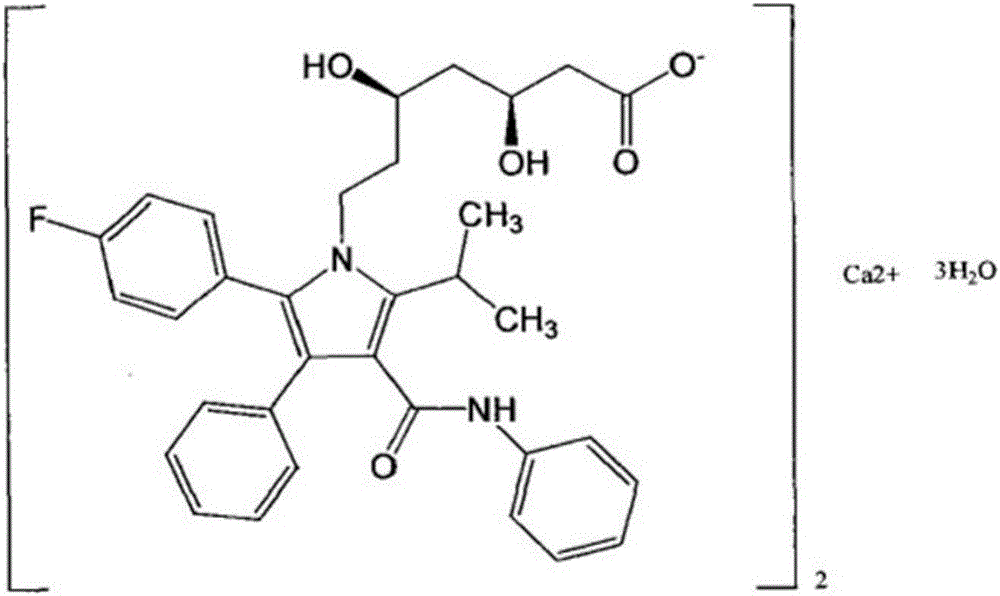
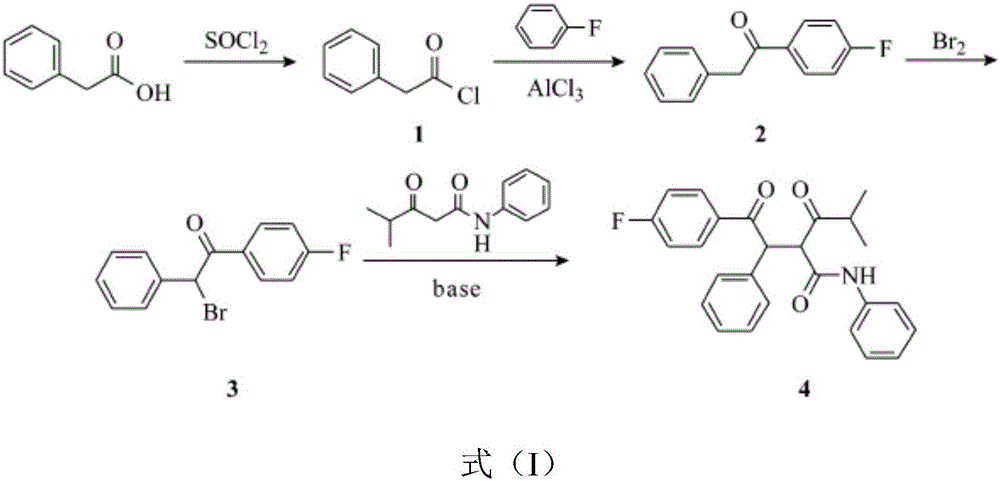
Preparation technology of atorvastatin
A technology for atorvastatin and preparation process, which is applied in the field of chemical synthesis, can solve the problems of complicated processing, decreased product yield and the like, and achieves the effects of improving reaction yield, improving reaction yield and facilitating separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In the first step, 6.8g of phenylacetic acid was dissolved in 40ml of chloroform, 7.8g of thionyl chloride was added into the reaction flask, and the temperature was raised to reflux for 2-3 hours. 6.31 g of phenylacetyl chloride, the yield is 82%.
[0020] In the second step, add 10.6 g of fluorobenzene into 100 ml of dichloromethane and stir, add 13 g of zeolite molecular sieves under ice bath, then add dropwise 40 ml of dichloromethane containing 15.4 g of phenylacetyl chloride to the above solution under ice bath, drop During the addition process, keep the temperature of the reaction solution not exceeding 10°C, continue the reaction for 2 hours after the dropwise addition, and TLC tracking shows that the reaction is complete. Filter and spin dry to obtain a light yellow solid, which is recrystallized to obtain 17.12 g of 4-fluorophenylacetophenone with a yield of 80%.
[0021] In the third step, dissolve 10.7g of 4-fluorophenylacetophenone in 100ml of glacial acet...
Embodiment 2
[0025] In the first step, 6.8g of phenylacetic acid was dissolved in 40ml of chloroform, 7.8g of thionyl chloride was added into the reaction flask, and the temperature was raised to reflux for 2-3 hours. 6.31 g of phenylacetyl chloride, the yield is 82%.
[0026] In the second step, add 10.6 g of fluorobenzene into 100 ml of dichloromethane and stir, add 13 g of zeolite molecular sieves under ice bath, then add dropwise 40 ml of dichloromethane containing 15.4 g of phenylacetyl chloride to the above solution under ice bath, drop During the addition process, keep the temperature of the reaction solution not exceeding 10°C, continue the reaction for 2 hours after the dropwise addition, and TLC tracking shows that the reaction is complete. Filter and spin dry to obtain a light yellow solid, which is recrystallized to obtain 17.12 g of 4-fluorophenylacetophenone with a yield of 80%.
[0027] In the third step, dissolve 10.7g of 4-fluorophenylacetophenone in 100ml of glacial acet...
Embodiment 3
[0031] In the first step, 6.8g of phenylacetic acid was dissolved in 40ml of chloroform, 7.8g of thionyl chloride was added into the reaction flask, and the temperature was raised to reflux for 2-3 hours. 6.31 g of phenylacetyl chloride, the yield is 82%.
[0032] In the second step, add 10.6 g of fluorobenzene into 100 ml of dichloromethane and stir, add 13 g of zeolite molecular sieves under ice bath, then add dropwise 40 ml of dichloromethane containing 15.4 g of phenylacetyl chloride to the above solution under ice bath, drop During the addition process, keep the temperature of the reaction solution not exceeding 10°C, continue the reaction for 2 hours after the dropwise addition, and TLC tracking shows that the reaction is complete. Filter and spin dry to obtain a light yellow solid, which is recrystallized to obtain 17.12 g of 4-fluorophenylacetophenone with a yield of 80%.
[0033] In the third step, dissolve 10.7g of 4-fluorophenylacetophenone in 100ml of glacial acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com