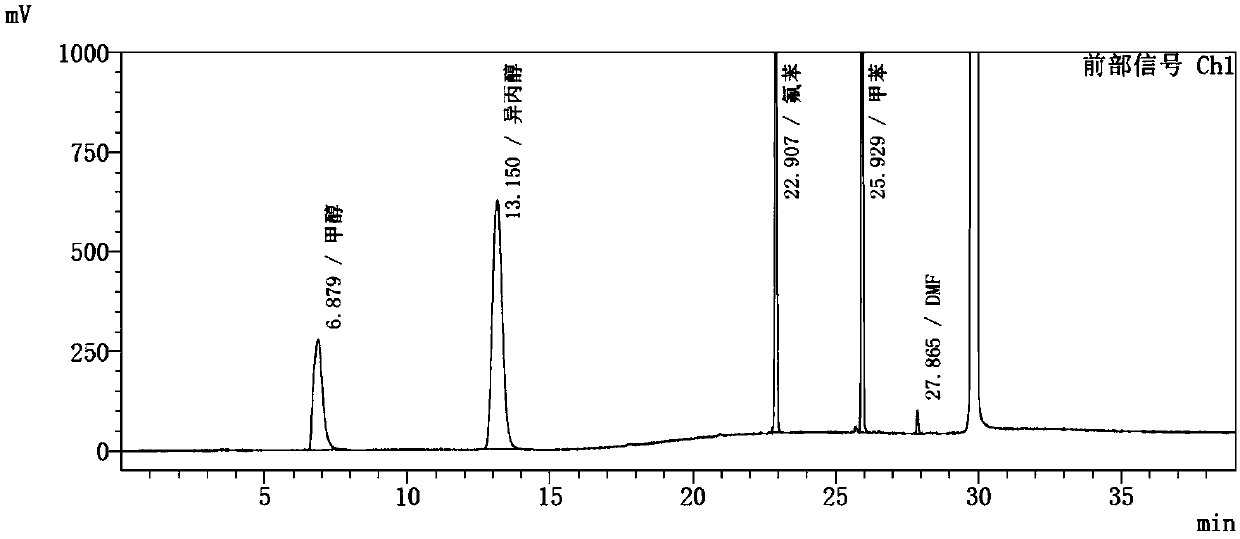
The invention discloses a method for determining solvents residual in an ezetimibe bulk drug The method adopts the following analysis conditions: the chromatographic column adopts a capillary chromatographic column with 6% cyanopropylphenyl 94% polydimethylsiloxane as the stationary phase; a reference substance solution is injected multiple times in a split flow manner, a sample solution is injected in the split flow manner, the carrier gas is nitrogen, the introduction is carried out by using a headspace injector, and detection is performed after temperature programming; and the detector adopts an FID detector. Methanol, isopropanol, fluorobenzene, toluene and N,N-dimethyl formamide in the ezetimibe bulk drug are rapidly and efficiently separated under same chromatographic conditions through the method in order to effectively control the quality of ezetimibe. The detection method has the advantages of strong specificity, high detection sensitivity, high precision, high accuracy, convenience in operation, and effective control of the product quality.
Owner:WATERSTONE PHARMA WUHAN
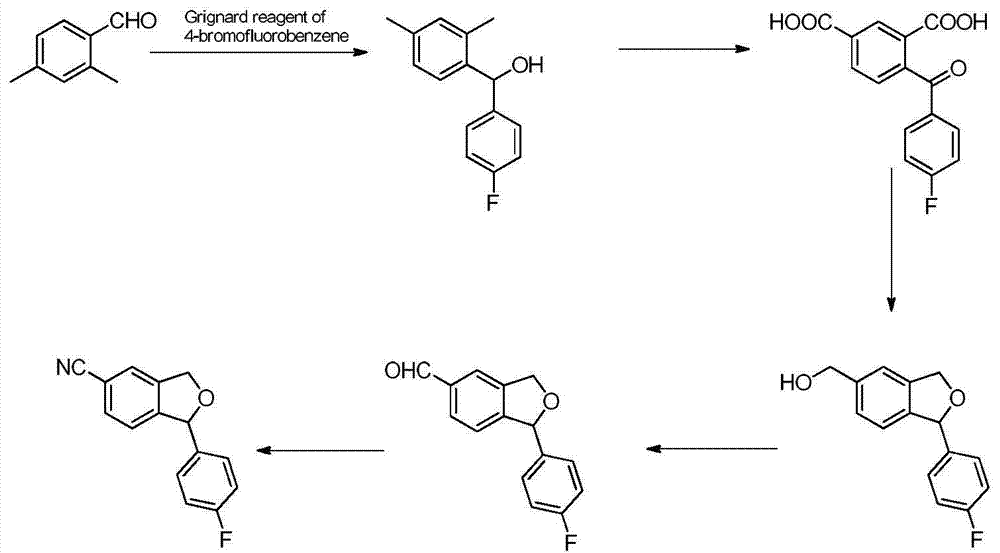
Preparation method of 5-cyanogen-1-(4-fluobenzene)-1,3-dihydrogenated-isobenzofuranone
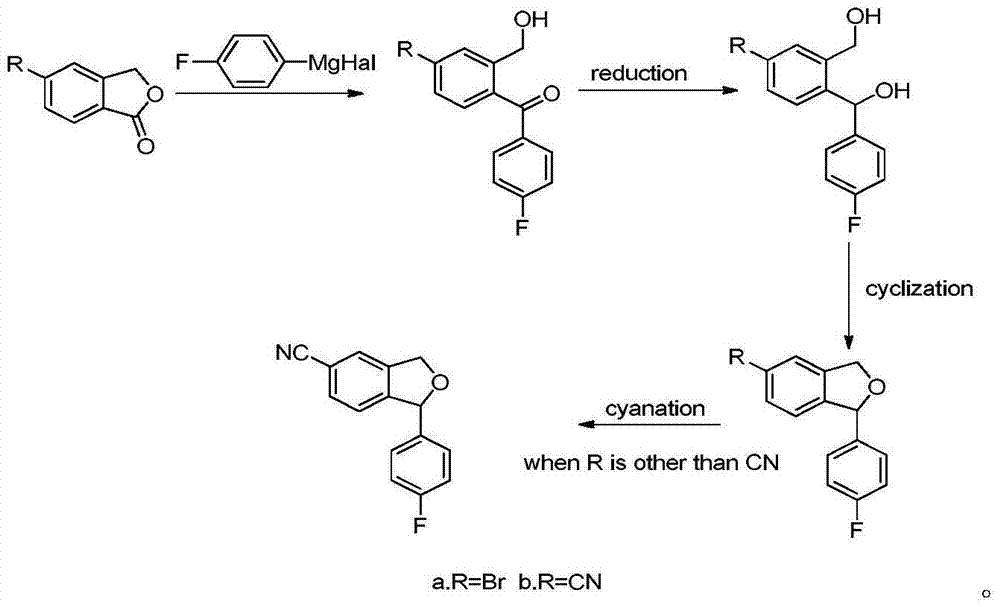
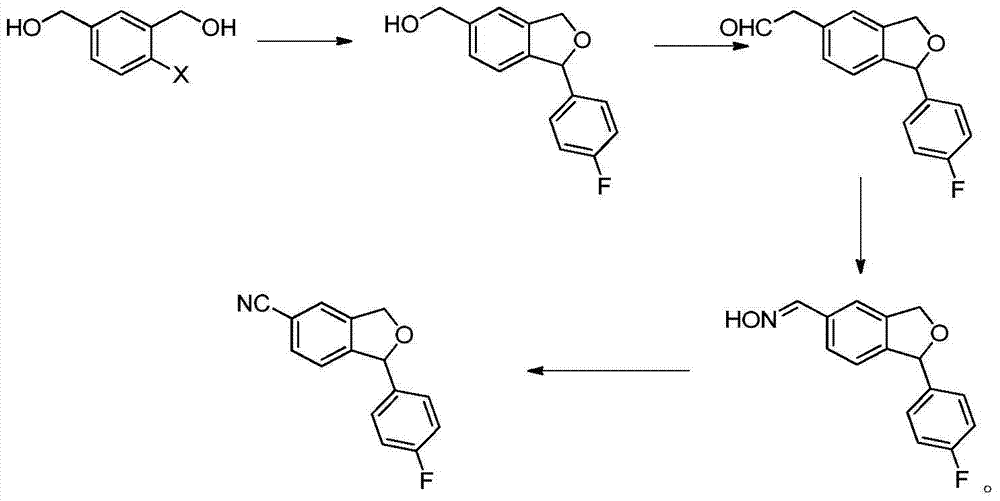
The invention provides a preparation method of 5-cyanogen-1-(4-fluobenzene)-1,3-dihydrogenated-isobenzofuranone. The preparation method comprises the following steps: reducing fluorobenzoic acid ester through hydrazine hydrate to obtain fluorobenzene formylhydrazine; enabling the fluorobenzene formylhydrazine to react with 5-bromine-2-hydroxybenzaldehyde under acidic