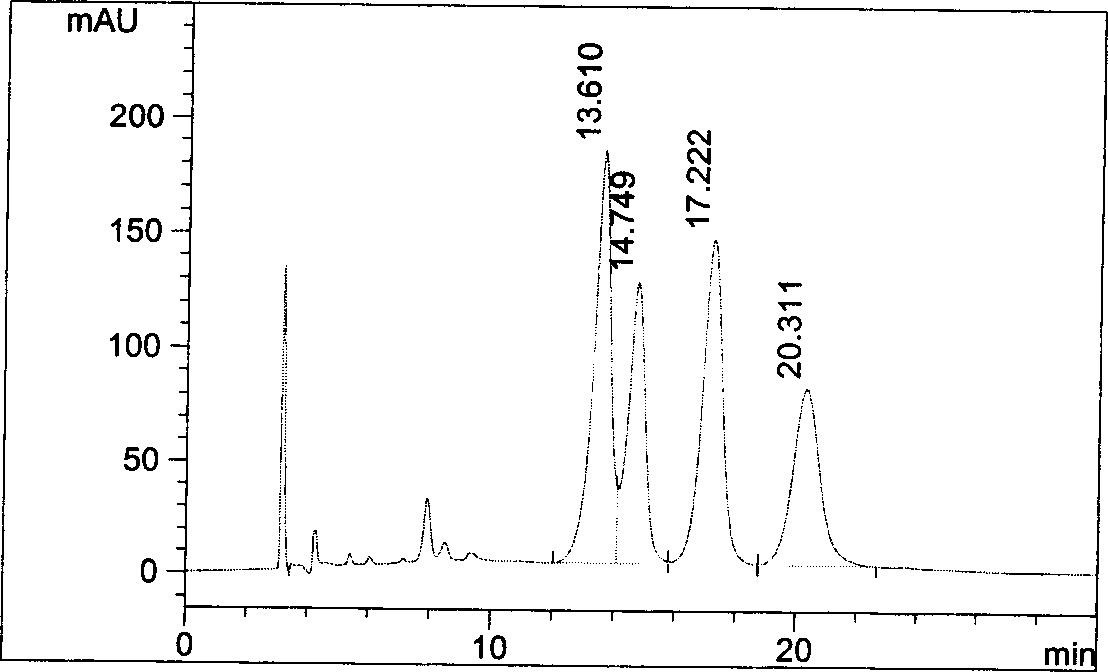
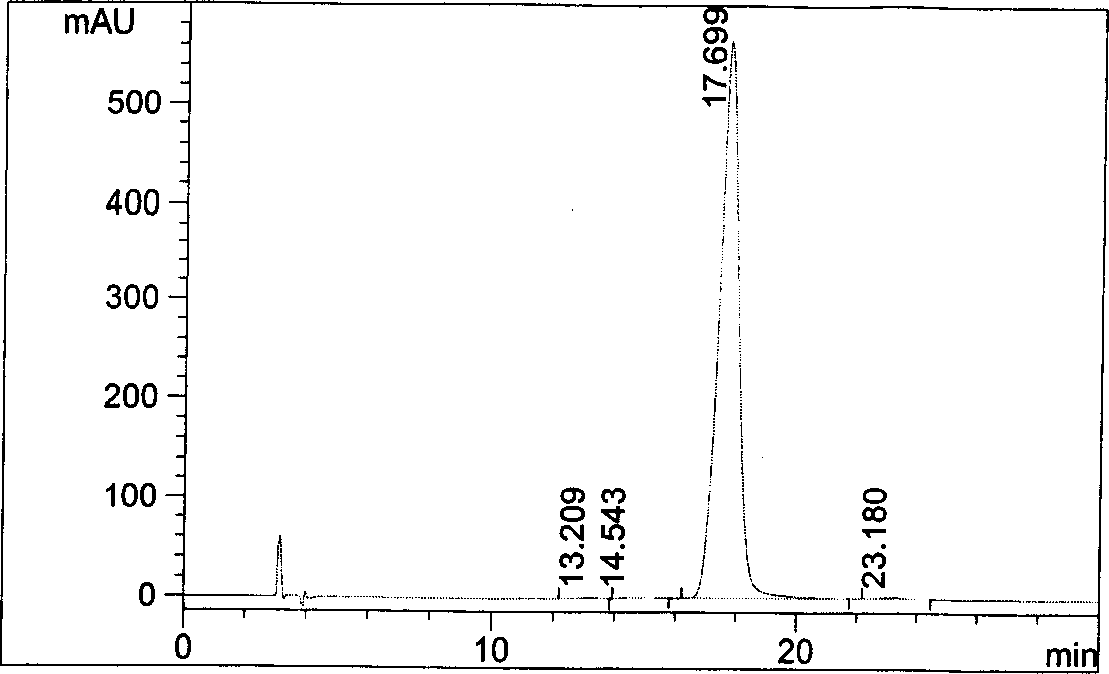
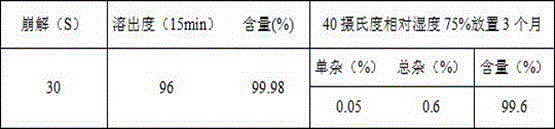
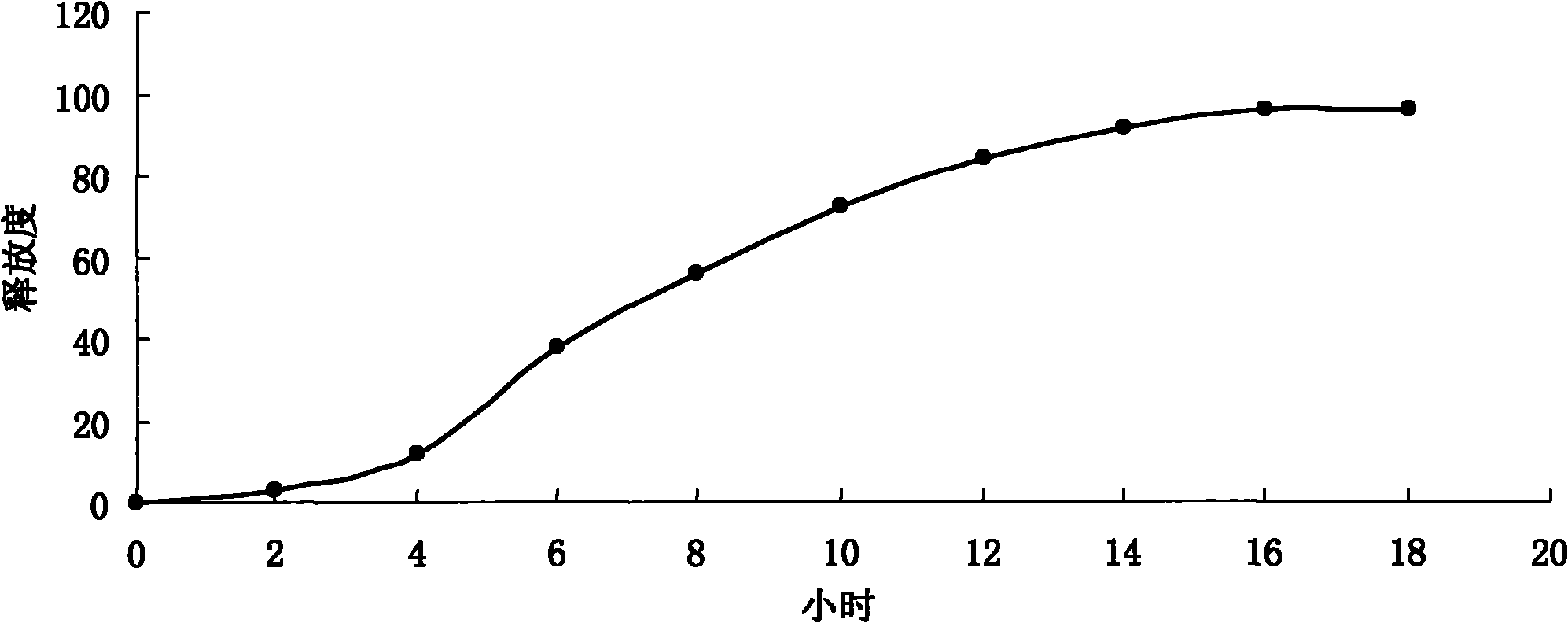
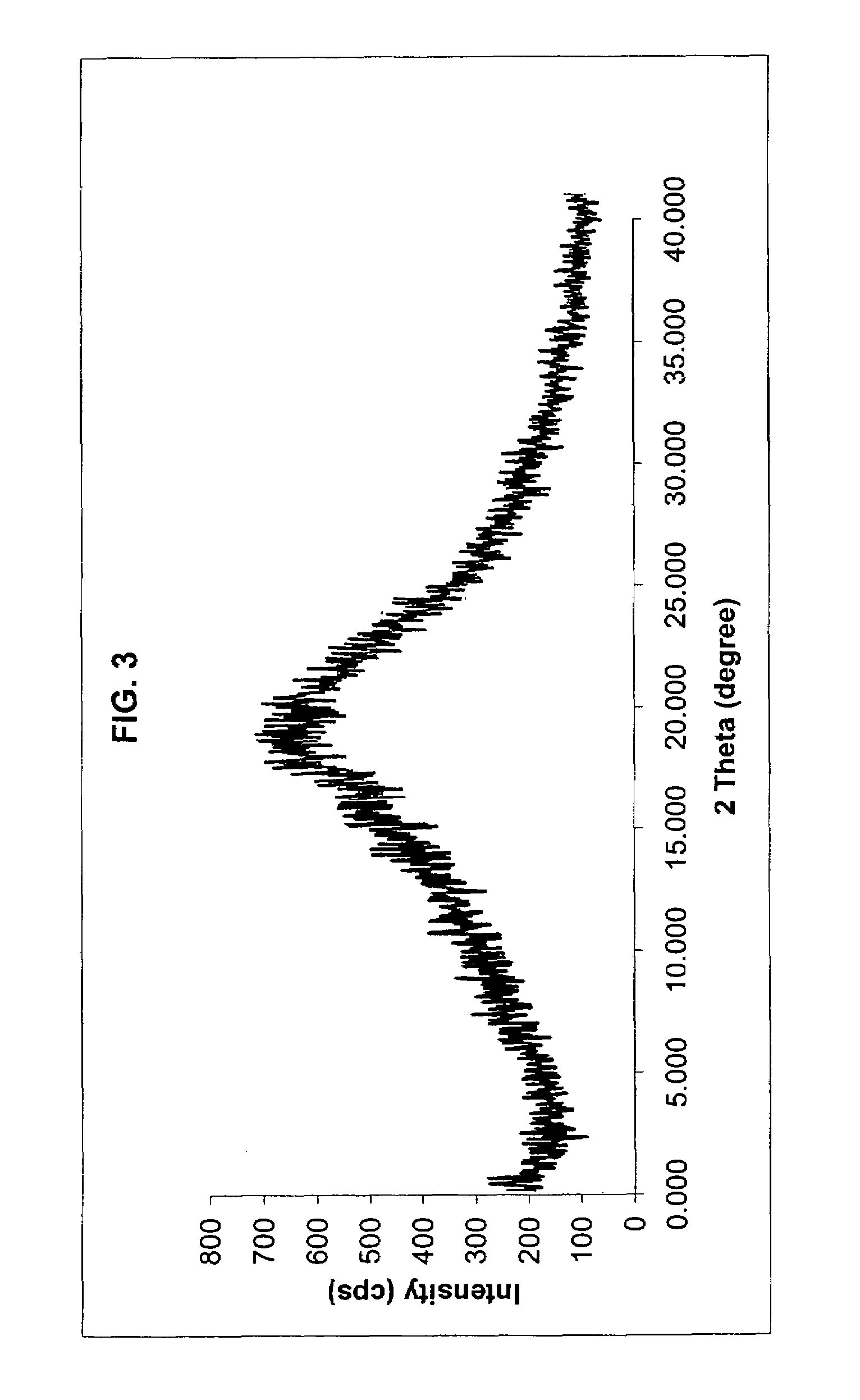
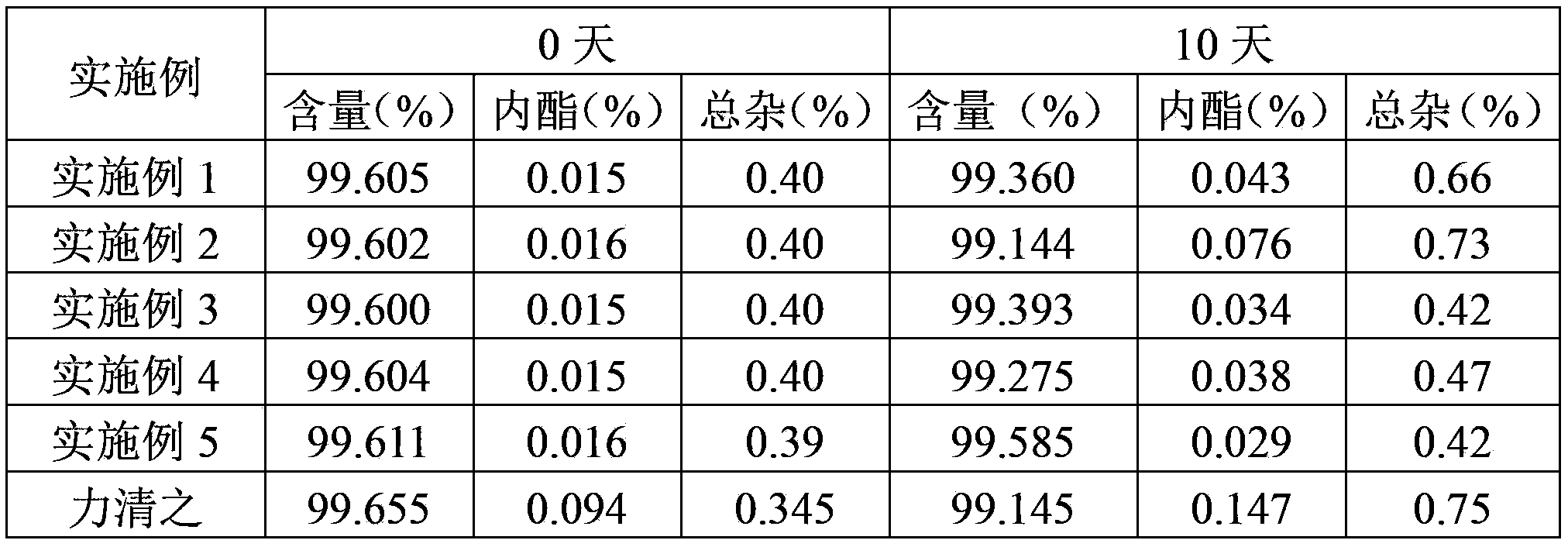
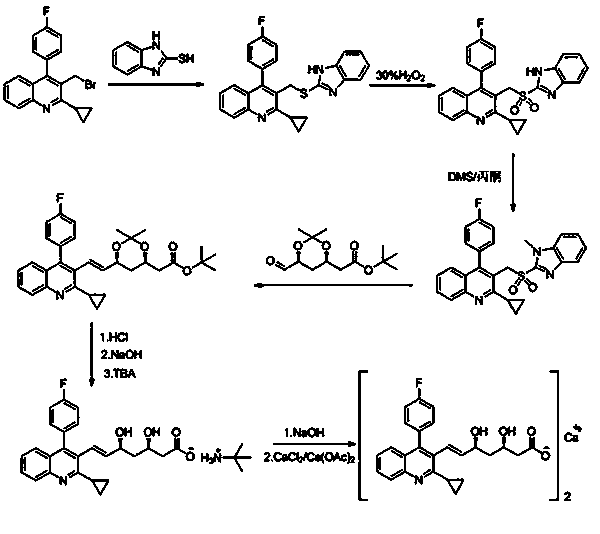
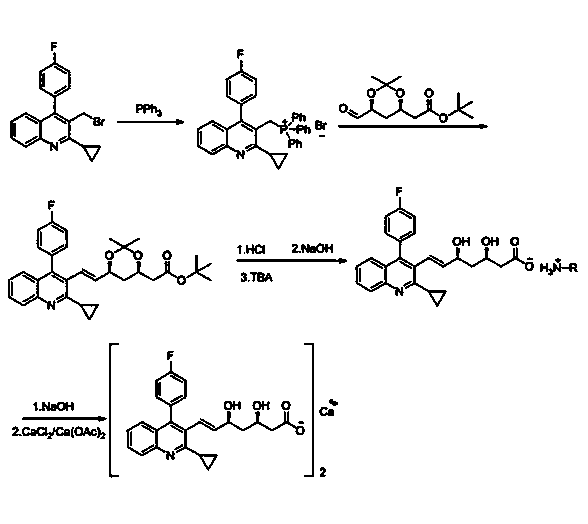
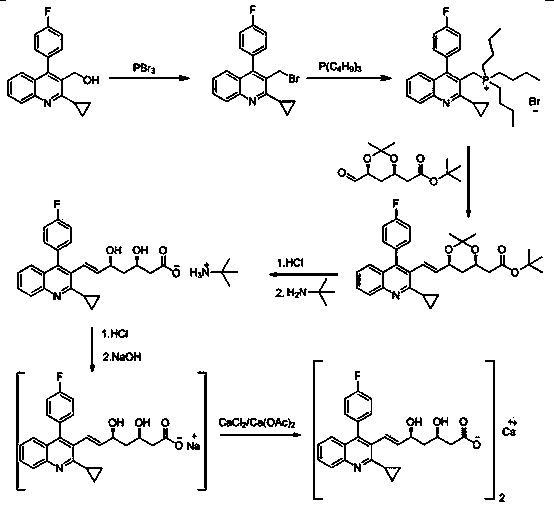
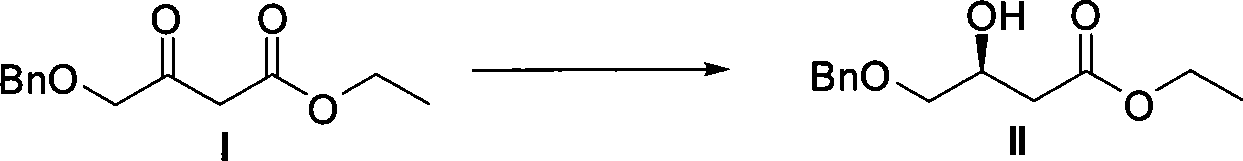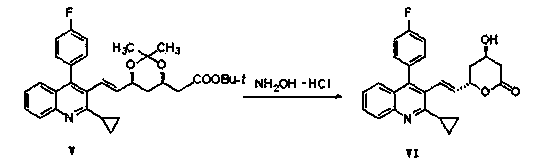Patents
Literature
