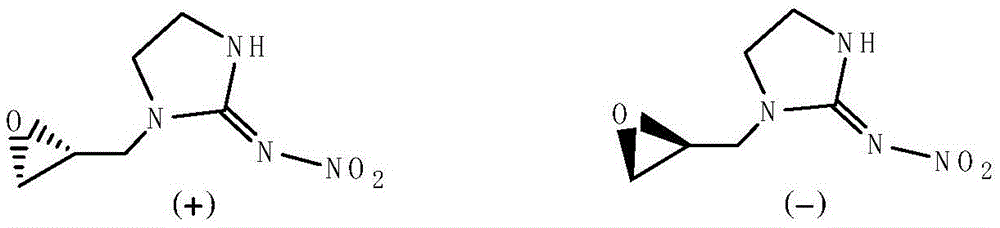Preparation method for cycloxylidin enantiomer with photoactivity
A technology of enantiomers and epoxy morphine, which is applied in the field of asymmetric catalytic synthesis of optically active drugs, to achieve the effects of reducing pollution, avoiding self-polymerization, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
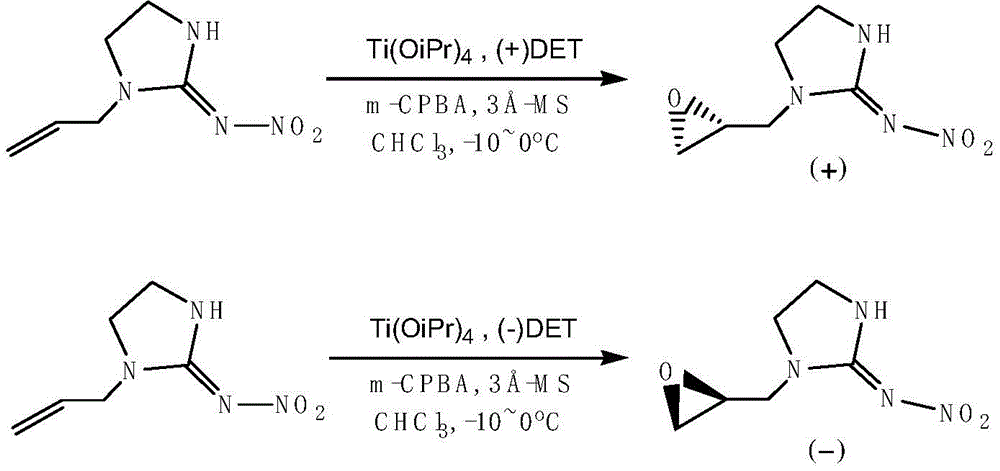
[0023] Example 1: The specific preparation method of the enantiomer of 1-(1,2-epoxypropyl)-N-nitroimidazolidin-2-ylamine provided by the present invention is as follows:
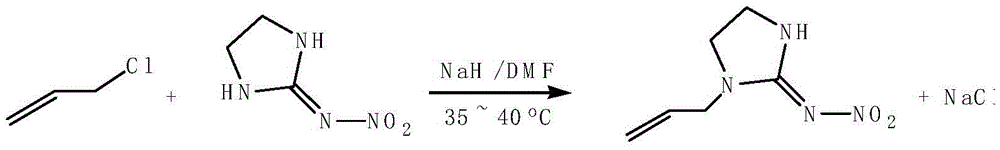
[0024] (1): Preparation of 1-allyl-N-nitroimidazolidin-2-ylamine:
[0025] Under the protection of nitrogen, 6.5g (0.05mol) of N-nitroiminoimidazolidine was added to 50mL of N,N'-dimethylformamide, stirred for 30 minutes to dissolve it completely, and 2.4g Sodium hydride was added in 3-5 times, and the stirring was continued for about 1 h to obtain a homogeneous light yellow solution. Slowly add 4.9mL (0.06mol) of chloropropene into the above solution with a constant pressure dropping funnel, and then slowly raise the temperature to 40°C after the dropwise addition, TLC or HPLC method is used to monitor the reaction process until the reaction is complete. Suction filtration through diatomaceous earth to remove the inorganic salts produced during the reaction to obtain a yellow transparent solution, which wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com