Method for determining solvents residual in ezetimibe bulk drug through headspace gas chromatography
A headspace gas chromatography and ezetimibe technology, which is applied in the field of drug analysis, can solve problems that need to be improved, and achieve rapid and efficient separation and content determination, convenient operation, and high precision.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
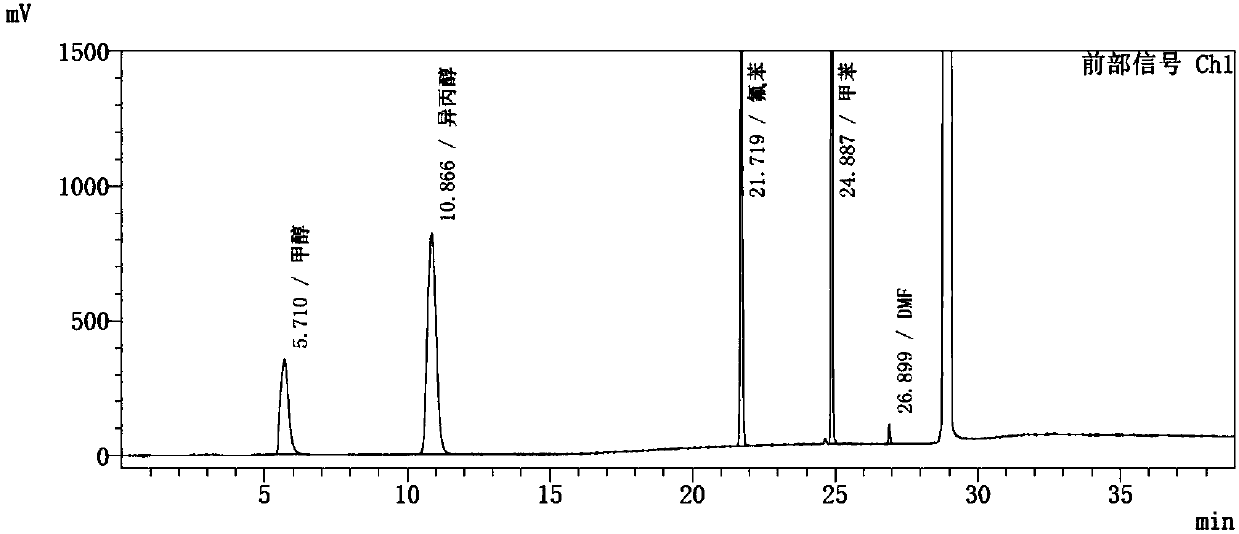
Embodiment 1
[0050] Instrument: Agilent 7890A-7697A headspace gas chromatograph, FID detector;
[0051] Chromatographic column: KB-624 (30m*0.32mm, 3.0μm);
[0052] Chromatographic parameters: carrier gas: nitrogen; carrier gas flow rate: 1.0ml / min; split ratio: 1:1;
[0053] Detector temperature: 250°C; inlet temperature: 200°C;
[0054] Column temperature: the initial temperature is 40°C for 15 minutes, then rise to 200°C at a rate of 10°C / min, and hold for 8 minutes.
[0055] Headspace detection parameters: heating box temperature: 90℃; quantitative loop temperature: 100℃; transmission line temperature: 110℃;
[0056] Headspace balance time: 30min; cycle time: 50min; sampling time: 0.5min;
[0057] Pressure balance time: 0.1min.
[0058] 1. Exclusiveness
[0059] Blank solution: dimethyl sulfoxide (DMSO).
[0060] Methanol stock solution: Take about 750mg of methanol, accurately weigh it, put it in a 25ml measuring flask, dilute and dissolve with DMSO to the mark, shake well, and get it;
[0061] Isopr...
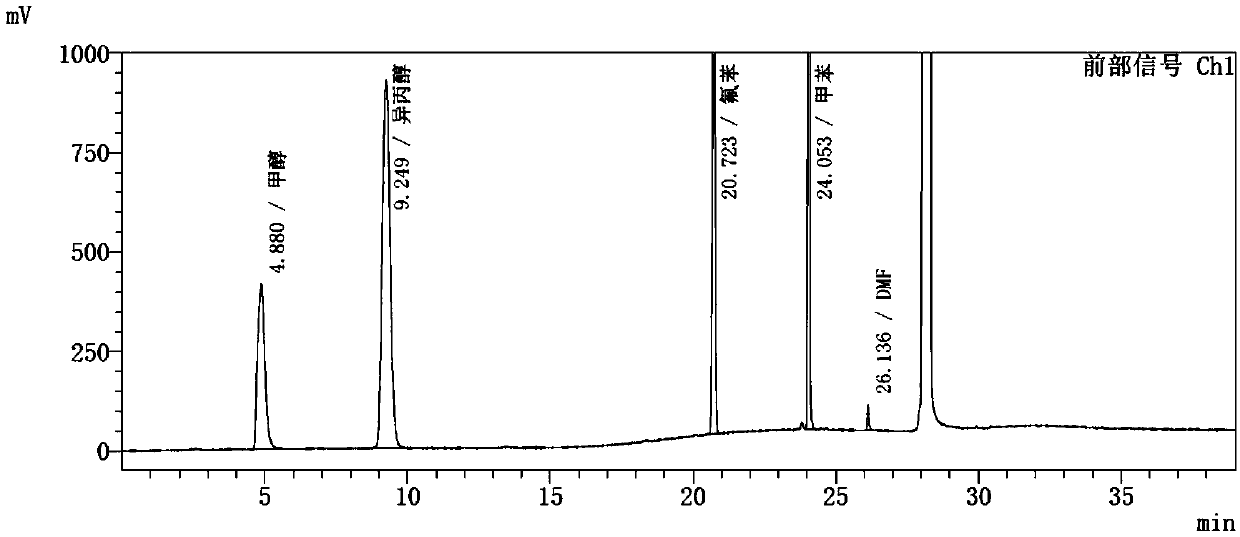
Embodiment 2
[0093] Instrument: Agilent 7890A-7697A headspace gas chromatograph, FID detector;
[0094] Chromatographic column: KB-624 (30m*0.32mm, 3.0μm);
[0095] Chromatographic parameters: carrier gas: nitrogen; carrier gas flow rate: 1.2ml / min; split ratio: 1:1;
[0096] Detector temperature: 250°C; inlet temperature: 200°C;
[0097] Column temperature: the initial temperature is 40°C for 15 minutes, then rise to 200°C at a rate of 10°C / min, and hold for 8 minutes.