122 results about "Pitavastatin calcium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
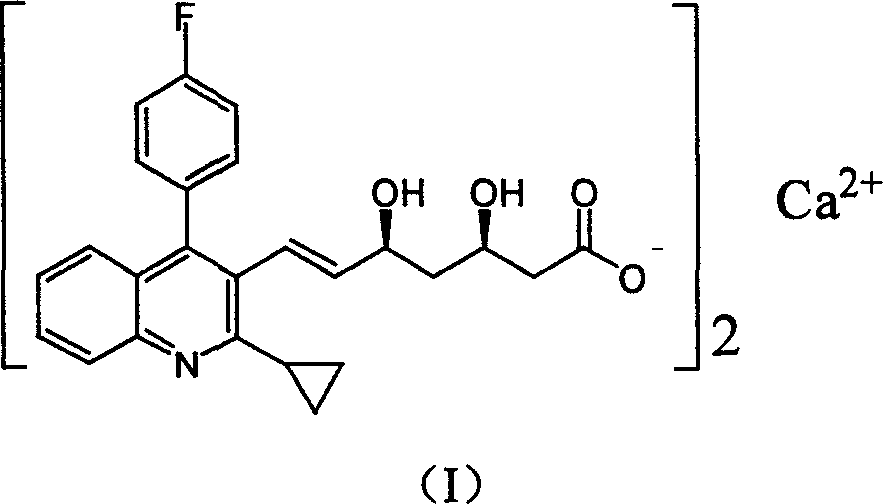
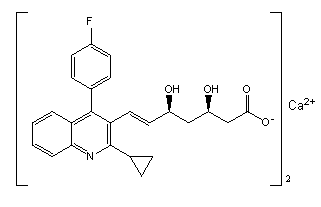
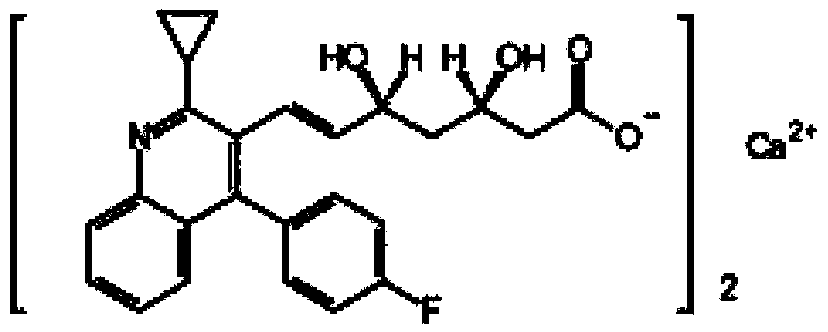
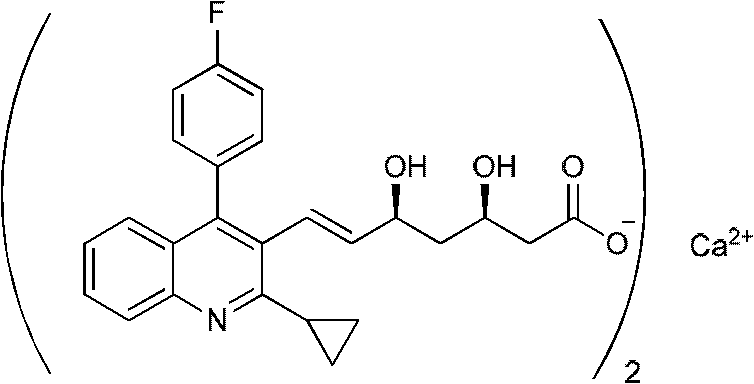
Pitavastatin (usually as a calcium salt) is a member of the blood cholesterol lowering medication class of statins, marketed in the United States under the trade name Livalo, and in European Union and Russia under the trade name Livazo.