conditions to obtain 4-fluorine-[(5-bromine-2- hydroxy phenyl) methene] hydrazide benzoic acid; oxidizing the 4-fluorine-[(5-bromine-2- hydroxy phenyl) methene] hydrazide benzoic acid through an oxidizing agent to obtain 5-bromine-2-(4-fluorine benzoyl)-benzaldehyde; reducing the 5-bromine-2-(4-fluorine benzoyl)-benzaldehyde through a reducing agent to obtain 4-bromine-Alpha 1-(4-fluorine phenyl)-1,2-xylyl alcohol; performing condensation reaction on the 4-bromine-Alpha 1-(4-fluorine phenyl)-1,2-xylyl alcohol by using toluenesulfonic acid as a catalyst to obtain 1-(4-fluobenzene)-5-bromine-1,3-dihydro- isobenzofuranone; enabling the 1-(4-fluobenzene)-5-bromine-1,3-dihydro- isobenzofuranone to react with copper cyanide to obtain the product. The method is low in cost, simple in technology, easy-operated in reaction, high in yield and suitable for industrialized production.
Owner:SOUTHEAST UNIV
Process for preparing p-fluoro thiophenol
InactiveCN1554644AShort processReduce the discharge of three wastesThiol preparationZincFluorobenzenes
The one-pot process of preparing p-fluoro thiophenol includes the following steps: reaction between fluorobenzene and chlorosulfonic acid to produce p-fluorophenyl sulfuryl chloride, and adding icy water to lower the temperature to 0-40 deg.c; and adding zinc powder to the reactor for direct reduction in two stages, including the first stage at 0-50 deg.c for 1-4 hr and the second stage at 70-95 deg.c for 1-4 hr. The one-pot process completes the chrlorosulfonation and reduction in one reactor, needs no filtering separation, and has short technological process, less apparatus used, saving in material, less exhaust, low cost and easy industrial application.
