Method for continuously producing prothioconazole by utilizing micro-channel reactor and micro-channel system
A microchannel reactor, prothioconazole technology, applied in chemical instruments and methods, chemical/physical/physical-chemical reactors, organic chemistry, etc., can solve the problem that the ring-closing reaction is difficult to control, unfavorable for industrial production, and the reaction steps are cumbersome. and other problems, to achieve the effect of accurately controlling the reaction temperature and reaction time, reducing the production of side reactions and three wastes, and increasing the efficiency of mass transfer and heat transfer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of compound (Ⅰ)
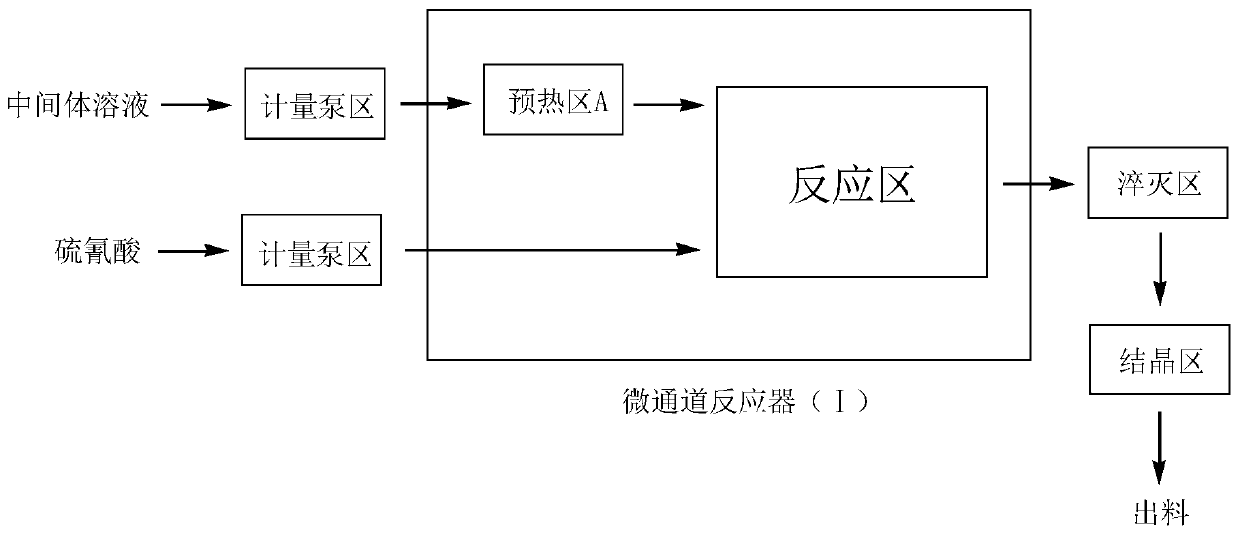
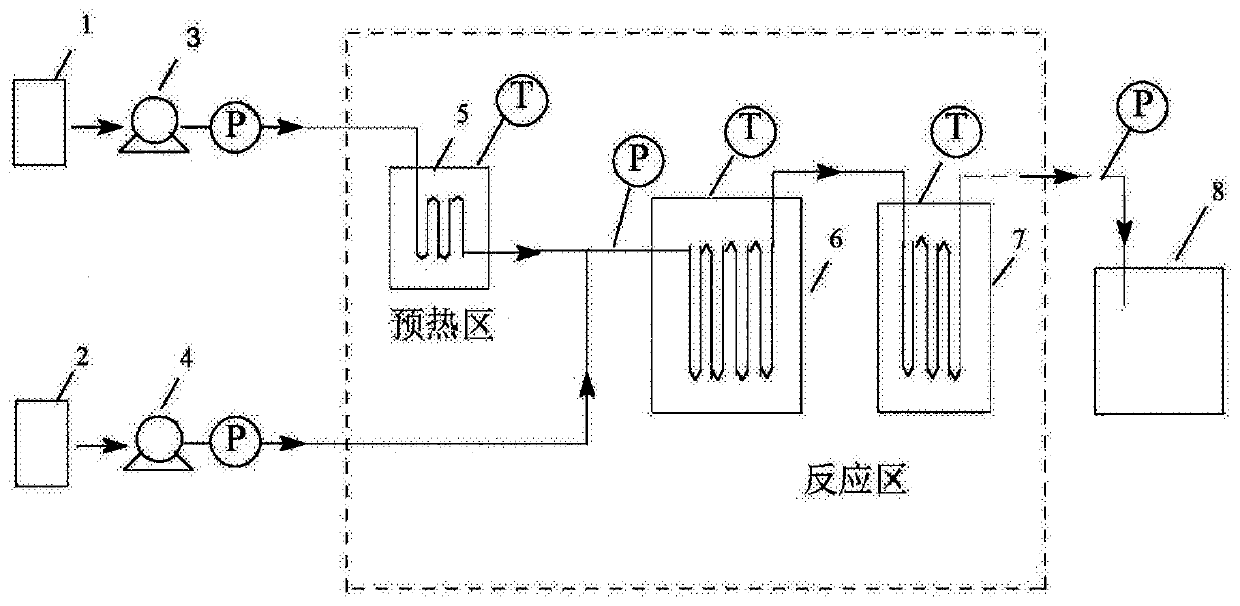
[0040] (1) Device: continuous flow microchannel reaction device (3a+3a), refer to image 3 Determine the connection mode of the microchannel reactor, the length of the microchannel is determined according to the flow rate and the reaction residence time, and the heat transfer medium is heat transfer oil.