Hyperlipemia therapeutic agent
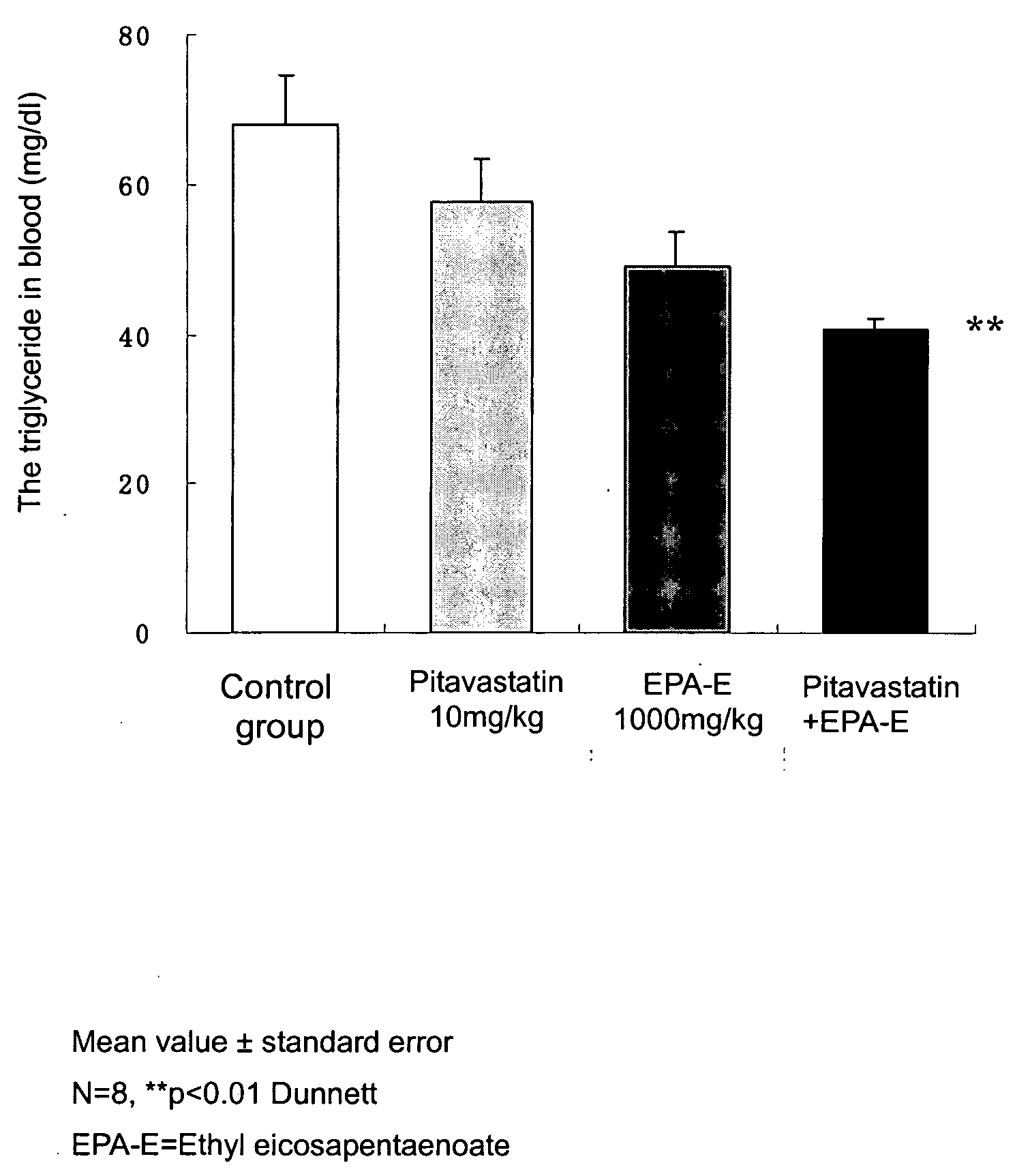
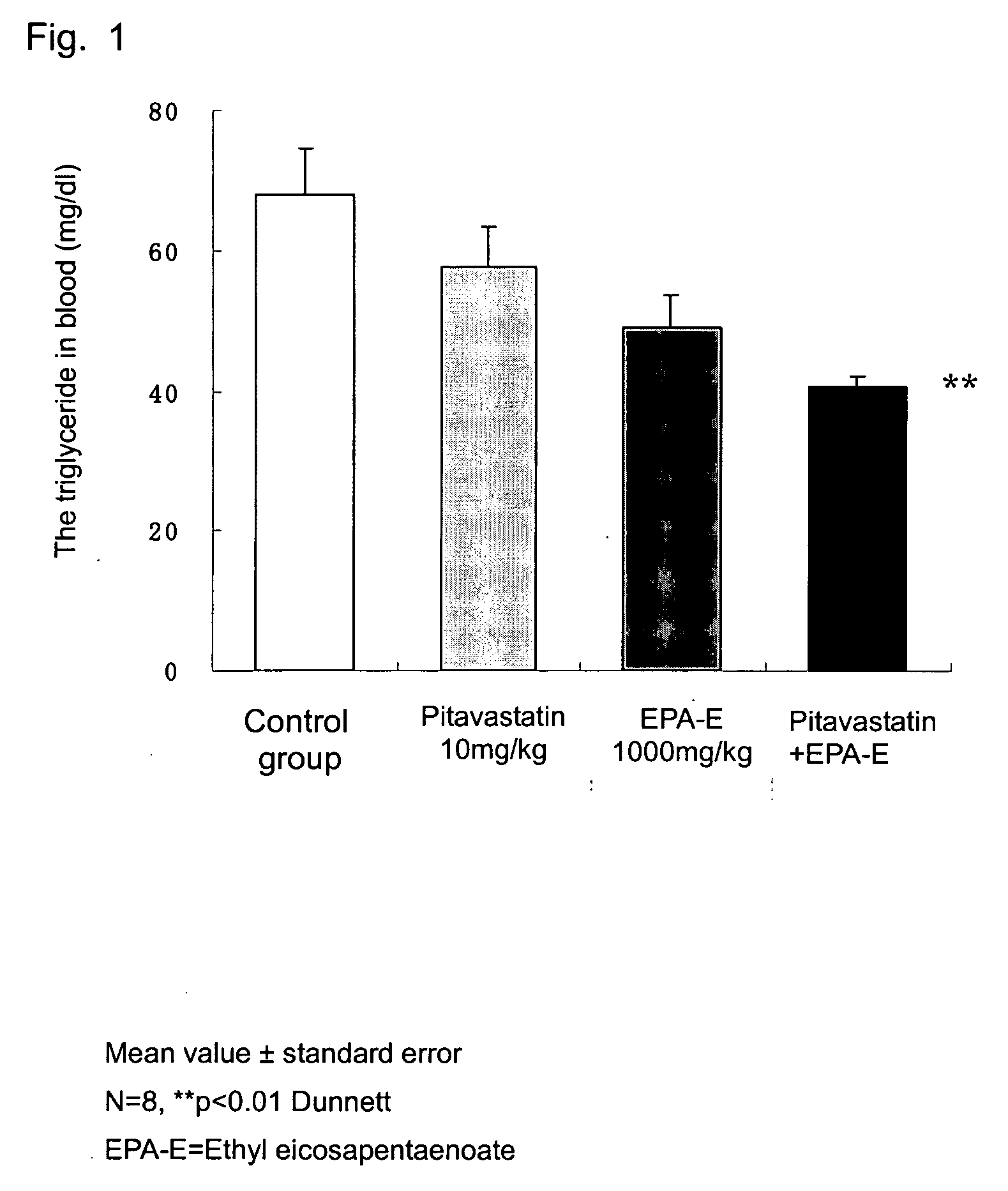
The present invention relates to a hyperlipemia therapeutic agent comprising pitavastatins and eicosapentaenoic acid or an ester derivative thereof as effective ingredients. According to the present invention, a type IIb and type IV hyperlipemia therapeutic agent having an excellent effect of lowering the cholesterol and triglyceride in blood is provided.
Owner:KOWA CO LTD +1
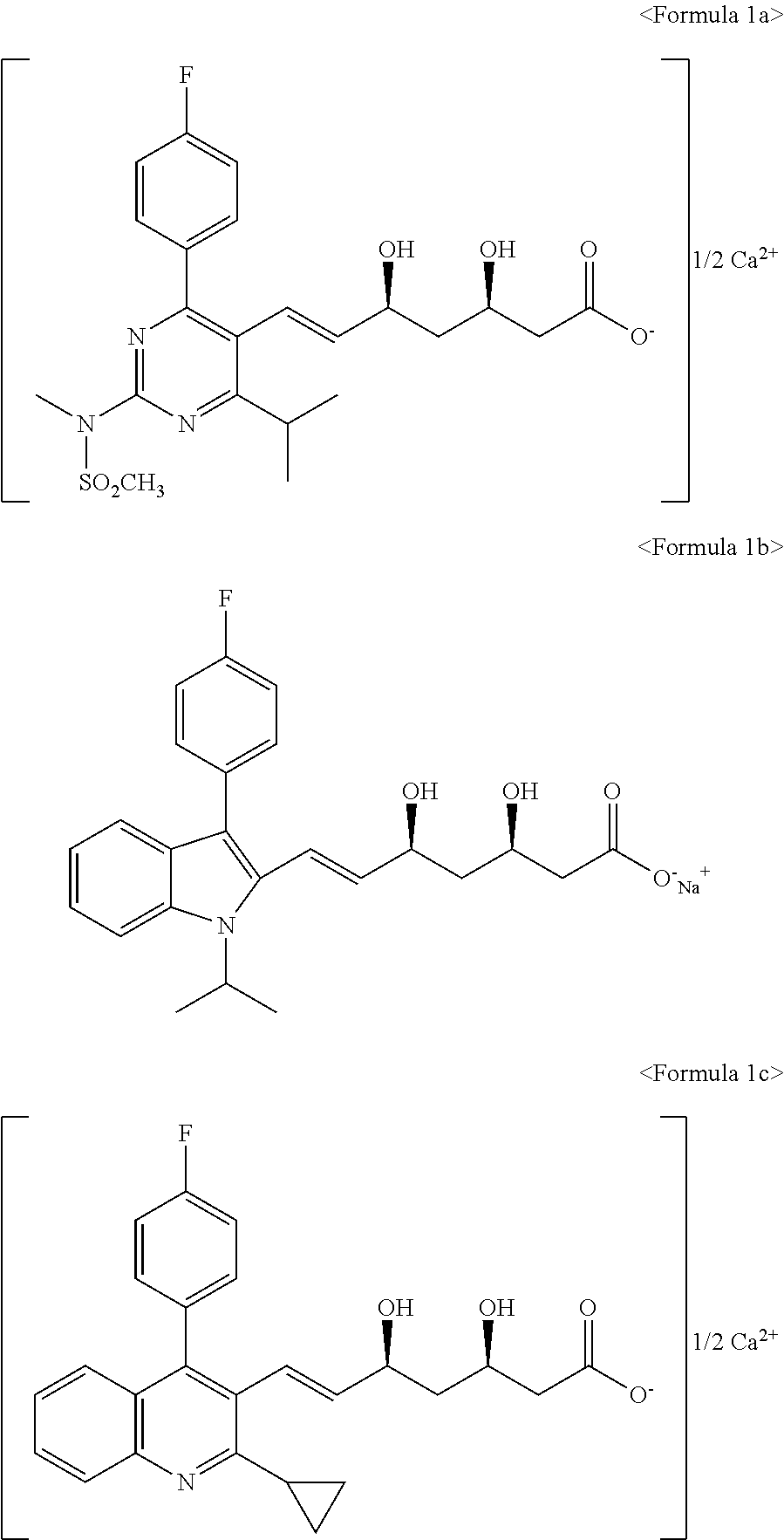
Novel anhydrous amorphous forms of rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium
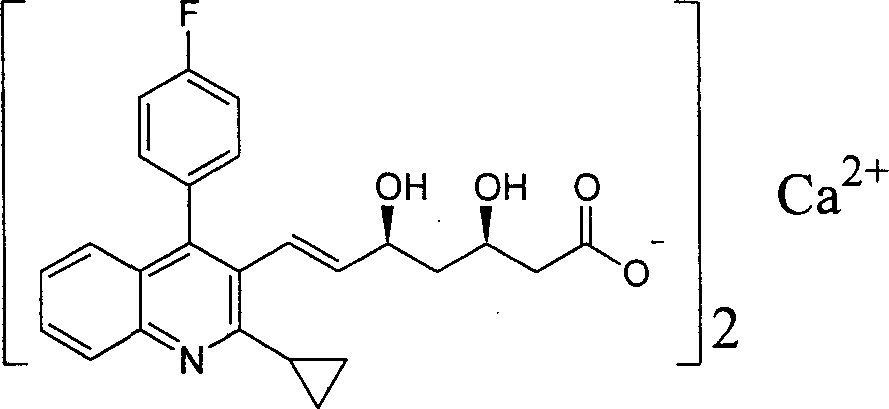
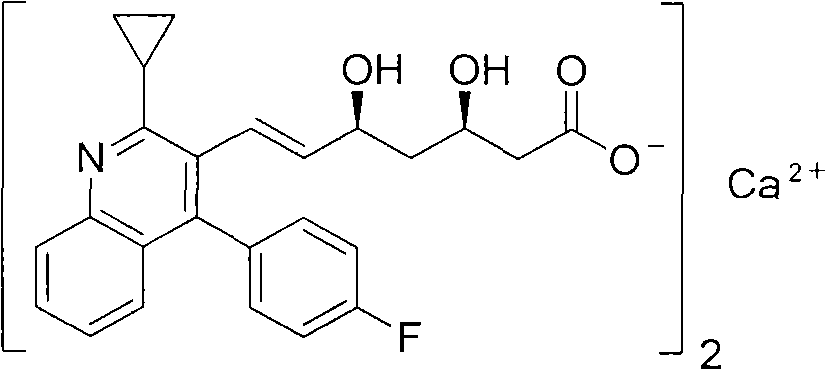
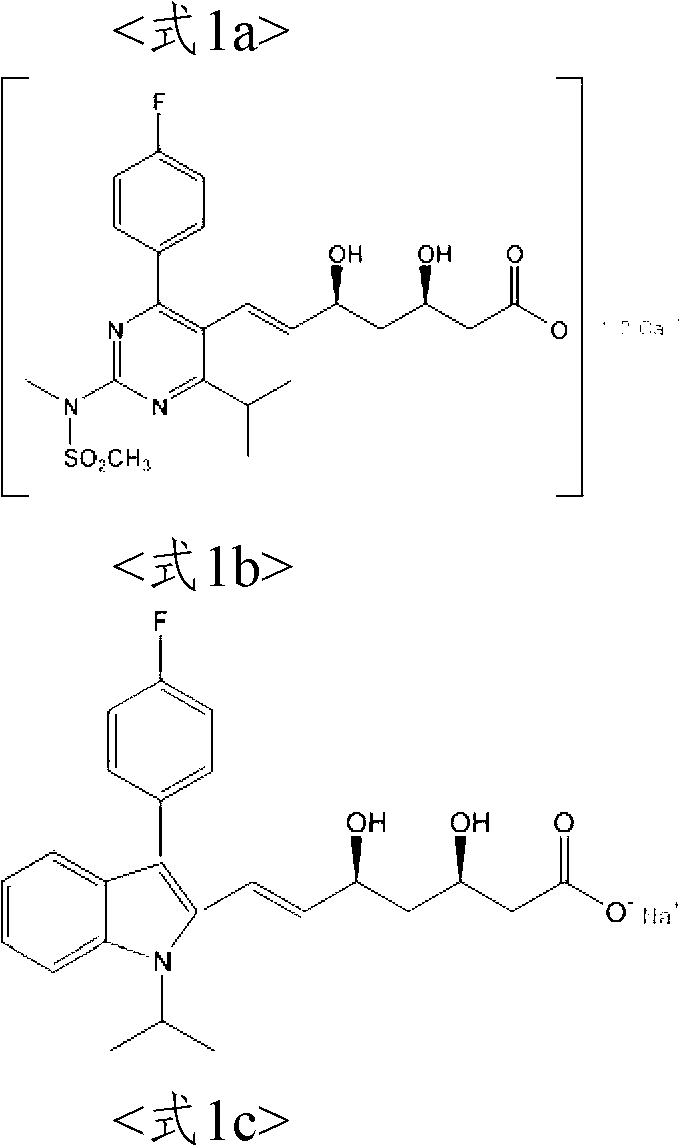
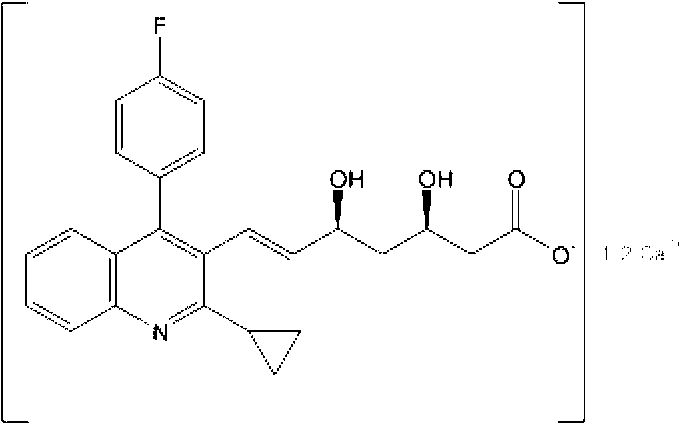
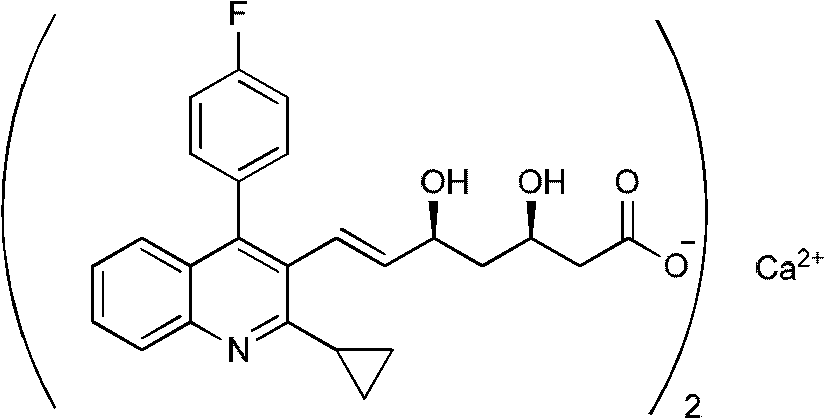
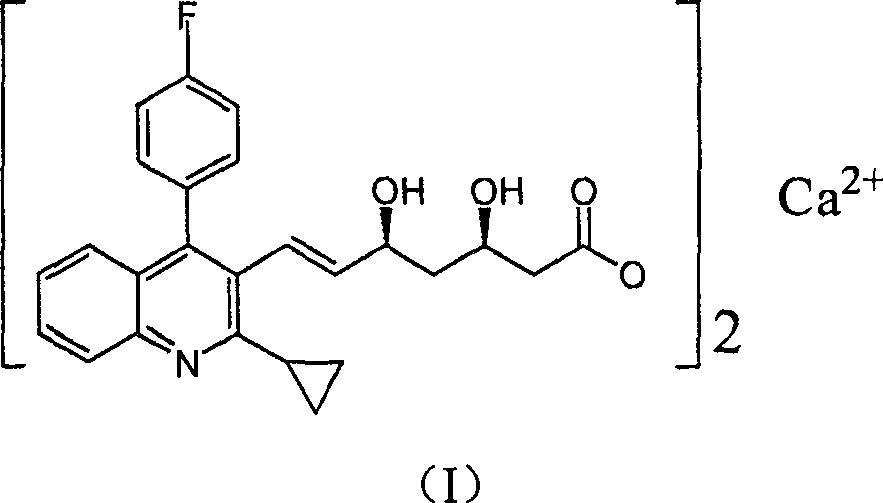