Owner:TIANJIN UNIV
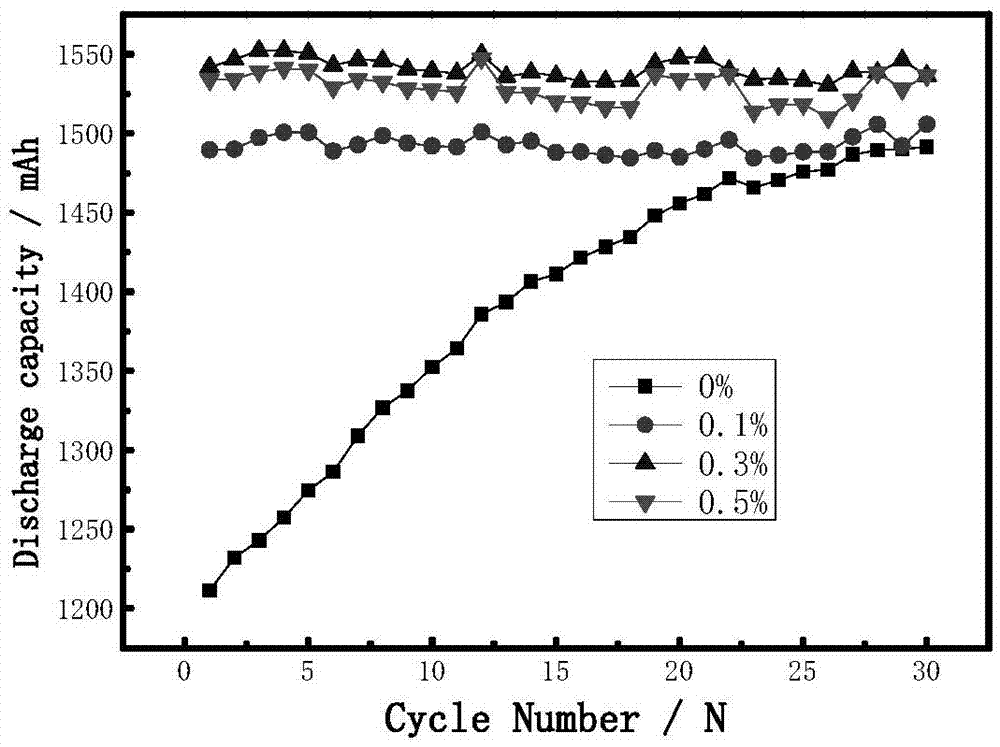
Electrolyte solution and lithium ion battery using said electrolyte solution
ActiveUS9601807B2Increase charging voltageImprove high-temperature intermittent cyclabilitySecondary cells servicing/maintenanceOrganic electrolytesOrganic solventInternal resistance
An electrolyte solution for a lithium-ion battery and a lithium-ion battery using the electrolyte solution are provided. The electrolyte solution includes organic solvents, an electrolyte lithium salt, and additives. The additives include succinonitrile, fluorobenzene, and lithium tetrafluoroborate. The percentage by mass of the fluorobenzene in the electrolyte solution is 0.1%-15%. The percentage by mass of the succinonitrile in the electrolyte solution is 0.1%-10%. The percentage by mass of the lithium tetrafluoroborate in the electrolyte solution is 0.01%-1%. The electrolyte solution may increase the charging voltage upper limit and improve the high-temperature intermittent cyclability of the lithium-ion battery. At the same time, the electrolyte may lower the battery swelling rate, reduce the internal resistance, and improve the stability and safety of the lithium-ion battery.
Owner:DONGGUAN AMPEREX TECH +1
Paddy field herbicide composition based on non-transgenic imidazolinone herbicide-resistant rice variety
The invention relates to a paddy field herbicidal composition based on non-transgenic imidazolinone herbicide-resistant rice varieties, comprising active ingredients a, b and c; wherein a is selected from imazethapyr, imazamox, imazethapyr, imazamox A kind of in acid, imidazolinic acid, imazapyr, imazamox, imazamox methyl, b and c are respectively selected from aryloxyphenoxypropionic acids, sulfonylureas, sulfonamides, triazoles Pyrimidine sulfonamides, amides, pyrimidinesalicylic acids, pyridyloxyacetic acids, arylpicolinates, quinoline carboxylic acids, phenoxyacetic acids, triketones, pyrazolopyridine rings, oxadi One of azole nitrogen-containing heterocycles, fluorophenyl ethers, triazolinones, organic heterocycles, dithiophosphoric acid esters, and benzonitrile, the categories of b and c can be the same but the specific compounds are different, The combination of a, b and c in a certain proportion has a synergistic effect on the target weeds, and is suitable for preventing annual weeds in paddy fields of non-transgenic rice varieties resistant to imidazolinone herbicides.
