Lithium battery electrolyte with wettability improved, and lithium battery
A lithium battery and electrolyte technology, applied in the field of electrochemistry, can solve the problems of battery performance damage, long industrialization process, etc., and achieve the effect of improved wettability and good high-temperature output characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
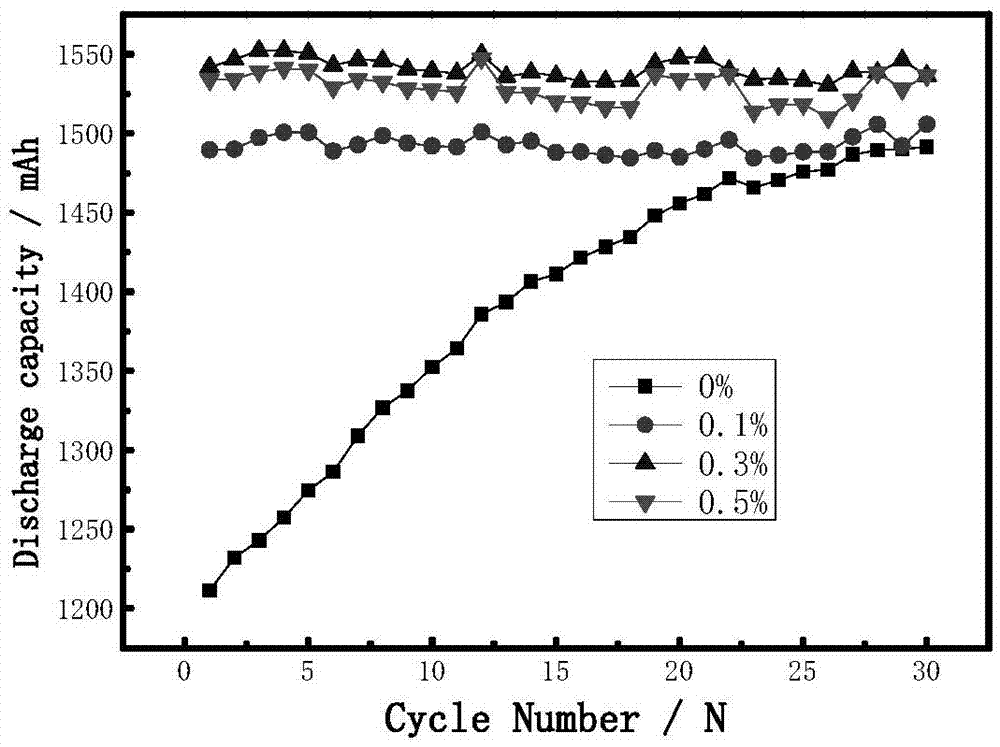
[0028] in an argon-filled glove box (H 2 O6 / VC / PS=35 / 10 / 35 / 15 / 2 / 3 mass ratio mix evenly, then add the total amount of electrolyte 0%, 0.05%, 0.1%, 0.3%, 0.5%, 1% respectively to the electrolyte And 2% of 2-[ethyl[(heptadecafluorooctyl)sulfonyl]amino]ethanol polyoxyethylene ether.
[0029] The ternary graphite battery was selected to be charged and discharged at 0.1C to form the battery, and the cycle performance of the battery at room temperature was tested. Test results such as figure 1 shown.
Embodiment 2
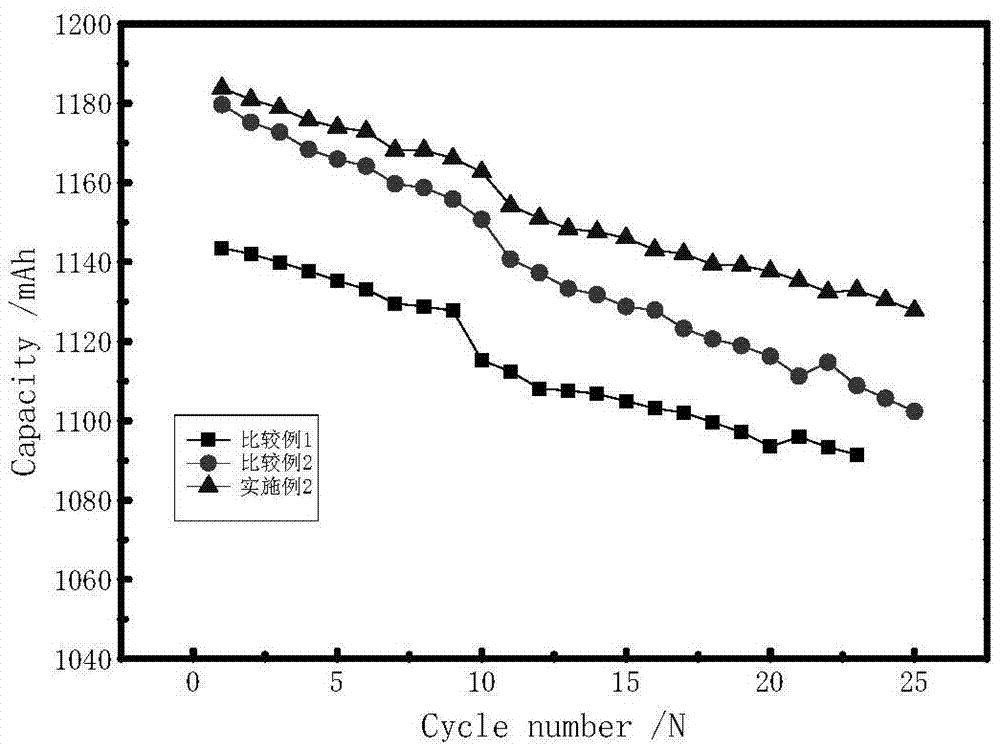
[0037] in an argon-filled glove box (H 2 O6 ) in which, 2-[ethyl[(heptadecafluorooctyl)sulfonyl]amino]ethanol polyoxyethylene ether of 0.5% of the total amount of the electrolyte and 1% of the total amount of the electrolyte of vinylene carbonate were added to the electrolyte ester.