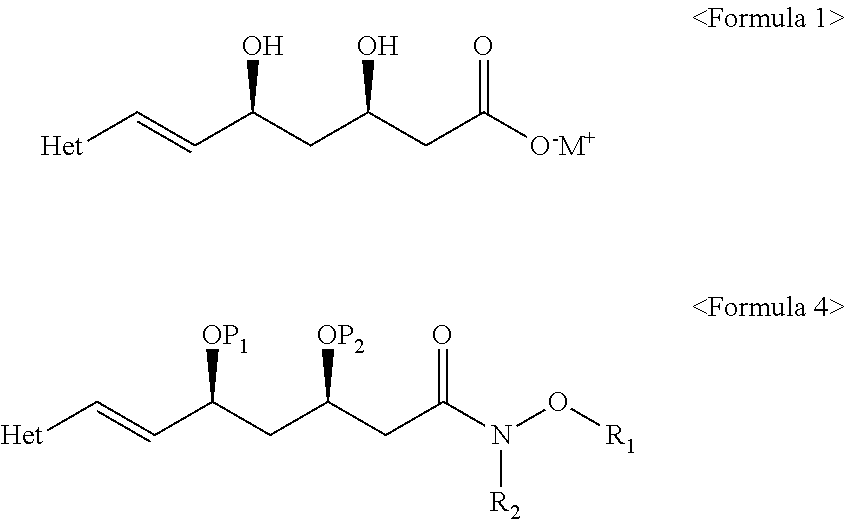
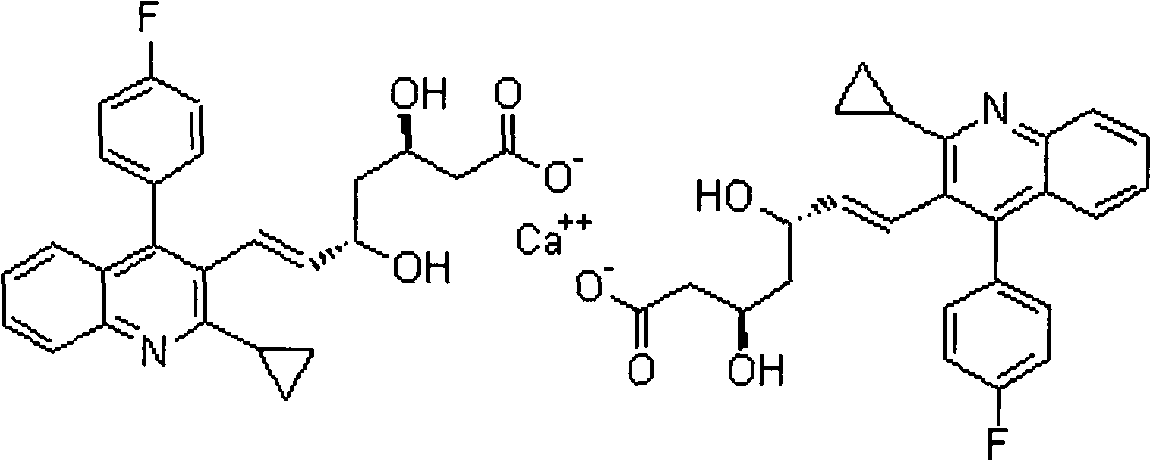
The present invention relates to novel anhydrous amorphous forms of bis[(E)[4-(4-fluorophenyl)isopropyl[methyl(methylsulfonyl)amino]pyrimidinyl](3R,5S)-3,5-dihydroxyhept enoic acid]calcium salt (rosuvastatin calcium), (±)7-(3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl)3,5-dihydroxy heptenoic acid monosodium salt (fluvastatin sodium) and bis[(E)-3,5-dihydroxy-7-[4′-(4″-fluorophenyl)-2′-cyclopropyl-quinolin-3′-hept-6-enoic acid]calcium salt (pitavastatin calcium), to processes for their preparation, to pharmaceutical compositions containing them and to methods of treatment using the same. The rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium obtained are known valuable agents useful in treating hyperlipidemia and hypercholestrolemia.