Owner:JIANGXI ZHONGXUN AGROCHEM
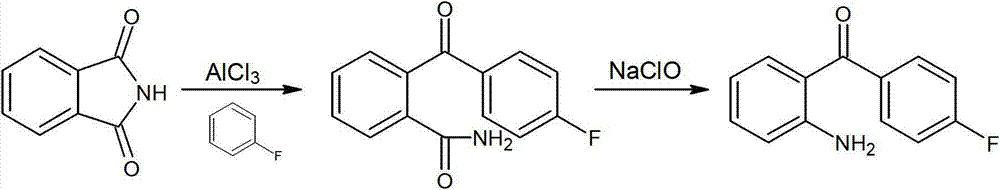
Synthesizing method of 2-amino-4'-fluoro-benzophenone
ActiveCN103086899AMeet technical requirementsShort reaction cycleOrganic chemistryOrganic compound preparationChemical synthesisBenzophenone
The invention belongs to the technical field of fine chemicals. The invention relates to a pharmaceutical chemical synthesis technology, and especially relates to a synthesizing method of 2-amino-4'-fluoro-benzophenone. According to the invention, o-phthalimide is adopted as an initial raw material for a first time, and is subjected to a Friedel-Crafts reaction with fluorobenzene, such that 2-p-luorobenzoyl benzamide is obtained; and through Hofmann degradation, 2-amino-4'-fluoro-benzophenone is obtained. The method has the advantages of short reaction period, high conversion rate, and good product quality. The content of produced 2-amino-4'-fluoro-benzophenone is higher than 99%, such that technical requirement by the market for 2-amino-4'-fluoro-benzophenone is satisfied. The operation steps and required devices are simple, and energy consumption is low.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
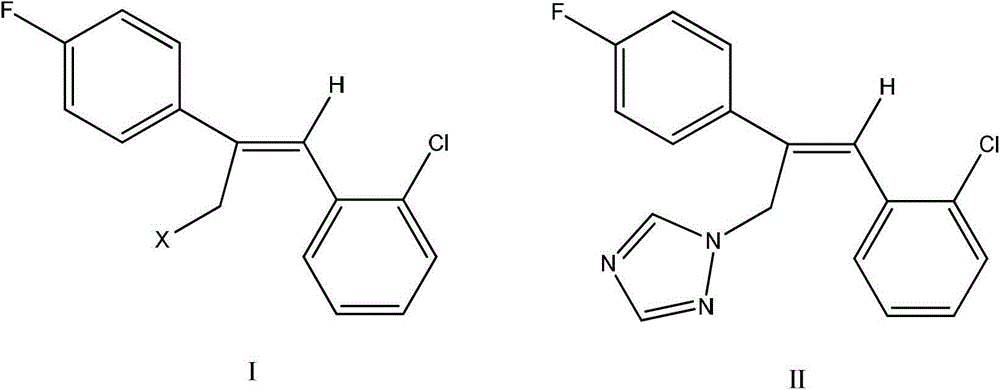
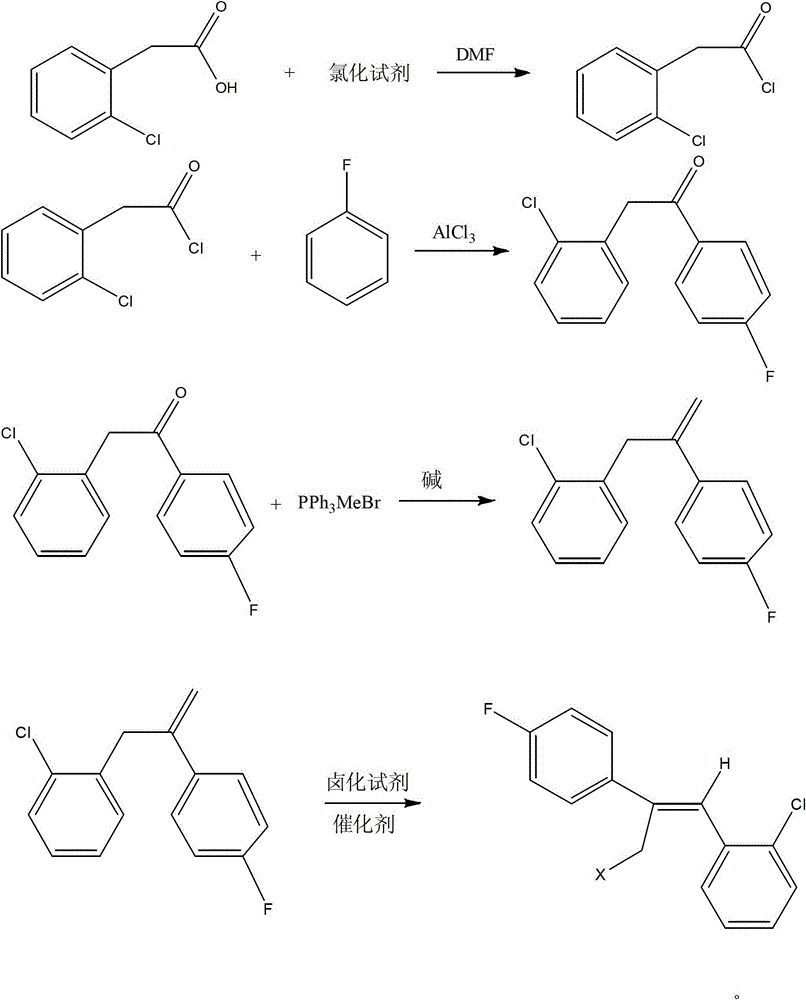
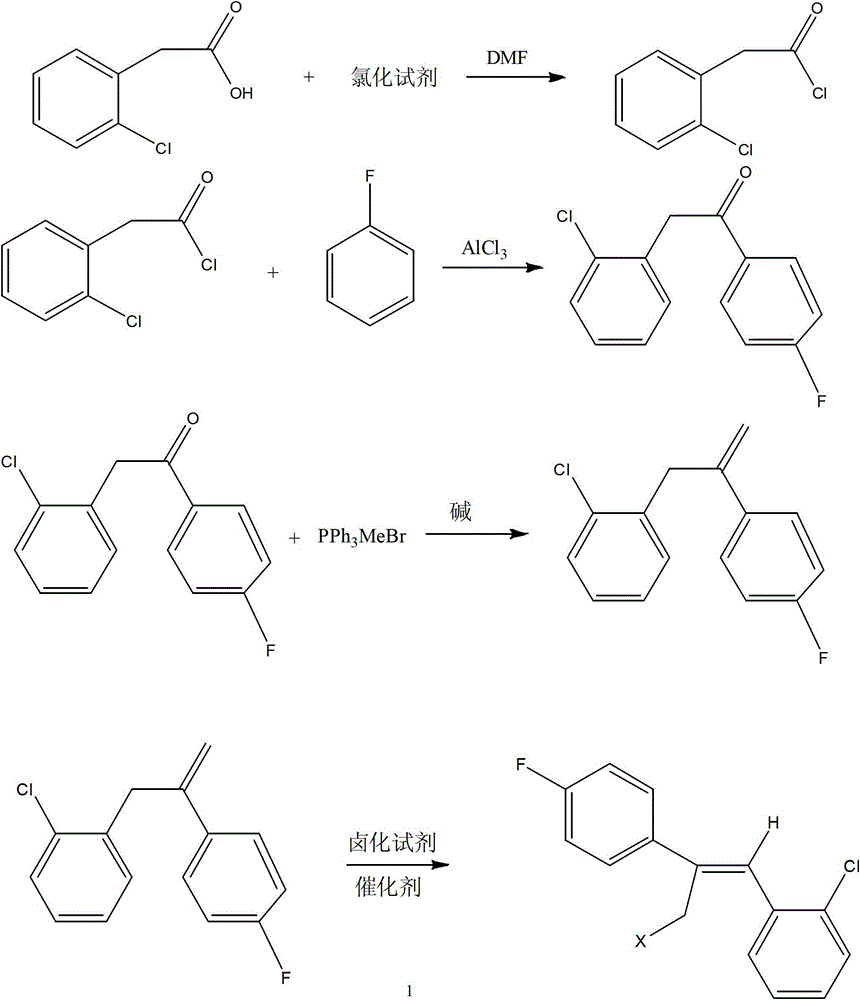
Preparation method of epoxiconazole intermediate (Z)-2-(4-fluorophenyl)-1-(2-chlorphenyl)-3-halogen propylene
ActiveCN103936552AGood choiceMild conditionsHalogenated hydrocarbon preparationAluminium chlorideEthyl Chloride
The invention relates to the field of pesticides, and particularly relates to a preparation method of an epoxiconazole intermediate (Z)-2-(4-fluorophenyl)-1-(2-chlorphenyl)-3-halogen propylene. The method includes: reacting o-clorophenylacetic acid and a chlorinating agent under the catalysis of dimethyl formamide to obtain o-chlorophenylacetyl chloride; subjecting the o-chlorophenylacetyl chloride and fluorobenzene to acylation under the catalysis of aluminium chloride to obtain 2-chlorophenyl-4'-fluoroacetophenone; subjecting the 2-chlorophenyl-4'-fluoroacetophenone and methyltriphenylphosphonium bromide under alkaline conditions to a Wittig reaction to obtain an olefin; and reacting the olefin with a halogenating agent to obtain the (Z)-2-(4-fluorophenyl)-1-(2-chlorphenyl)-3-halogen propylene. The method has characteristics of simple process, mild reaction conditions, high selectivity of the needed isomer after halogenation (namely high ratio of the Z isomer), and high content and yield of the target product.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Preparation method of high surface smoothness polystyrene hollow microspheres
ActiveCN108889253AImprove applicabilityReduce surface roughnessMicroballoon preparationMicrocapsule preparationFiltrationMicrosphere
