Novel crystal form of pitavastatin and preparation method of novel crystal form
A crystal form and crystallization technology, applied in the field of compound preparation, can solve the problems of difficult purification, low yield, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 (-)-(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-2-yl]-3,5-dihydroxy-6(E) -Ethyl heptenoate crystallized from ethyl acetate
[0107] Diastereoisomer detection method:
[0108] HPLC conditions:
[0109] Chromatographic column: Agilent C8 (4.5*15mm, 5um)
[0110] Gradient elution: A: 1ml 85% phosphoric acid dissolved in 1000ml water
[0111] B: Isopropanol
[0112] C: Methanol
[0113] See Table 1 for specific conditions.
[0114] Table 1 gradient elution conditions
[0115]
[0116] Flow rate: 0.8ml / min
[0117] Detection wavelength λ=242nm
[0118] Column temperature: 30°C
[0119] Sample Preparation:
[0120] Take an appropriate amount of sample and add 90% methanol to make a 0.5mg / ml solution. Inject 20 μL.
[0121] Enantiomer detection method:
[0122] Chromatographic column: CHIRALCEL OJ-H (4.6*250mm, 5μm)
[0123] Mobile phase: (n-hexane: ethanol: trifluoroacetic acid) - (80:20:0.1)
[0124] Flow: 1.0ml / min λ=242nm Column temperat...
Embodiment 2
[0128] Example 2 (-)-(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-2-yl]-3,5-dihydroxy-6(E) -Ethyl heptenoate crystallized from a mixture of methanol and water (2:1 by volume)
[0129] The detection method of diastereoisomers and enantiomers is the same as in Example 1.
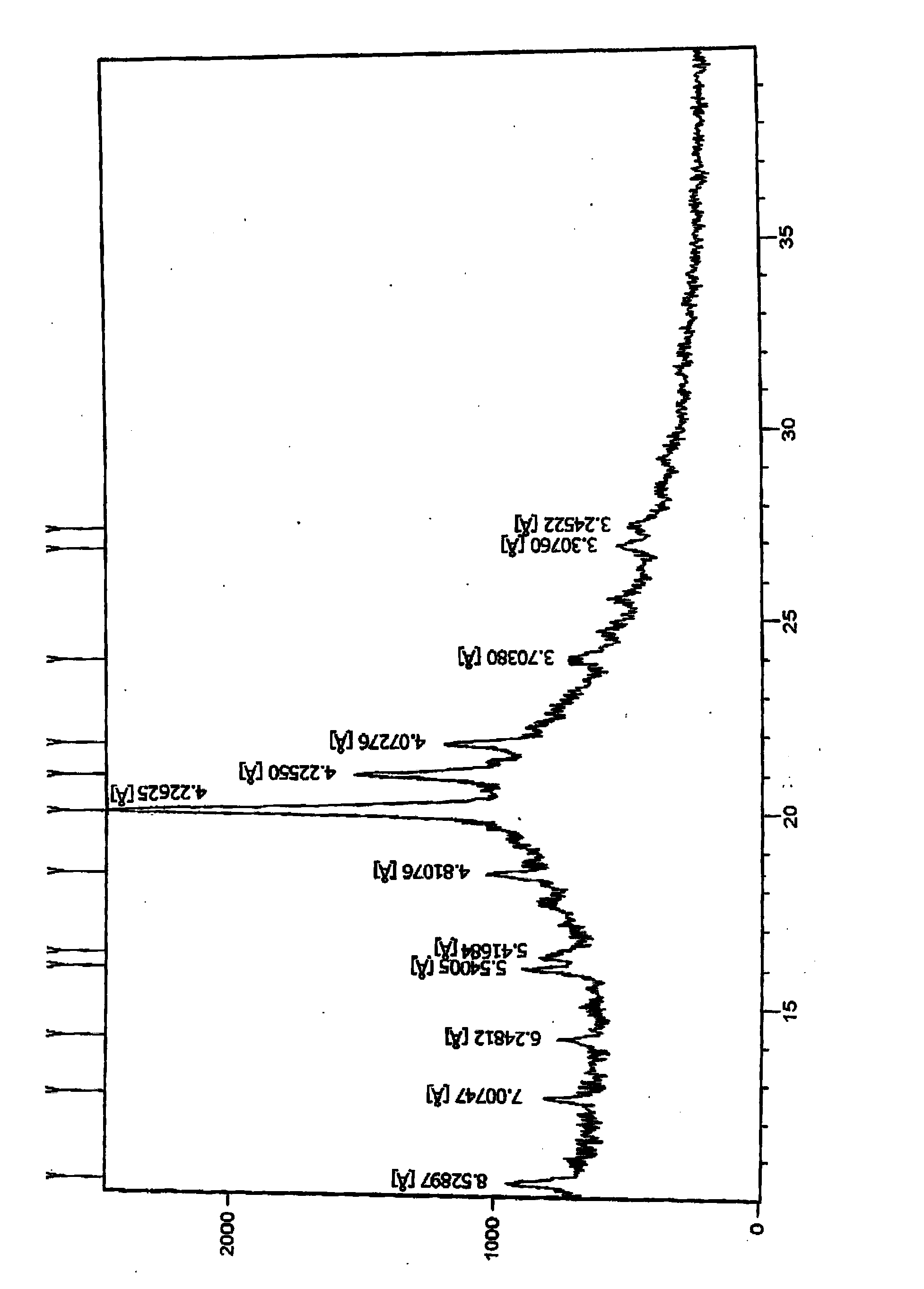
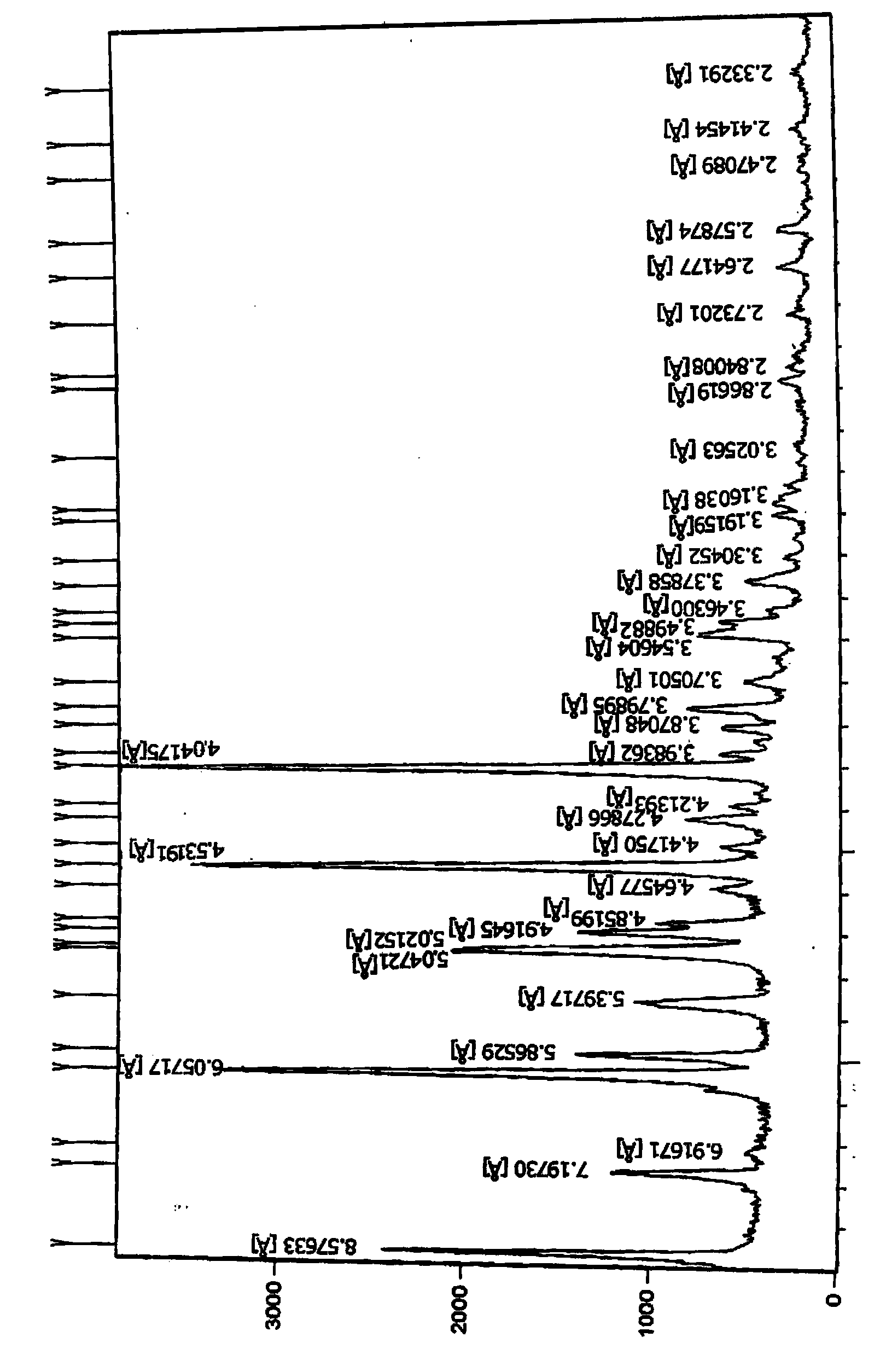
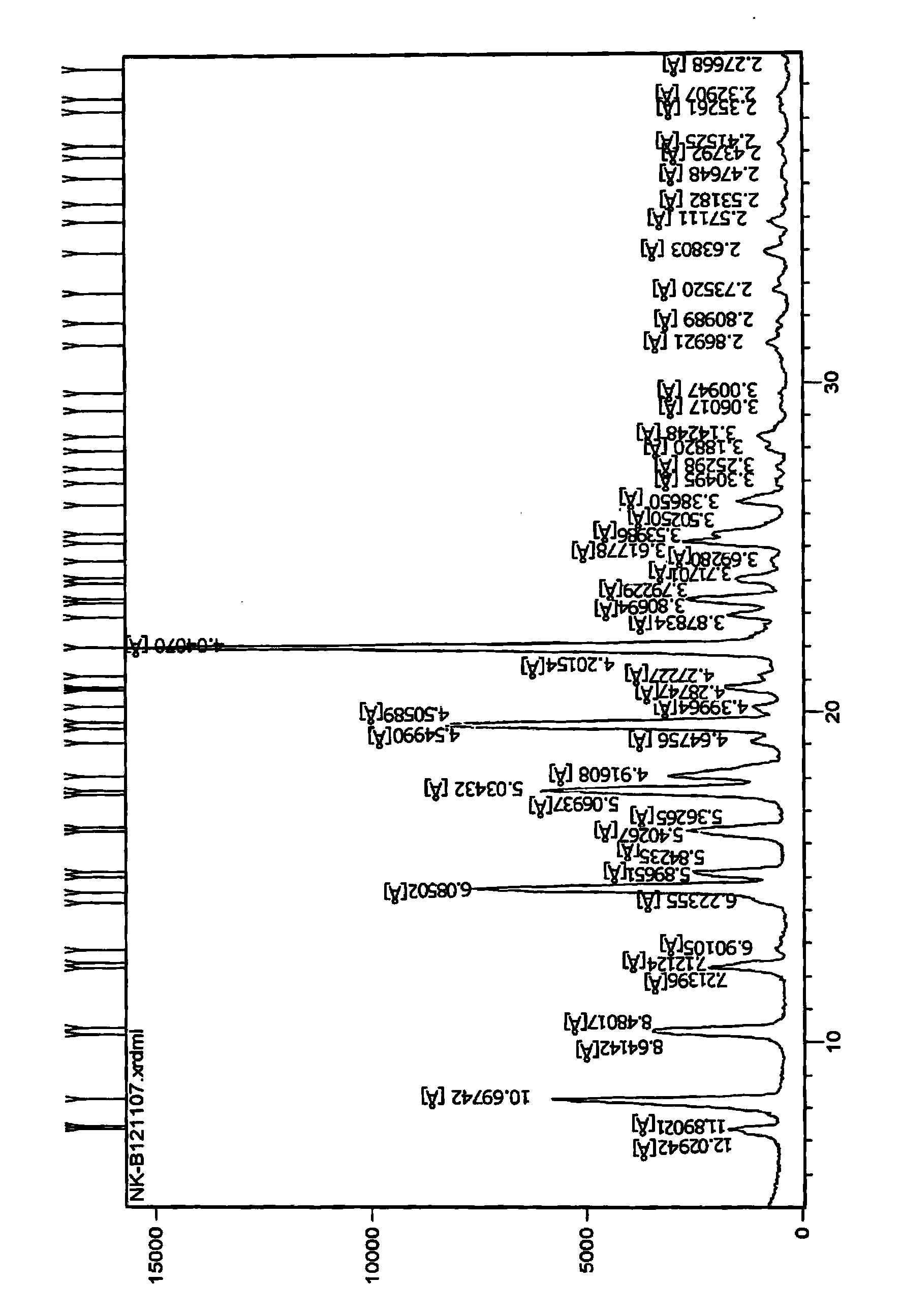
[0130] 10g of pitavastatin ethyl ester (1.0% diastereoisomer 0.7% enantiomer) was dissolved in 25mL methanol and water mixture (the volume ratio of methanol to water was 2:1) and then cooled to room temperature (40°C), and then put it in the refrigerator (-10°C) to freeze and crystallize, precipitate the crystals and filter, and dry the filter cake at 50°C under reduced pressure to obtain 8.3 g of pitavastatin ethyl ester, of which enantiomer The isomeric level was 0.50%, and the diastereomeric level was 0.27%. The resulting (-)-(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-2-yl]-3,5-dihydroxy-6(E)- X-ray Powder Diffraction of Ethyl Heptenoate figure 2 There are peaks at positions 10.3528, 12...
Embodiment 3
[0131] Example 3 (-)-(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-2-yl]-3,5-dihydroxy-6(E) -Ethyl heptenoate crystallized from a mixture of methanol and water (5:3)
[0132] The detection method of diastereoisomers and enantiomers is the same as in Example 1.
[0133] 10g of pitavastatin ethyl ester (1.0% diastereoisomer 0.7% enantiomer) was dissolved in 25mL methanol and water mixture (the volume ratio of methanol to water was 5:3) and then cooled to room temperature (20°C), then put it in the refrigerator (-20°C) to freeze and crystallize, precipitate the crystals and filter, and dry the filter cake at 40°C under reduced pressure to obtain 8.4 g of pitavastatin ethyl ester, of which enantiomer The isomeric level was 0.48%, and the diastereomeric level was 0.20%.
[0134] The resulting (-)-(3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-2-yl]-3,5-dihydroxy-6(E)- X-ray Powder Diffraction of Ethyl Heptenoate figure 2 There is a peak at the θ position, and the 2θ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com