Owner:MAI DE
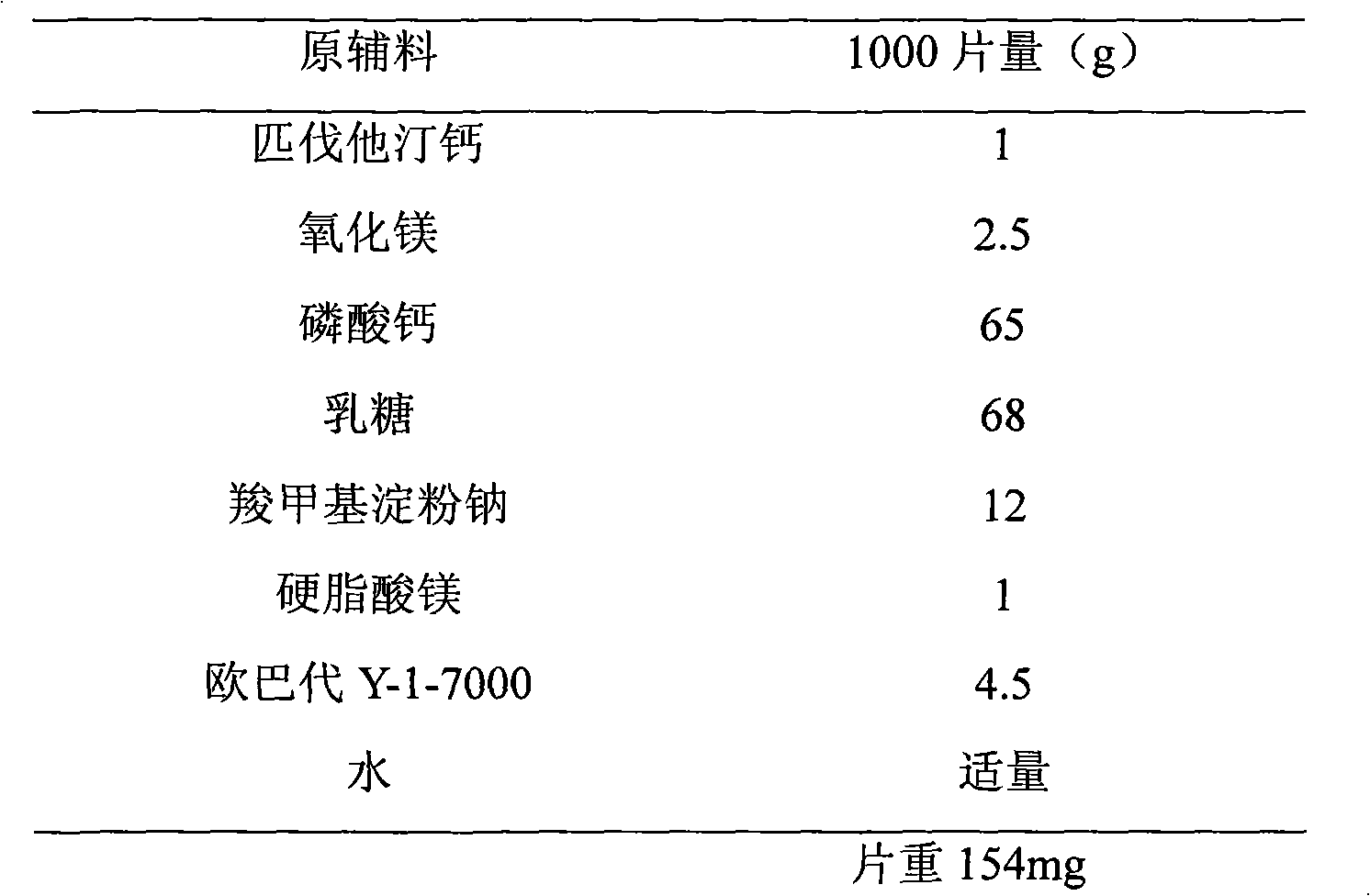
Stable pharmaceutical composition containing pitavastatin calcium and preparation process thereof
InactiveCN1969849AAvoid the effects of pitavastatin calciumAvoid influenceMetabolism disorderPharmaceutical non-active ingredientsChemistryPitavastatin calcium
Owner:上海药明康德新药开发有限公司
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
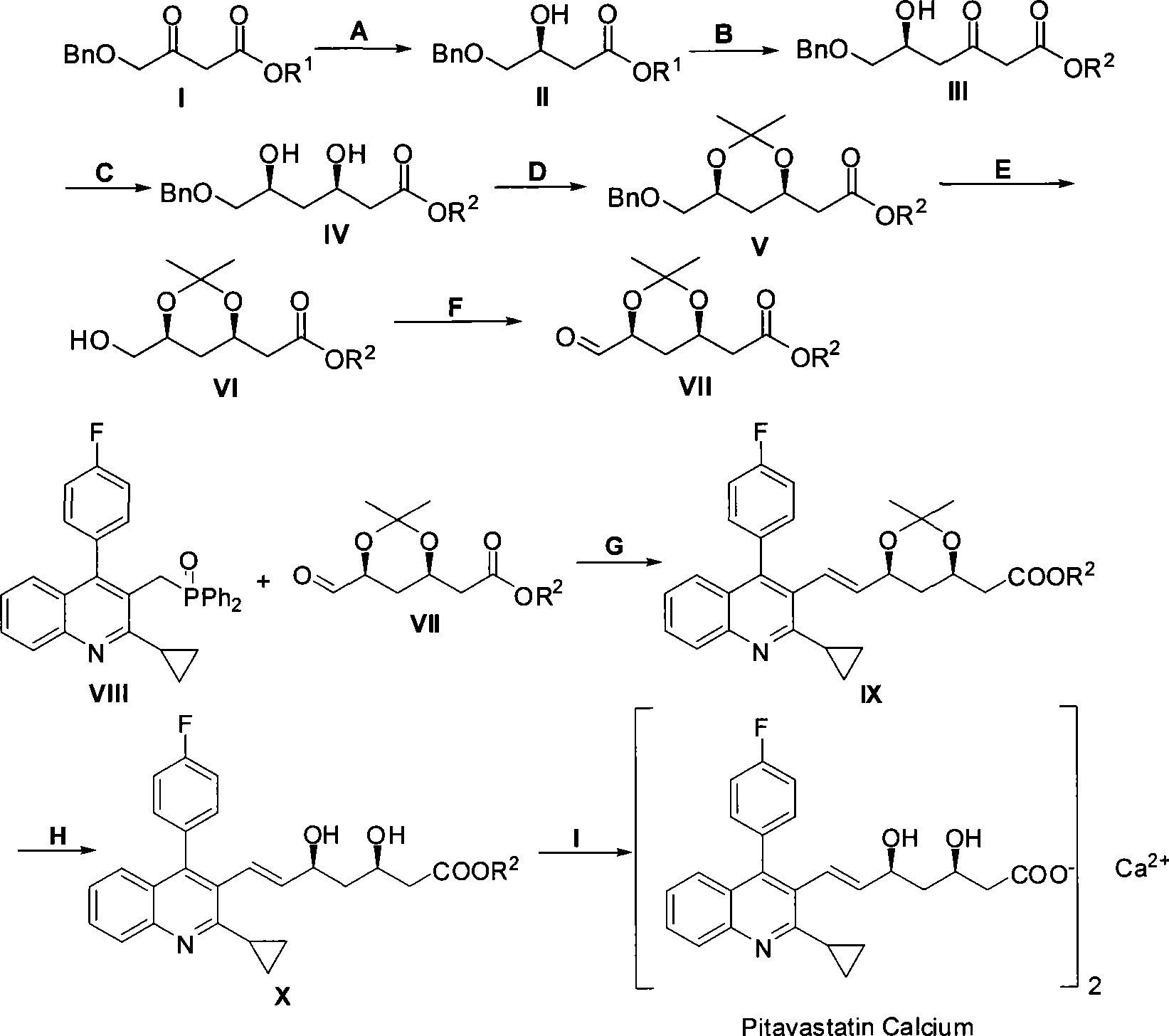
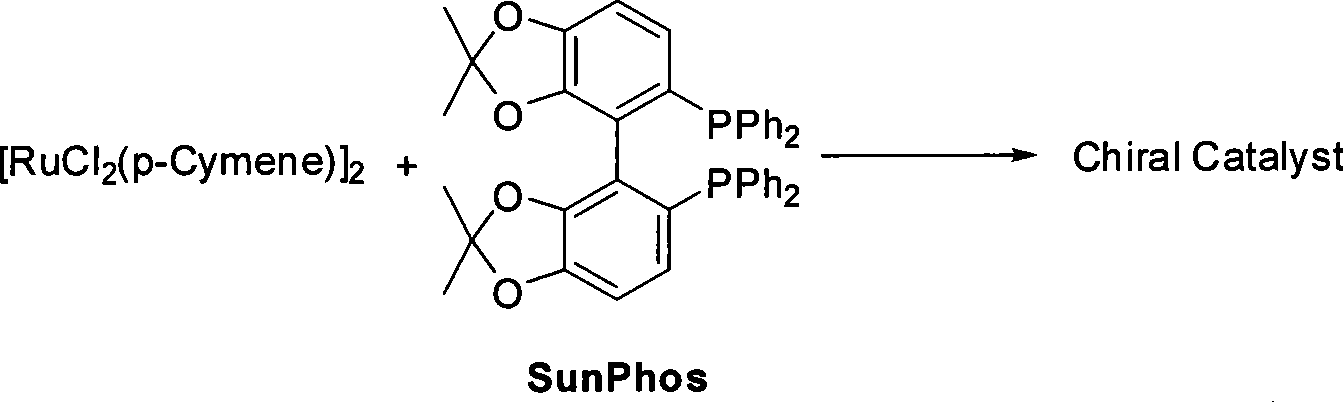
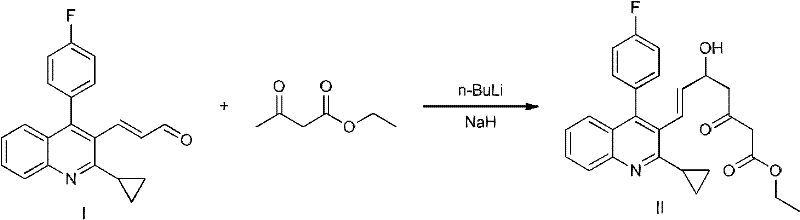
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
Method for preparing high optical purity pitavastatin calcium raw material drug
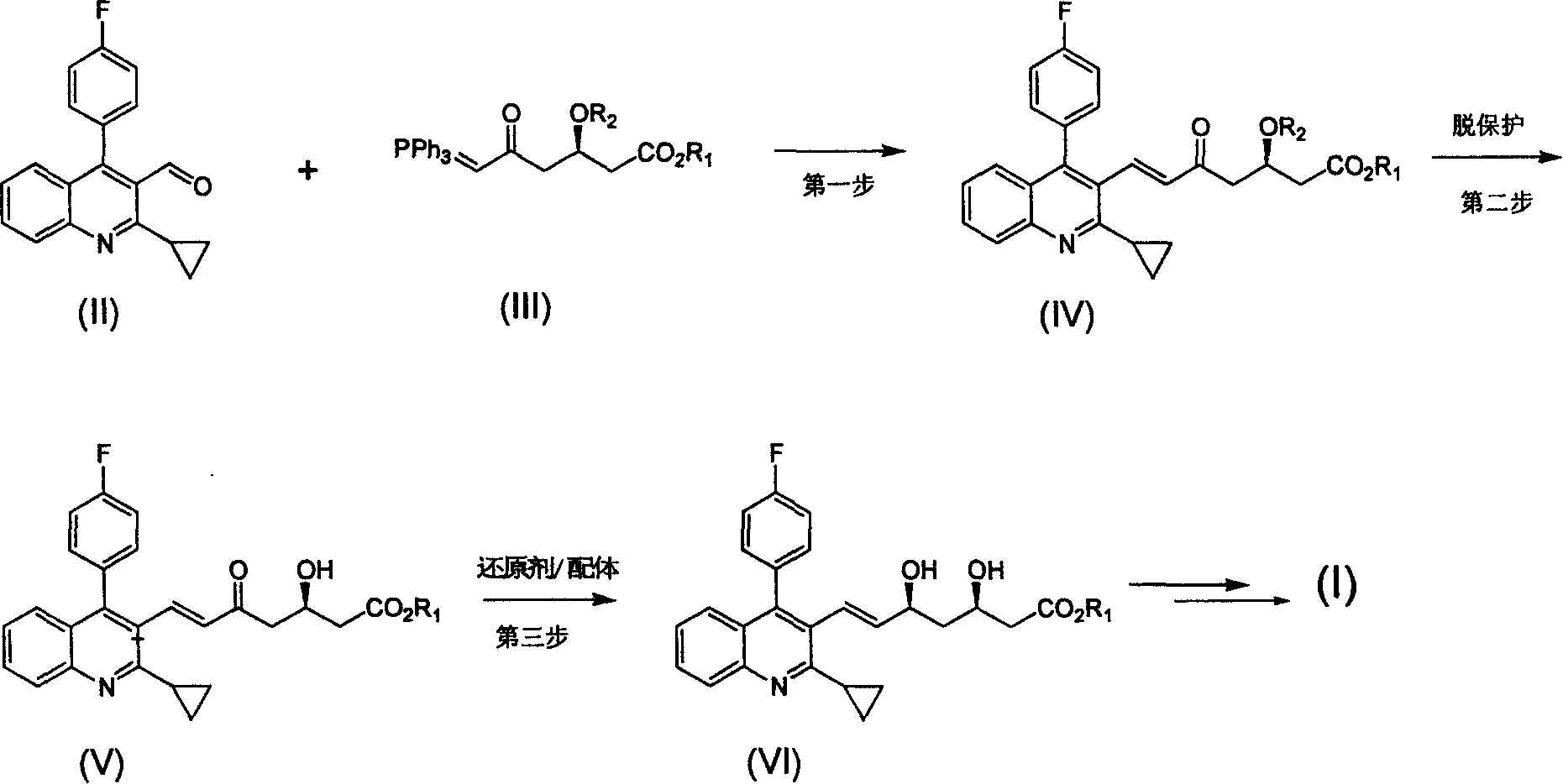
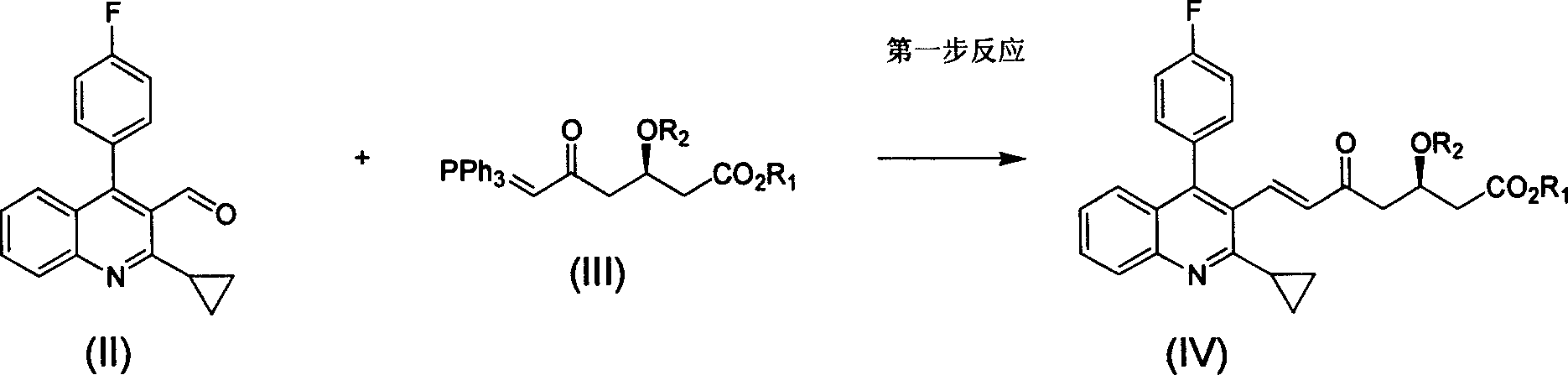
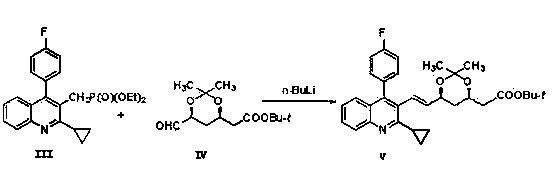
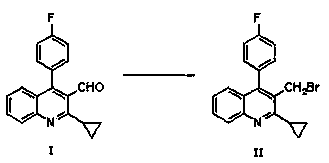
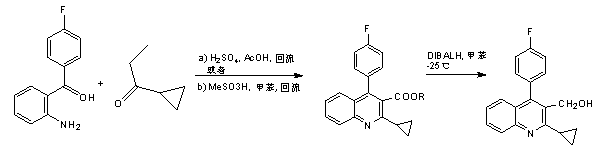
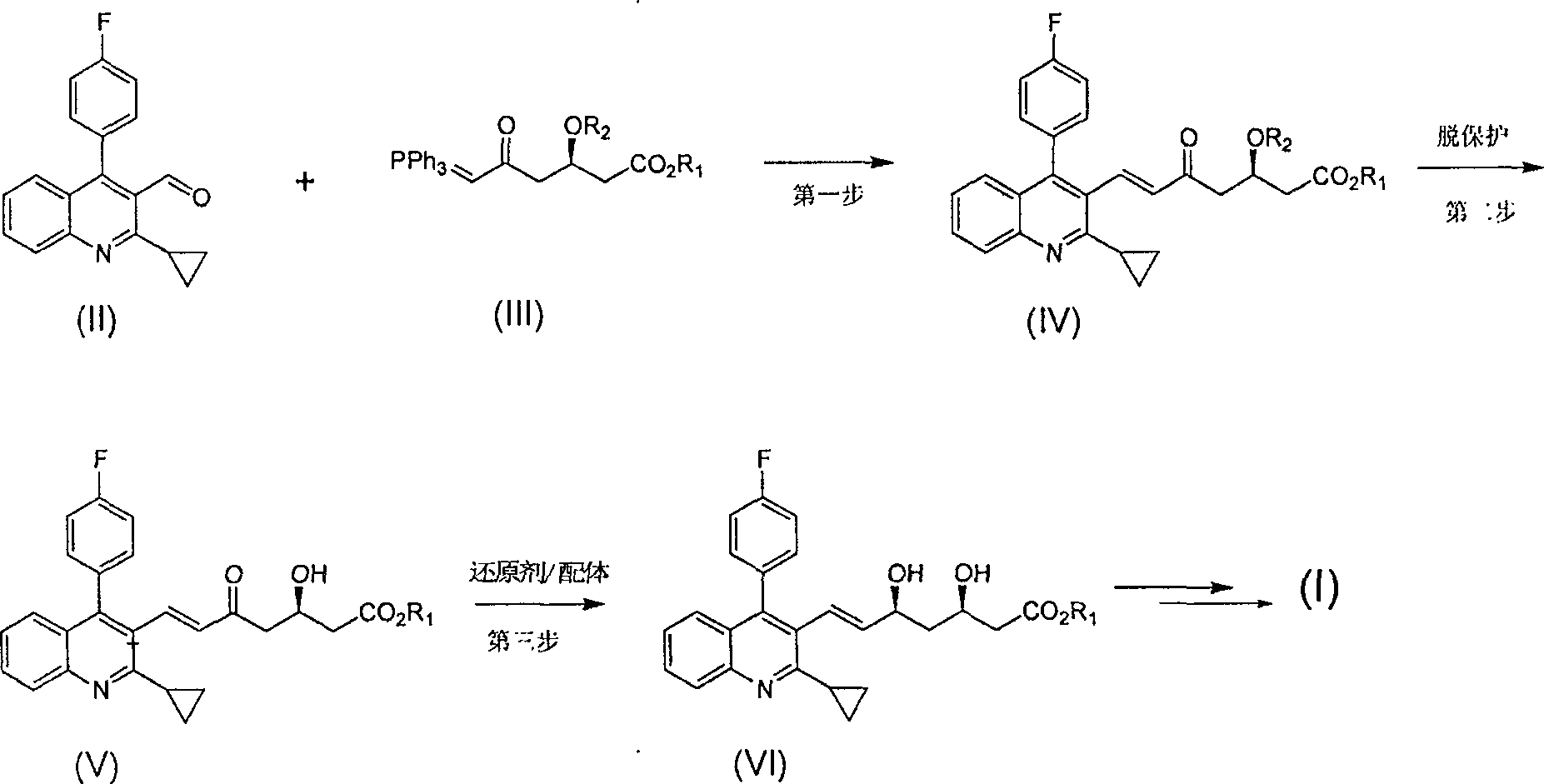
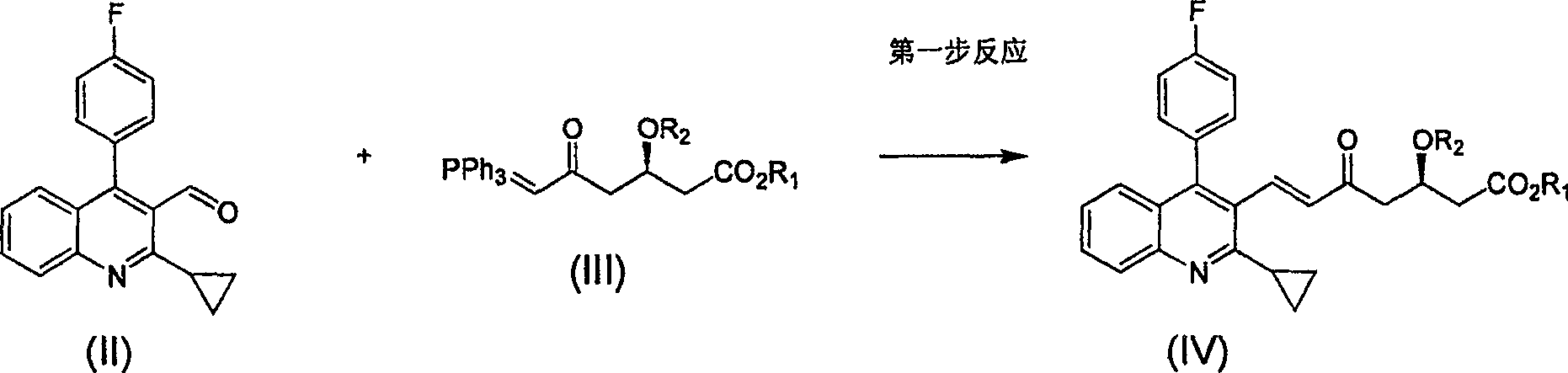
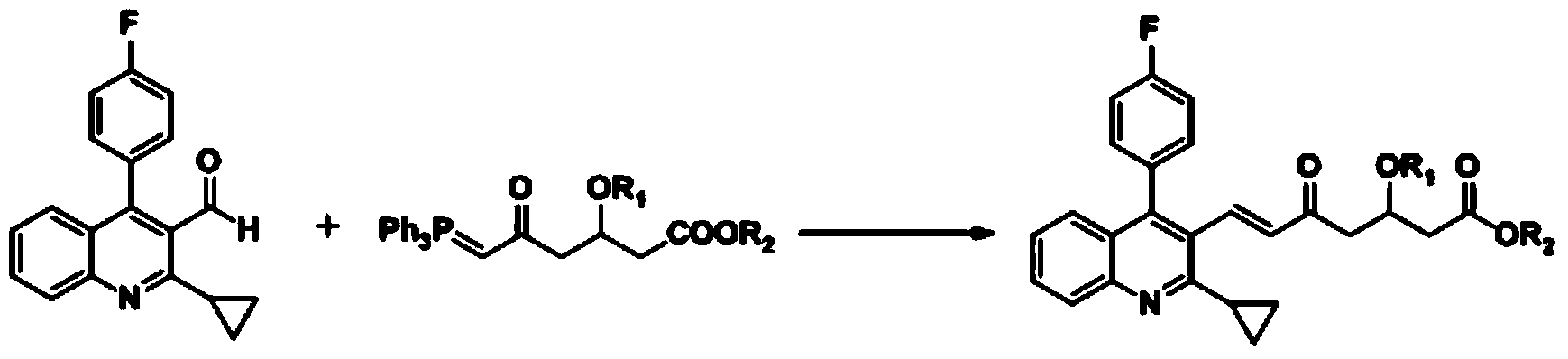
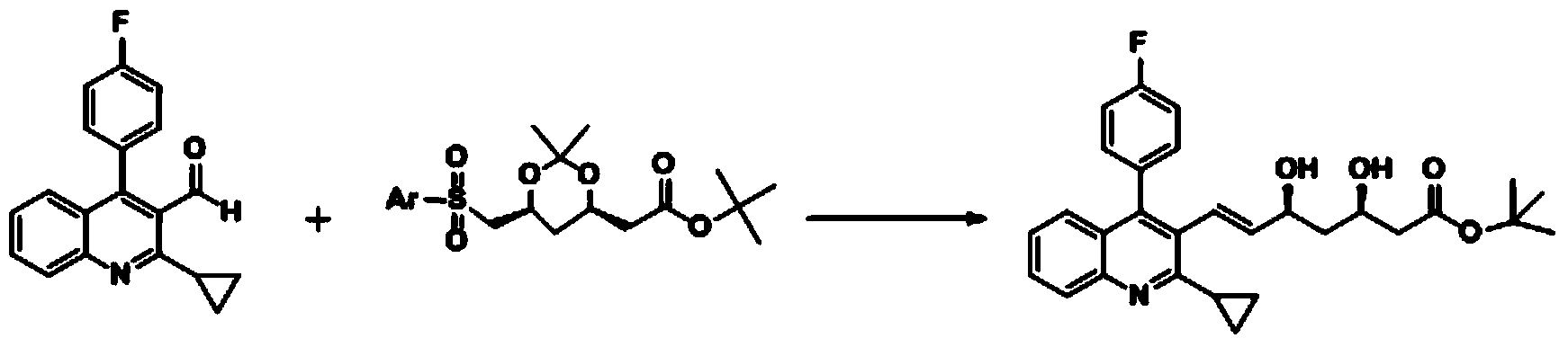
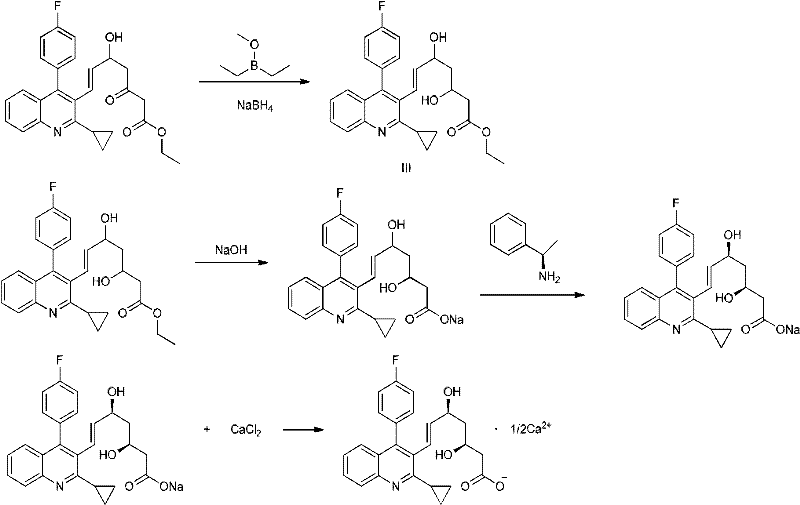
The invention relates the method of preparing the raw material of high optical purity pravastatin calcium. The method comprises the following steps: adding the 2- cyclopropyl-4-(4- fluorophenyl)-3-quinoline aldehyde II and (3R)-3- alkoxy silane-5- carbonyl-6- triphenyl phosphor heptene acid ester III in dissolvent, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)-3- alkoxy silane-6- triphenyl phosphor heptene acid ester IV, removing the protection of IV, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)- hydroxyl -6- triphenyl phosphor heptene acid ester V, deacidizing it in the mixture dissolvent of alcohol and ether with NaBH4 or KBH4 at -100-0Deg.C, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-(3R, 5S)- dihydroxy -6- triphenyl phosphor heptene acid ester VI, hydrolyzing it with alkali, and getting pravastatin calcium. The material is used to prepare HMG-CoA reductase inhibiting agent.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Pitavastatin calcium composition stabilized by using alkaline reagent and preparation method thereof
InactiveCN101890013AImprove stabilitySuitable for long term storageMetabolism disorderInorganic non-active ingredientsClinical efficacyCurative effect
The invention relates to a medicinal composition, in particular to a pitavastatin calcium composition stabilized by using an alkaline reagent and a preparation method thereof. The pitavastatin calcium composition is characterized in that: the alkaline reagent is magnesium oxide, the pH of the aqueous solution or the suspension of the magnesium oxide is more than 9 and less than 12, and the alkaline reagent can remarkably improve the stability of the pitavastatin calcium composition. The pitavastatin calcium composition is suitable for long-term storage and has good clinical effect. The preparation method for the pitavastatin calcium composition is simple, convenient to operate and suitable for industrialized production.