The invention discloses a preparation method of high surface smoothness polystyrene hollow microspheres. The preparation method comprises the following steps: (1) purifying polystyrene, fluorobenzene,heavy water and ethanol; (2) dissolving polystyrene in fluorobenzene, and carrying out filtration, wherein the filtrate is taken as an oil phase; the heavy water and deionized distilled water are mixed as an inner water phase; a surfactant A and an electrolyte A are dissolved in the deionized distilled water and filtered, and the filtrate is taken as an outer water phase A; and a surfactant B isdissolved in deionized distilled water and is filtered, and the filtrate is taken as an outer water phase B; (3) preparing oil in water and water in oil compound emulsion particles with the inner water phase, the oil phase and the outer water phase A separately by adopting a microfluid technology; and (4) putting the microspheres into ethanol, carrying out ultrasonic treatment, standing, repeatedultrasonic treatment and standing, continuously standing, collecting microspheres on the surface of ethanol, and drying the microspheres to obtain the high surface smoothness polystyrene hollow microspheres. The high surface smoothness polystyrene hollow microspheres are applied to the fields of laser inertial confinement fusion, astrophysics of lab and high field physics and the like.
Owner:LASER FUSION RES CENT CHINA ACAD OF ENG PHYSICS
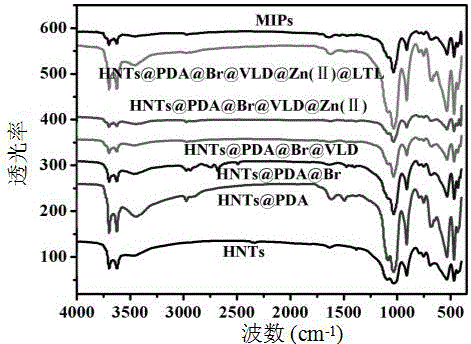
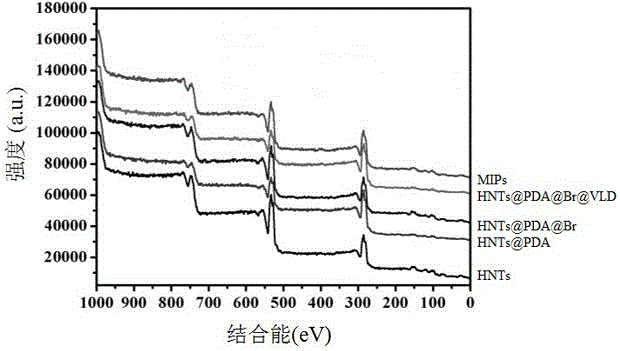
Preparation method of halloysite surface initiated boron affinity imprinted polymer adsorbent
InactiveCN106243295AFast adsorption kineticsStrong specific adsorption capacityOther chemical processesHalloysitePolymer adsorbent
The invention relates to a preparation method of a halloysite surface initiated boron affinity imprinted polymer adsorbent, and belongs to the technical field of preparation of environmental functional materials. The method comprises the following steps: purifying halloysite (HNTs), and wrapping the surface of halloysite with a layer of dopamine to form HNTs@PDA; connecting a bromine initiator to the surface of the HNTs@PDA to obtain HNTs@PDA@Br; connecting vinyl imidazole to the HNTs@PDA@Br through an atom free radical polymerization technology to obtain HNTs@PDA@Br@VLD; and adsorbing zinc ions, adsorbing luteolin, introducing fluorophenylboronic acid, and carrying out ATRP polymerization in order to obtain the imprinted polymer. Imprinted polymer microspheres prepared through the method have the advantages of good chemical stability, high adsorption capacity, good acid-alkali effect, realization of reversible adsorption / release functional luteolin with the acid-base value, and specific and high selective identification performance.