[0038] The lithium cobaltate graphite battery is selected, and the battery is formed by charging and discharging at 0.1C, and the first charge and discharge efficiency is measured, and it is cycled at room temperature. The test results are shown in Table 1 and figure 2 shown.
[0039] Table 1
[0040]
Embodiment 3
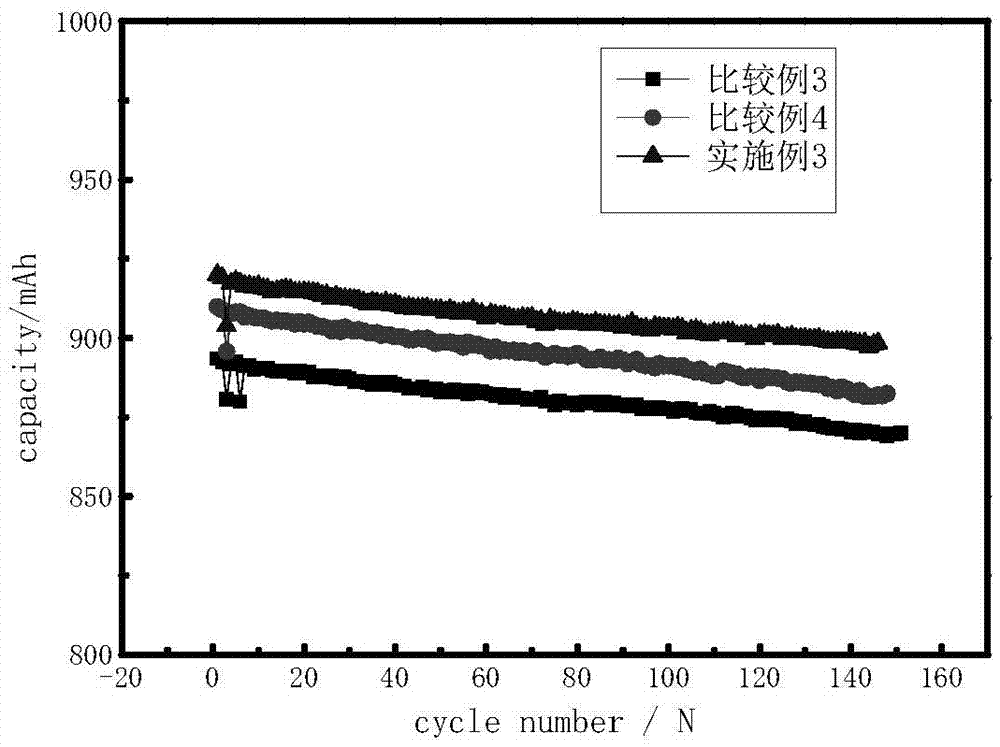
[0048] in an argon-filled glove box (H 2 O6 ) in which, 2-[ethyl[(heptadecafluorooctyl)sulfonyl]amino]ethanol polyoxyethylene ether of 0.05% of the total amount of the electrolyte and 1% of the total amount of the electrolyte of vinylene carbonate were added to the electrolyte ester.
[0049] Lithium cobaltate graphite battery was selected, and the battery was formed by charging and discharging at 0.1C, and the first charge and discharge efficiency was measured, and it was cycled at room temperature and high temperature and placed at 85°C for 4H. The test results are shown in Tables 2, 3, 4 and image 3 As shown, among them, Table 2 and Table 3 are the test results of 85°C high-temperature storage for 4H, and Table 4 is the test result of room temperature 45C rate.
[0050] Table 2
[0051]
[0052] table 3
[0053]
[0054] Table 4
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com