Owner:北京华禧联合科技发展有限公司
Method for treating hyperlipidemia
InactiveUS20060046996A1Easy to moveEffective treatmentBiocideMetabolism disorderMedicineSecondary hyperlipidemia
Provided is a method for treating hyperlipidemia or hypercholesterolemia, which comprises administering effective doses of ezetimibe and pitavastatin or a salt or lactone derivative thereof.
Owner:KOWA CO LTD +1
Method for separating and determining pitavastatin and its optical isomer by means of liquid chromatography
InactiveCN1790012AEffective separation assayGuaranteed stabilityOther chemical processesComponent separationPitavastatin calciumMass spectrography
The invention discloses a segregation detection method of drapery tartan calcium and optical isomer (impurity), which is characterized by the following: adapting high effective gas-chromatography or high effective gas-chromatography mass spectrography to detect the optical isomer of drapery tartan calcium; segregating the drapery tartan calcium and optical isomer of drapery tartan calcium.
Owner:CHONGQING PHARMA RES INST +1
Novel pitavastatin calcium oral disintegrating tablet composition and preparation method thereof
InactiveCN104367560ASubstance increaseMetabolism disorderPill deliveryDrugs preparationsPharmaceutical Aids
The invention relates to the field of medicinal preparations, and in particular relates to a novel pitavastatin calcium oral disintegrating tablet composition and a preparation method thereof. The composition is mainly formed by combining soluble auxiliary materials and insoluble auxiliary materials in a certain proportion. The pitavastatin calcium composition has the characteristic of being rapidly disintegrated in the oral cavity to release medicines so as to promote effects of absorption and utilization.
Owner:万全万特制药江苏有限公司
Pitavastatin calcium enteric sustained-release micropill preparation and preparation method thereof
InactiveCN102048701APermeableCholesterol-lowering effectMetabolism disorderGranular deliveryCholesterolBULK ACTIVE INGREDIENT
The invention provides a pitavastatin calcium enteric sustained-release micropill preparation and a preparation method thereof, which can solve the problems that: (1) pitavastatin calcium is easily subjected to inversion of configuration under the condition that gastric juice has a low pH value, and has low stability, and (2) common tablets are released too quickly, so that active ingredients cannot fully achieve the effect of reducing cholesterol in the prior art. The technical scheme is that: the pitavastatin calcium enteric sustained-release micropill preparation comprises a medicine-containing pill core, an isolation layer, a sustained-release layer and an enteric layer from inside to outside, wherein the medicine-containing pill core comprises pitavastatin calcium and pharmaceutical excipients. The invention also provides a preparation method for the micropill preparation. The prepared micropill is not released in the gastric juice, and medicines are prevented from being exposed in the acid environment; by adopting microporous film coating technology, the medicines can be slowly released from the pill core, the blood concentration is kept balanced, the medicine taking frequency is reduced and the compliance of the patient is improved.
Owner:QINGDAO HANHE PHARMA
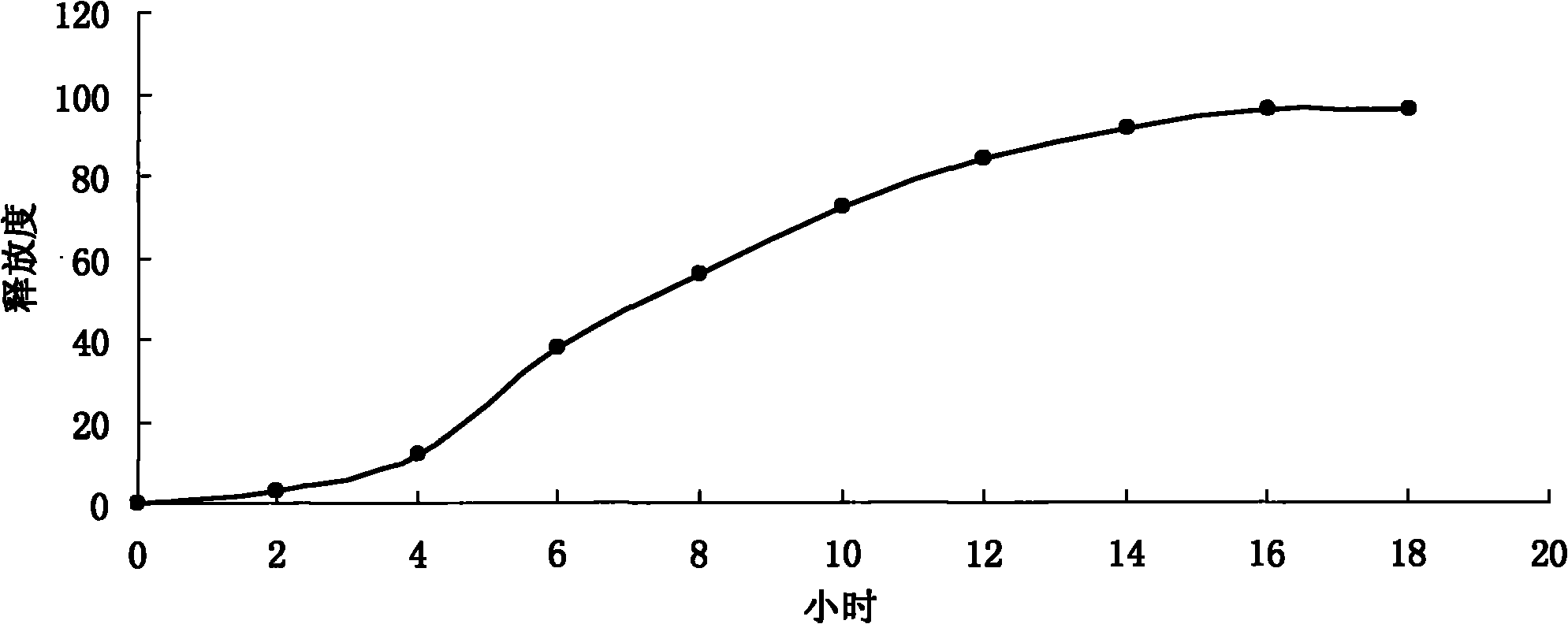
Process for Preparing Pitavastatin, Intermediates and Pharmaceuctically Acceptable Salts Thereof
Owner:MSN LAB PTE LTD
Preparation method of pitavastatin calcium
InactiveCN103508947AReasonable designSimple post-processingOrganic chemistryPhosphonium saltPhosphonium
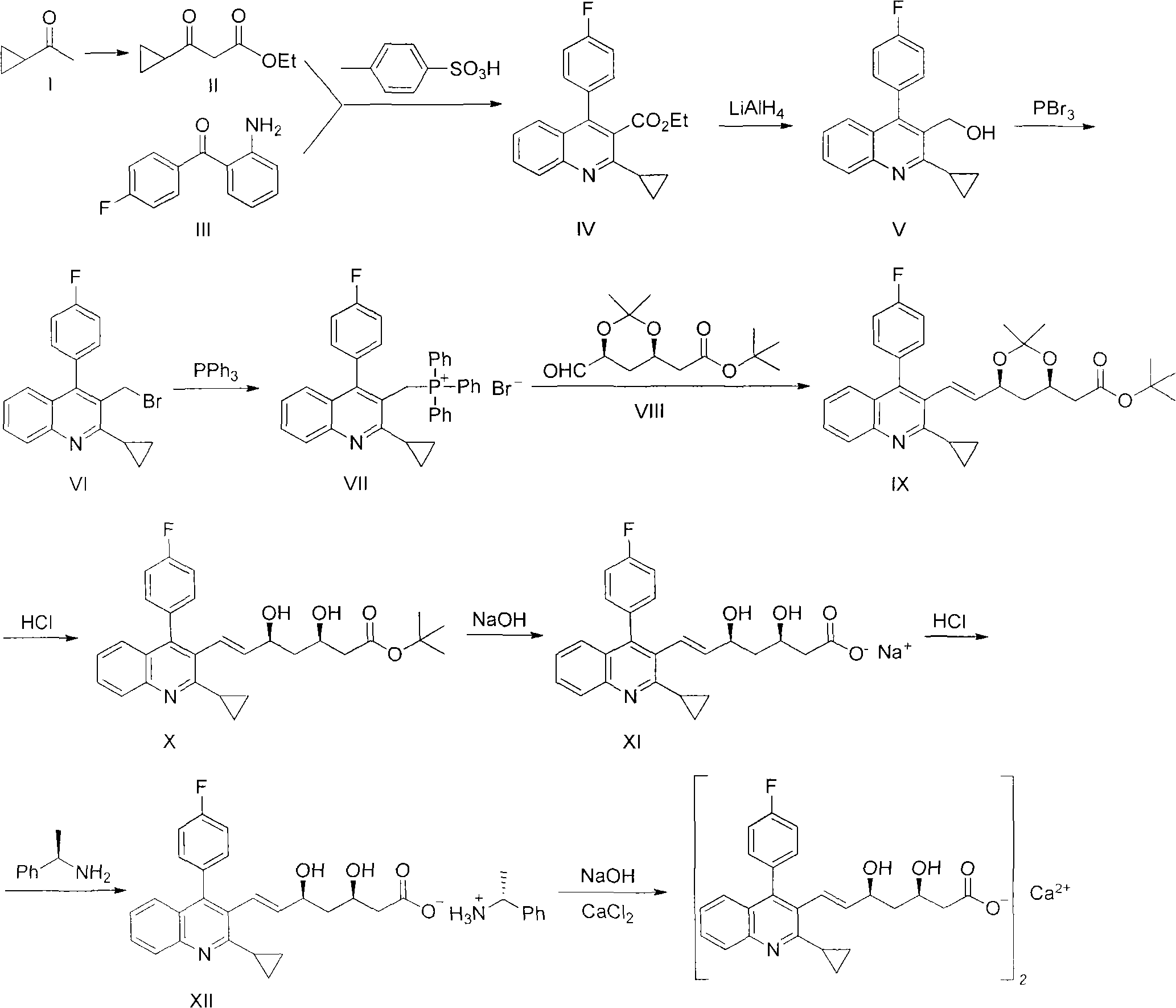
The invention relates to a preparation method of pitavastatin calcium for treating hyperlipidemia. The preparation method comprises the following steps: performing cyclization on 2-amino-4'-fluorobenzophenone (III) and ethyl 3-cyclopropyl-3-oxo-propanoate (II), and then reducing with LiAlH4 to obtain 2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline methanol (V); bromizing the V to obtain 2-cyclopropyl-3-bromomethyl-4-(4-fluorophenyl) quinoline (VI); reacting the VI with triphenylphosphine to obtain (2-cyclopropyl-4-(4-fluorophenyl)-quinoline-3-yl) methyltriphenylphosphonium bromide (VII), performing alkali treatment on a phosphonium salt, then forming phosphonium ylide, performing condensation with (3R, 5S)-6-oxo-3, 5-isopropylidene-dioxo-6-heptenoic acid tert-butyl ester (VIII) to obtain a compound IX; and acidifying the compound IX with hydrochloric acid, performing hydrolysis deprotection with sodium hydroxide, performing salt formation and purification with chiral amine and forming a calcium slat to obtain a final product. The whole route has reasonable design, the process flow is simple, starting raw materials and reagents used by the preparation method can be purchased from the market, the reagents with severe toxicity and serious pollution are not used in a reaction process, the post-treatment of an intermediate is simple, and the preparation method has the advantages of high yield and is easy to purify.