Owner:JIANGSU UNIV
Production of a wide gamut of structural colors using binary mixtures of particles with a potential application in ink jet printing
ActiveUS20200171869A1Avoid absorptionSimple and efficientDuplicating/marking methodsPretreated surfacesNanoparticleColloidal particle
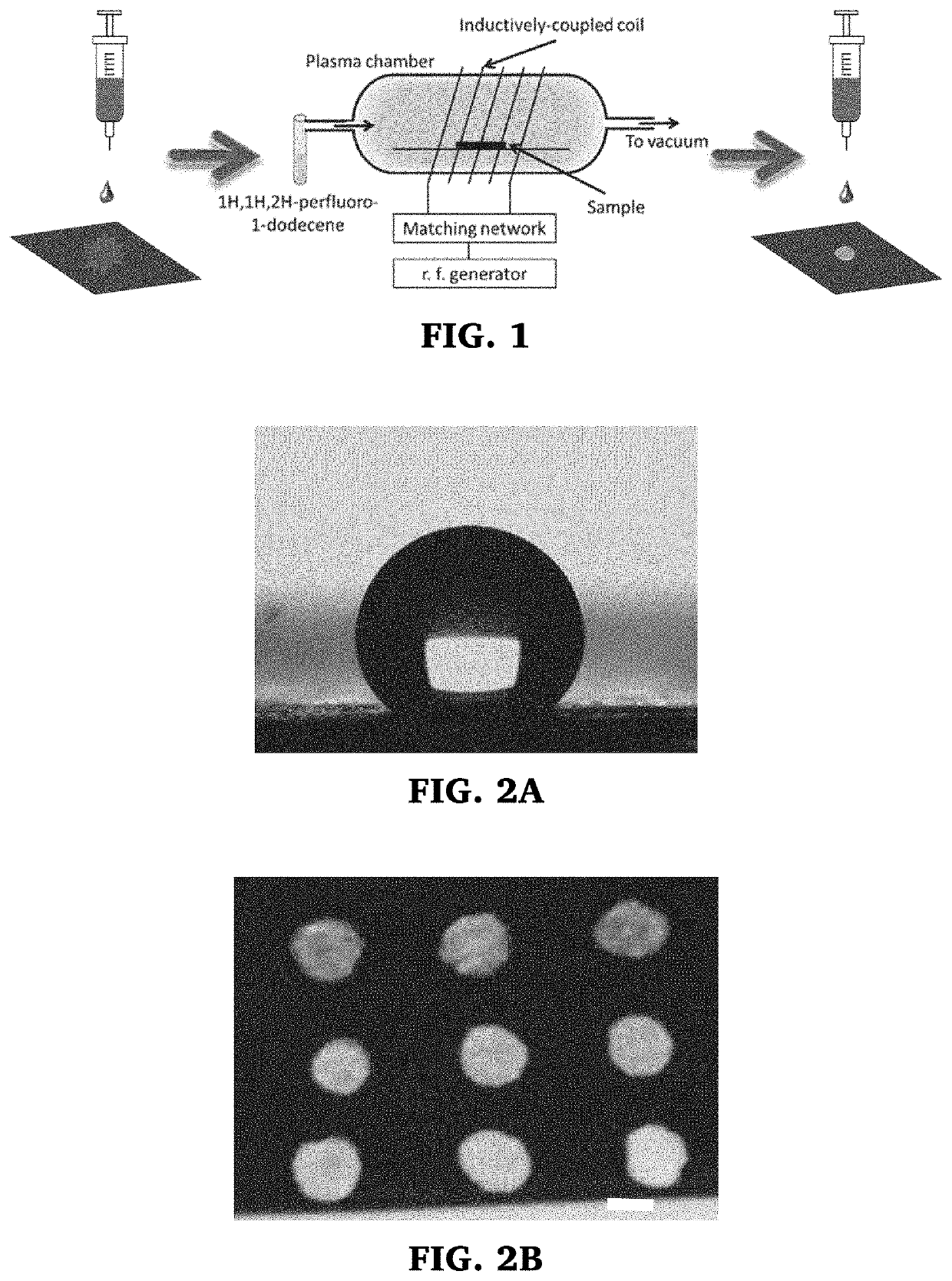
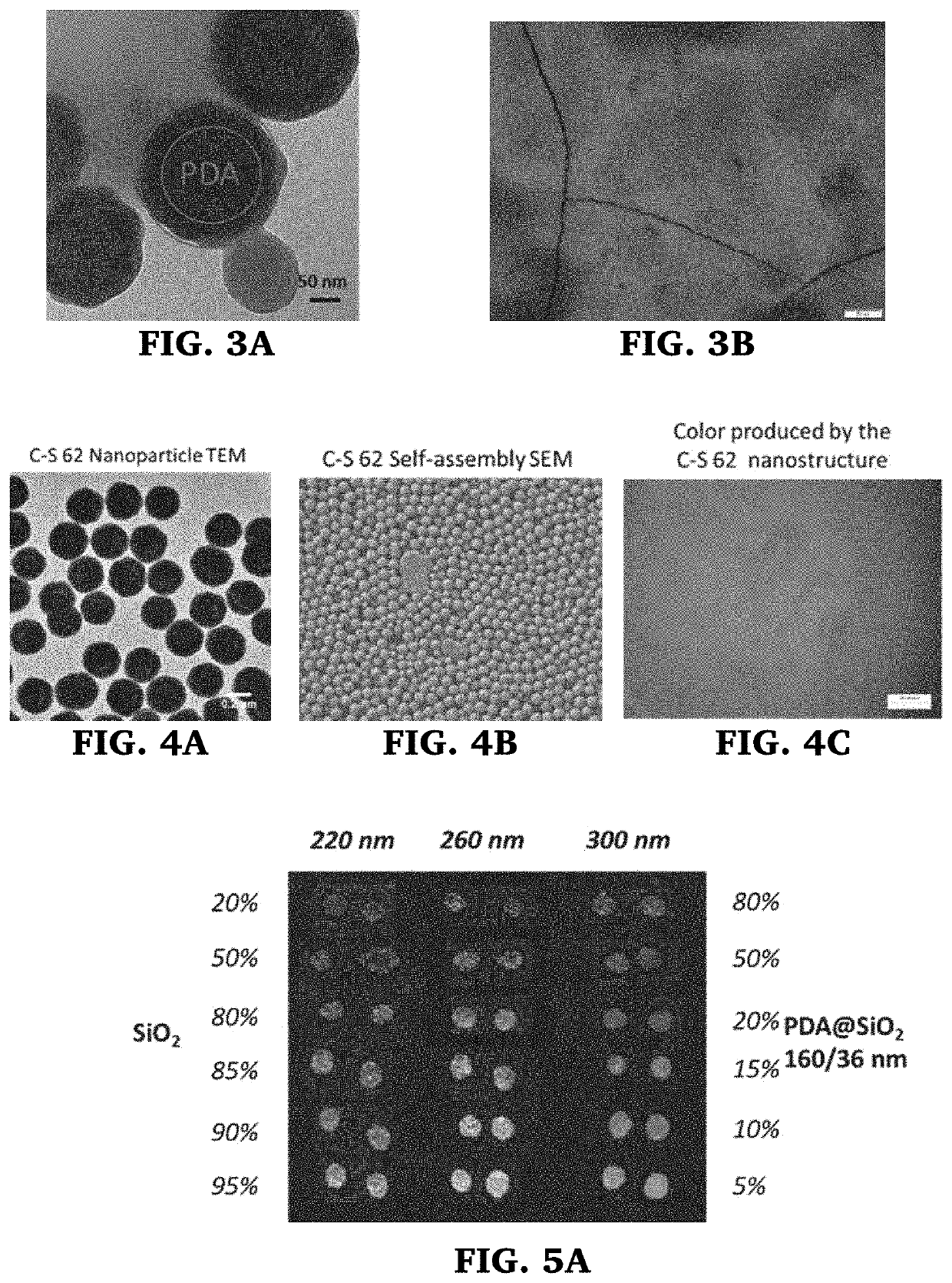
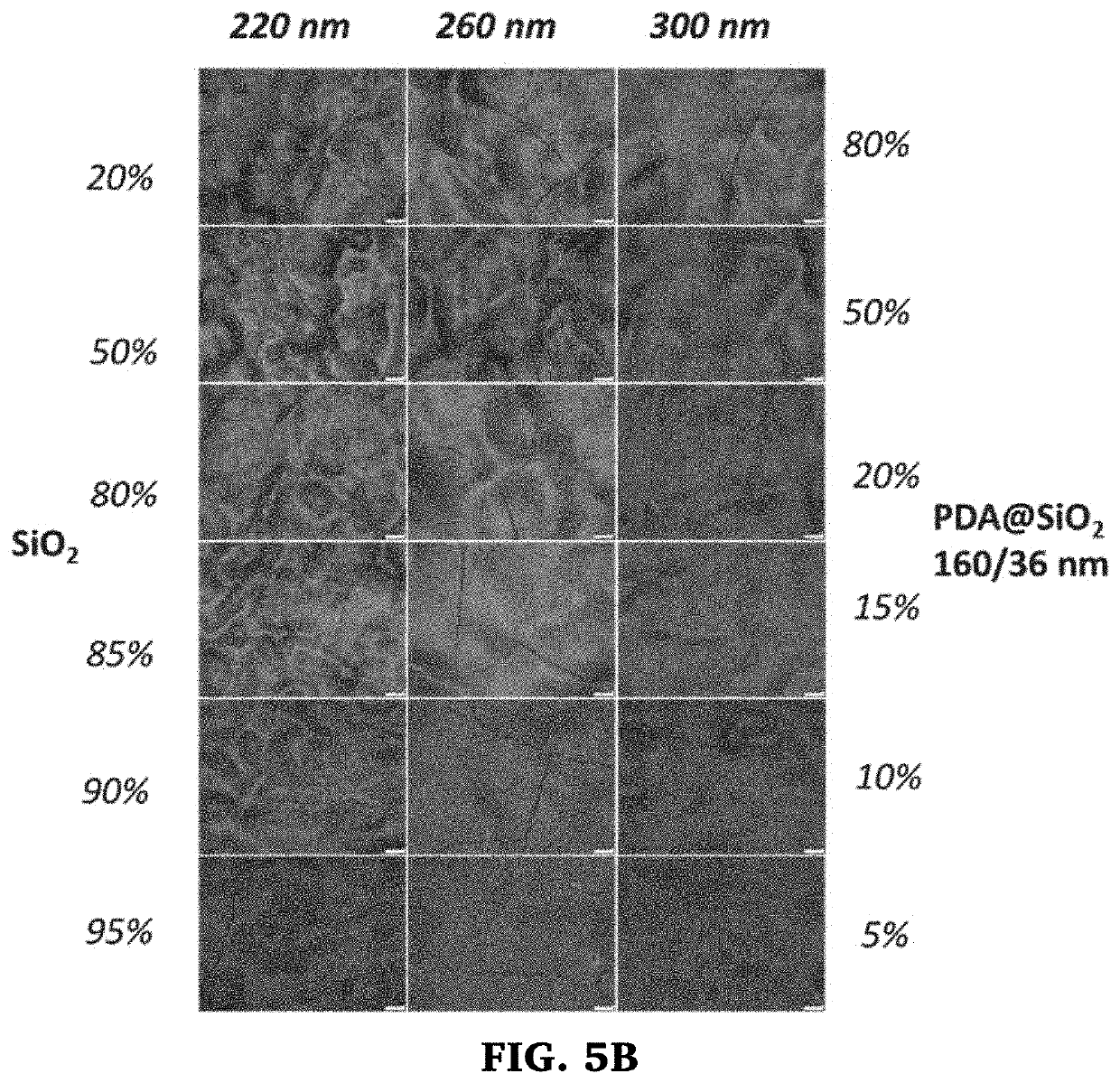
In one or more embodiments, the present invention provides a method of applying or printing structural colors to a substrate that involves pre-treatment of the substrate surface to prevent absorption of the fluid containing the particles. This allows the fluid to maintain their sessile drop shapes and as the water evaporates, the colloidal particles spontaneously assemble within the confined geometry into semi-ordered structures that interact with light to produce structural color. While the pre-treatment may be done in a variety of ways, application of a, hydrophobic and / or oleophobic coating, like 1H-IH,2H-perfluoro-1-dodecene (C10F21—CH═CH2) (perfluoro) monomer, fluoroalkyls, fluorohydroalkyls, cyclo-fluoroalkyls, fluorobenzen, by