[0041] (2) With the microchannel system as the reactor, at 0°C, 41g (0.149mol) 2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-hydrazinopropane-2 -alcohol, 100ml toluene, 0.4g (0.0074mol) sodium methylate catalyst and 15g 36% (0.18mol) formaldehyde aqueous solution are mixed, form reaction intermediate solution under constant stirring, pass in the microchannel reactor (Ⅰ) respectively through metering pump and set the preheating temperature and reaction temperature to be 35°C, barium thiocyanate and sulfuric acid are mixed in 500g water at a molar equivalent ratio of 1:1 at 0°C, a precipitate is formed under constant stirring, and the precipi...
Embodiment 2
[0046] Synthesis of compound (Ⅰ)
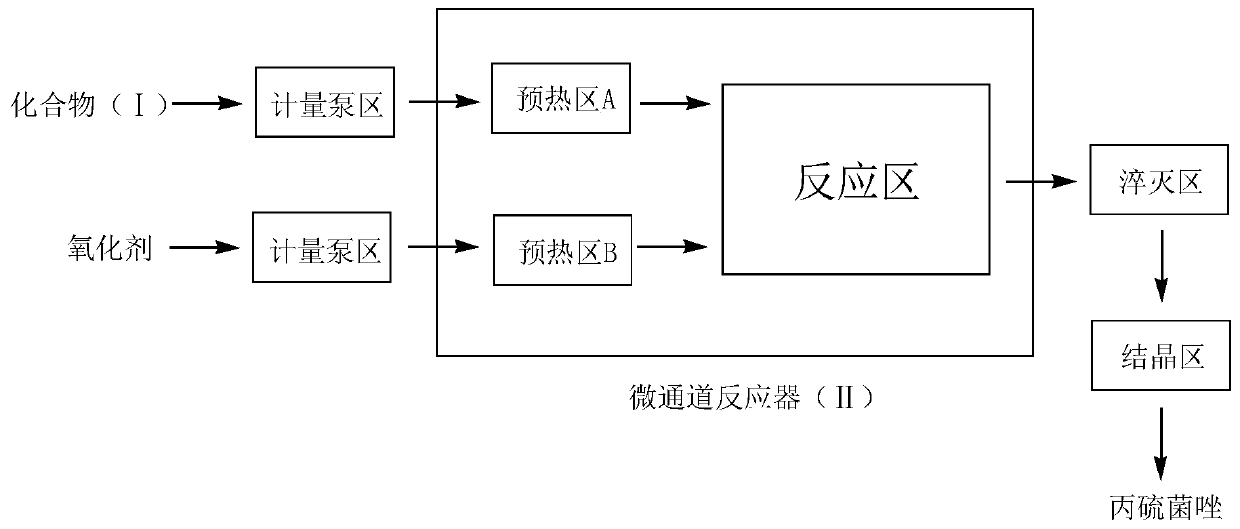
[0047] (1) Device: continuous flow microchannel reaction device (3a+3a), refer to image 3 Determine the connection mode of the microchannel reactor, the length of the microchannel is determined according to the flow rate and the reaction residence time, and the heat transfer medium is heat transfer oil.
[0048] (2) With the microchannel system as the reactor, at 10°C, 41g (0.149mol) 2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-hydrazinopropane-2 -Alcohol, 100ml dichloromethane, 0.3g (0.0074mol) sodium hydroxide catalyst mixes with 12.43g36% (0.149mol) formaldehyde aqueous solution, forms reaction intermediate solution under constant stirring, passes into microchannel reactor ( In Ⅰ), set the preheating temperature and reaction temperature to 35°C, mix potassium thiocyanate and 37% hydrochloric acid aqueous solution in 500g water at a molar equivalent ratio of 1:1 at 0°C, and generate Precipitate, filter the precipitate to obtain thiocyani...
Embodiment 3
[0053] Synthesis of compound (Ⅰ)
[0054] (1) Device: continuous flow microchannel reaction device (3a+3a), refer to image 3 Determine the connection mode of the microchannel reactor, the length of the microchannel is determined according to the flow rate and the reaction residence time, and the heat transfer medium is heat transfer oil.
[0055] (2) With the microchannel system as the reactor, at 0°C, 41g (0.149mol) 2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-hydrazinopropane-2 Alcohol, 100ml dimethylbenzene, 0.71g (0.0074mol) sodium tert-butoxide catalyst mixes with 15g 36% (0.18mol) formaldehyde aqueous solution, forms reaction intermediate solution under constant stirring, passes into microchannel reactor ( In Ⅰ), set the preheating temperature and reaction temperature to 35°C, mix ammonium thiocyanate and sulfuric acid in 500g water at a molar equivalent ratio of 1:1 at 0°C, and form a precipitate under constant stirring, filter Precipitation obtains thiocyanic acid s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com