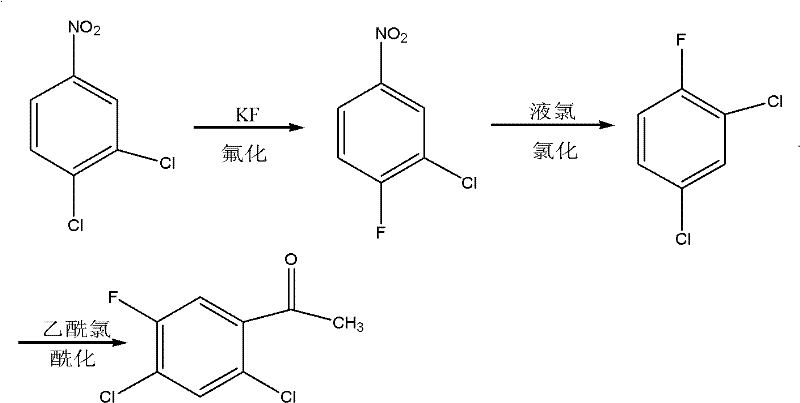
Method for coproducing key intermediates of quinolone medicines by using o-dichlorobenzene as raw material
A technology of o-dichlorobenzene and quinolones, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of diazonium and fluorine decomposition hazards, unfriendly environment, high price, etc., to reduce pollution, solve high production costs, and clean the environment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
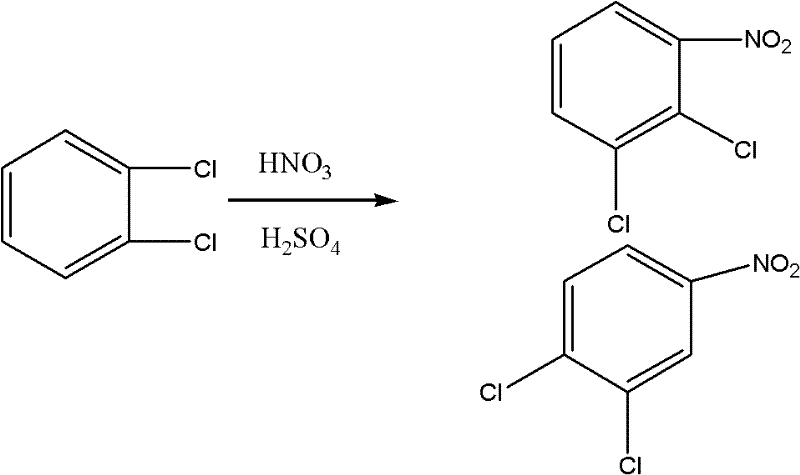
[0035] Put 210g of o-dichlorobenzene into a 500ml four-necked flask, raise the temperature to 90°C, add dropwise 340g of mixed acid (100g of 69% nitric acid and 240g of 98% sulfuric acid), control the reaction at 95-100°C, keep warm for 2 hours after the addition, and divide the mixture after cooling. layer, reclaim sulfuric acid, wash the organic phase with water, neutralize, and obtain 210g3, 4-dichloronitrobenzene (content ≥ 99.5%), 53g 2, 3-dichloronitrobenzene (content ≥ 99.6%), the total yield was 95.8%.
[0036] 2. Step (2)
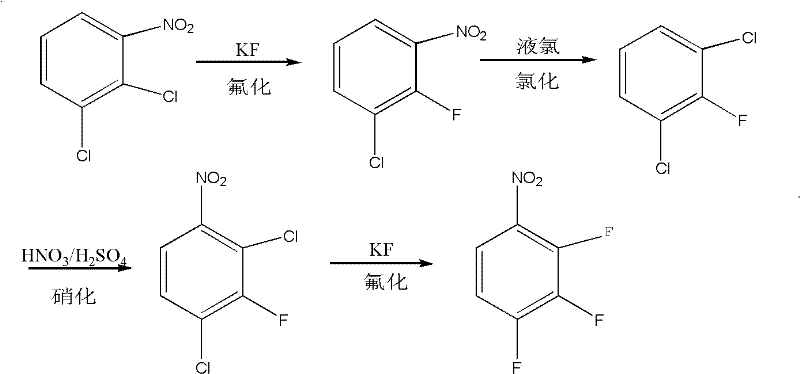
[0037] 1. 2,3-dichloronitrobenzene is fluorinated to obtain 2-fluoro-3-chloronitrobenzene, then chlorinated to obtain 2,6-dichlorofluorobenzene, and nitrated to obtain 2,4-dichloro-3 - fluoronitrobenzene, finally fluorinated to give 2,3,4-trifluoronitrobenzene,
[0038] 1.1. Fluorination reaction
Embodiment 2
[0040] Put 400g (2.08mol) of 2,3-dichloronitrobenzene into a 500ml four-neck flask, raise the temperature to 140°C and put in 112g (1.93mol) of KF, carry out dehydration under reduced pressure, keep warm at 140-150°C for 3 hours, after dehydration Raise the temperature to 140°C, add 6.6g (0.06mol) tetramethylammonium chloride into the reaction kettle between 150°C and 160°C, keep it at 165±5°C for 8 hours after adding, stop the reaction, cool, wash with water, and separate After the layer, the organic phase was first distilled and then rectified, and 100 g of 2,3-dichloronitrobenzene was recovered to generate 240 g of 2-fluoro-3-chloronitrobenzene, with a content of 99.6% and a yield of 87.6%.
Embodiment 3
[0042] According to embodiment 2, phase transfer catalyst tetramethylammonium chloride is reduced to 3.3g (0.03mol) direct reaction, reclaims 2,3-dichloronitrobenzene 230g, generates 2-fluoro-3-chloronitrobenzene 108g, Content 97.8%, yield 69.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com