[0098] Headspace detection parameters: heating box temperature: 90℃; quantitative loop temperature: 100℃; transmission line temperature: 110℃;
[0099] Headspace balance time: 30min; cycle time: 50min; sampling time: 0.5min;
[0100] Pressure balance time: 0.1min.
[0101] experiment procedure:
[0102] Blank solution: dimethyl sulfoxide (DMSO).
[0103] System suitability solution: Precisely pipette 1.0 ml of each positioning solution in Example 1 into a 100 ml volumetric flask containing about 30 ml of DMSO, dilute to the mark with th...
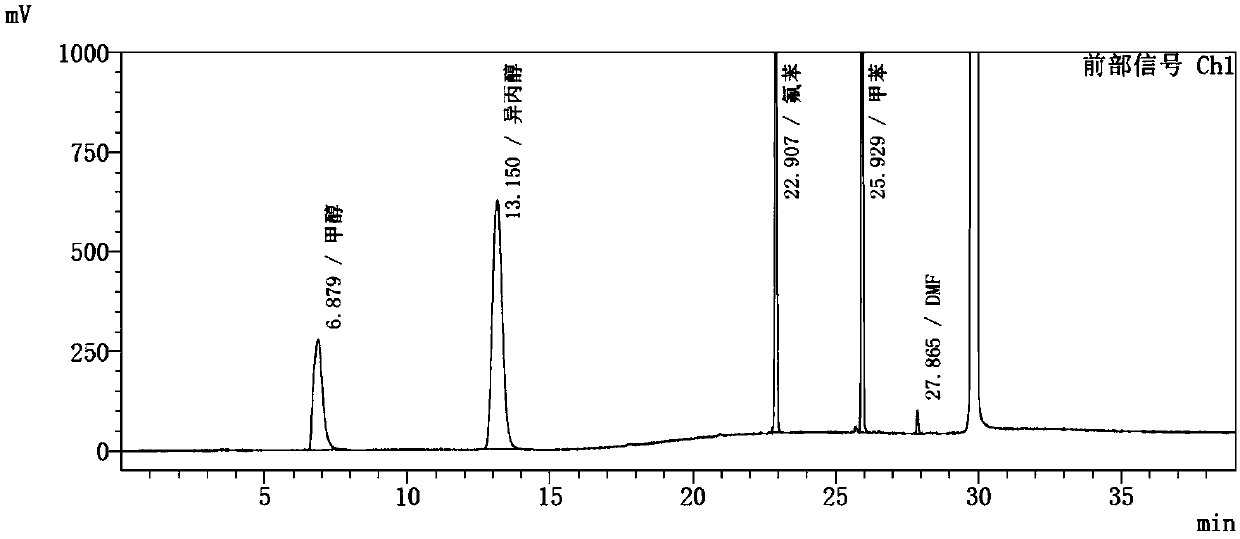
Embodiment 3
[0108] Instrument: Agilent 7890A-7697A headspace gas chromatograph, FID detector;
[0109] Chromatographic column: KB-624 (30m*0.32mm, 3.0μm);
[0110] Chromatographic parameters: carrier gas: nitrogen; carrier gas flow rate: 0.8ml / min; split ratio: 1:1;
[0111] Detector temperature: 250°C; inlet temperature: 200°C;
[0112] Column temperature: the initial temperature is 40°C for 15 minutes, then rise to 200°C at a rate of 10°C / min, and hold for 8 minutes.
[0113] Headspace detection parameters: heating box temperature: 90℃; quantitative loop temperature: 100℃; transmission line temperature: 110℃;
[0114] Headspace balance time: 30min; cycle time: 50min; sampling time: 0.5min;
[0115] Pressure balance time: 0.1min.
[0116] experiment procedure:
[0117] Blank solution: dimethyl sulfoxide (DMSO).
[0118] System suitability solution: Precisely pipette 1.0 ml of each positioning solution in Example 1 into a 100 ml volumetric flask containing about 30 ml of DMSO, dilute to the mark with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com