plasma-enhanced chemical vapor deposition (cold plasma treatment) has been found to be effective, particularly for printing applications. These treated substrates allow production of a wide range of structural colors using binary systems of nanoparticles.
Owner:THE UNIVERSITY OF AKRON
Pharmaceutical composition for treating or preventing respiratory and digestive diseases of birds and livestocks
ActiveCN1961881AEnhanced inhibitory effectEnhanced antibacterial activityDigestive systemAnimal feeding stuffQuinoxalineDisease
The invention relates to an antibacterial drug, wherein each 100g comprises 1-30 deals of fluorobenzene and 1-3 deals of antibacterial agent as quinoxaline antibiotic or amoxicillin. The invention can treat respiratory disease, etc. And it can be made into oral, injection types.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Novel pesticidal combinations
The present invention relates to combinations of a diamide insecticidal compound selected from broflanilide, chlorantraniliprole, cyantraniliprole, cyclaniliprole, cyhalodiamide, flubendiamide or tetraniliprole in combination with at least one multi-site fungicidally active compound and at least another insecticidal compound. The said combinations demonstrate excellent efficacy in the control of unwanted pests.
Owner:UNITED PHOSPHORUS LTD
Novel crystal form of pitavastatin and preparation method of novel crystal form
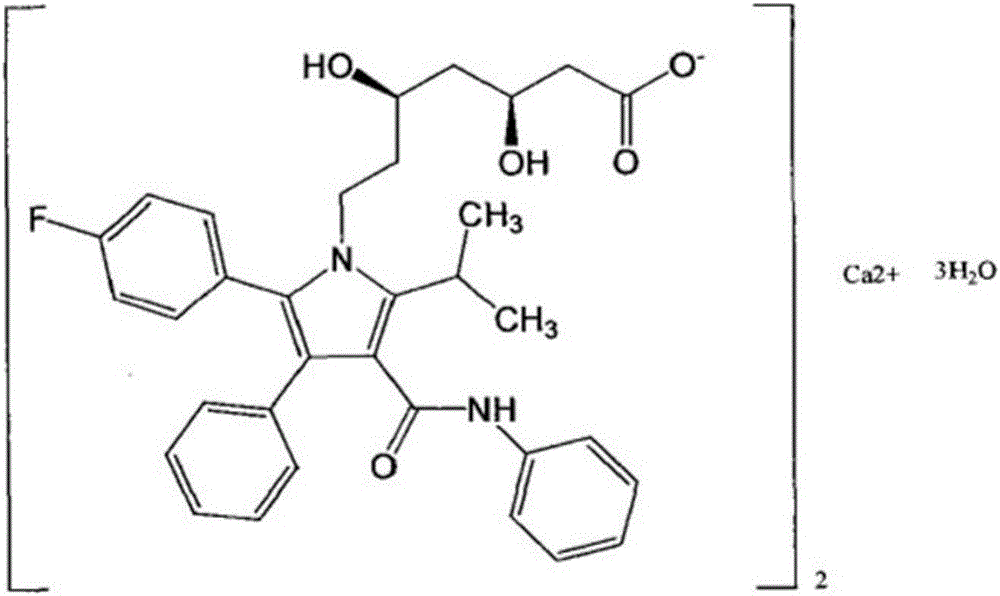
The invention relates to the preparation field of compounds and particularly relates to a novel crystal form of (-)-(3R,5S)-7-[2- cyclopropyl-4-(4- fluorobenzene group) quinoline-2-group]-3,5- dyhydroxyl-6(E)- heptylate and a preparation method of the novel form. By virtue of solvent crystallization, the method can obtain the crystal form of the (-)-(3R,5S)-7-[2- cyclopropyl-4-(4- fluorobenzene group) quinoline-2-group]-3,5- dyhydroxyl-6(E)- heptylate with enantiotopic impurity less than 0.50% and diastereomeric impurity less than 0.30%.
Owner:ANHUI QINGYUN PHARMA & CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200041.PNG)
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200042.PNG)
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200051.PNG)