Owner:WEIHAI WEITAI PHARMA TECH DEV
Preparation method of pitavastatin calcium
ActiveCN103508946AAchieve halogenationHigh yieldPhosphorus organic compoundsHydroxylamineCholesterol
The invention relates to a preparation method of a cholesterol reduction drug, particularly relates to a preparation method of pitavastatin calcium as a crude drug of the cholesterol reduction drug, and aims at the problems that the pitavastatin calcium synthetic technology in the prior art is long in steps and complicated in operation, and uses strongly corrosive reagents which is environmentally unfriendly, causes serious corrosion to equipment, and may not facilitate industrial production. The invention provides the new preparation method of the pitavastatin calcium, the new preparation method is as follows: 3-bromomethyl-2-cyclopropyl-4-(4-fluorophenyl)quinoline is prepared from 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinecarboxaldehyde by a one step reaction, and then reacts with an organophosphorus reagent to obtain pitavastatin calcium intermediate phosphorus ylide, on the basis of improving of the yield to 80%, the reaction steps are reduced, and the reaction difficulty is reduced, and hydroxylamine hydrochloride is selected as a deprotection reagent, so that the new preparation method is mild in reaction conditions, environmentally friendly, high in yield, and beneficial to industrial production.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Process For The Preparation Of HMG-COA reductase inhibitors and intermediates thereof
ActiveCN103025727AAvoid formingFew reaction stepsOrganic active ingredientsOrganic chemistryHMG-CoA reductaseRosuvastatin Calcium
Owner:YUHAN
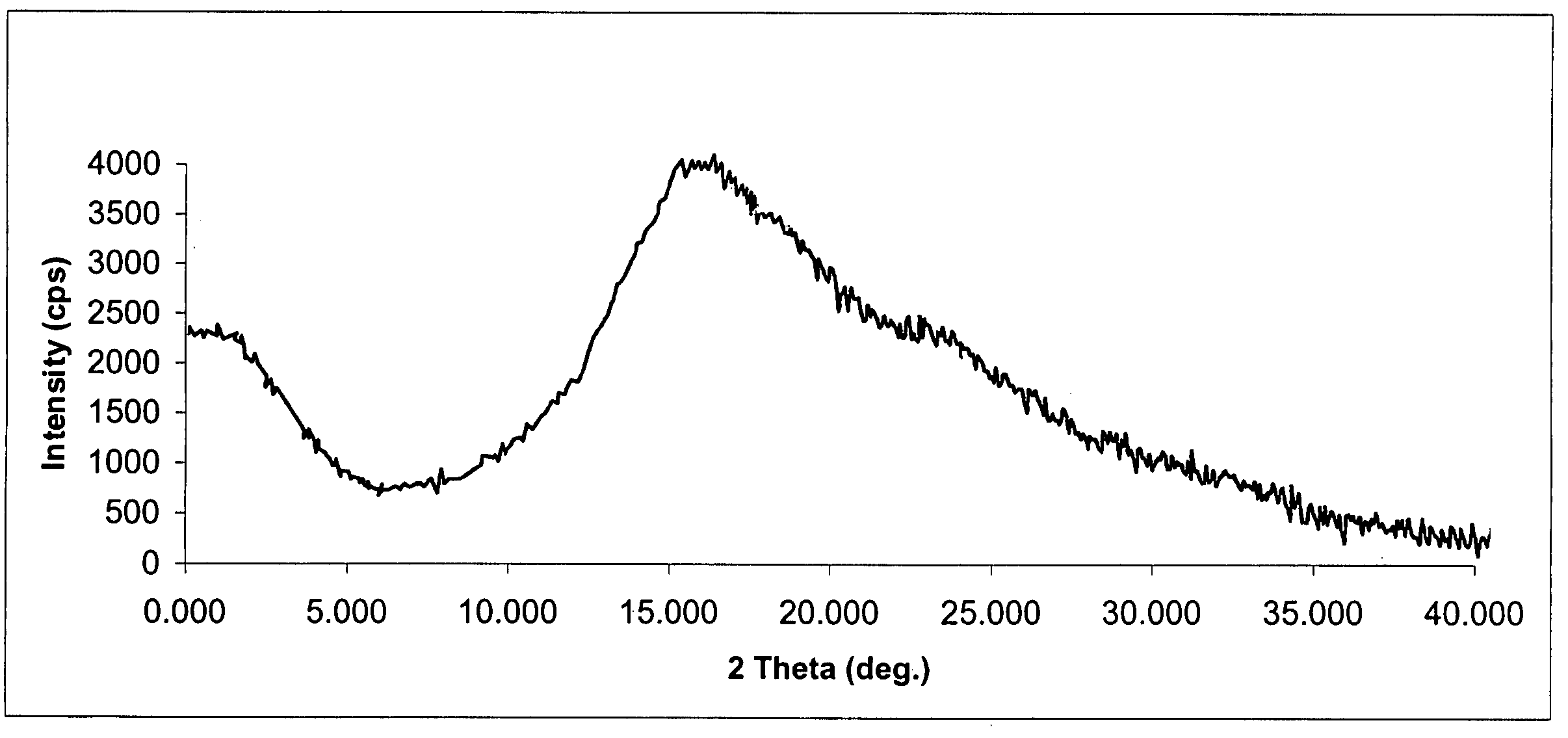
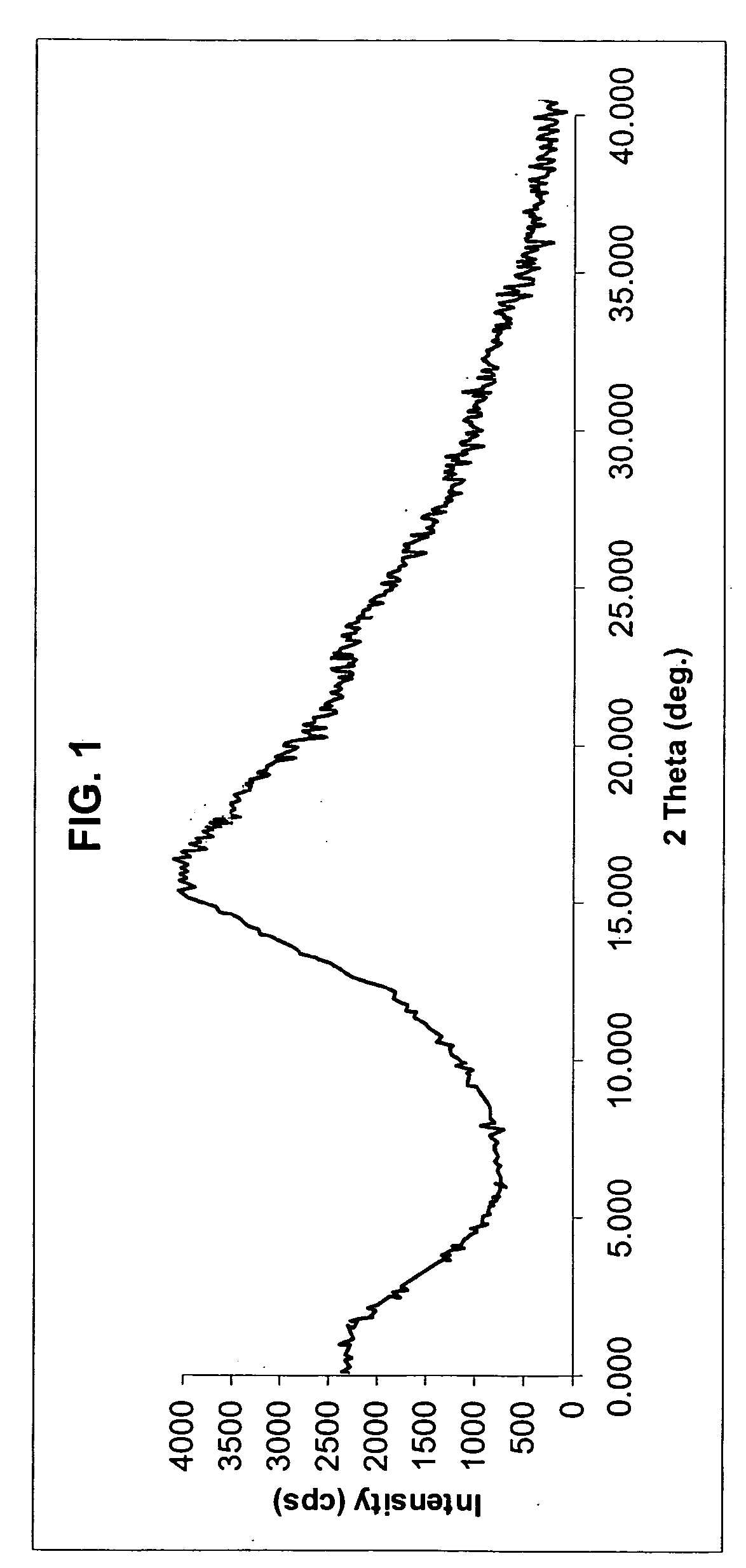
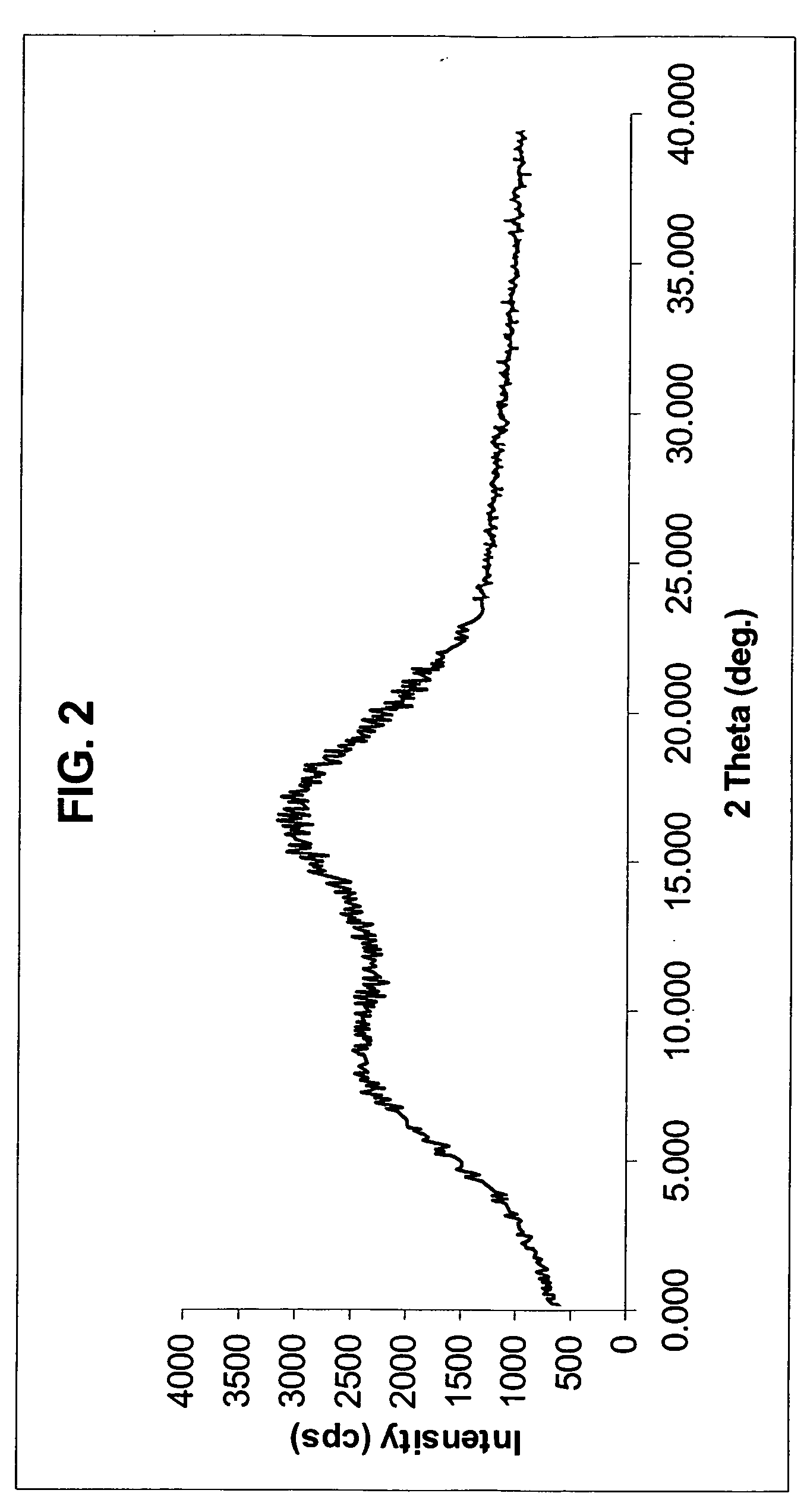
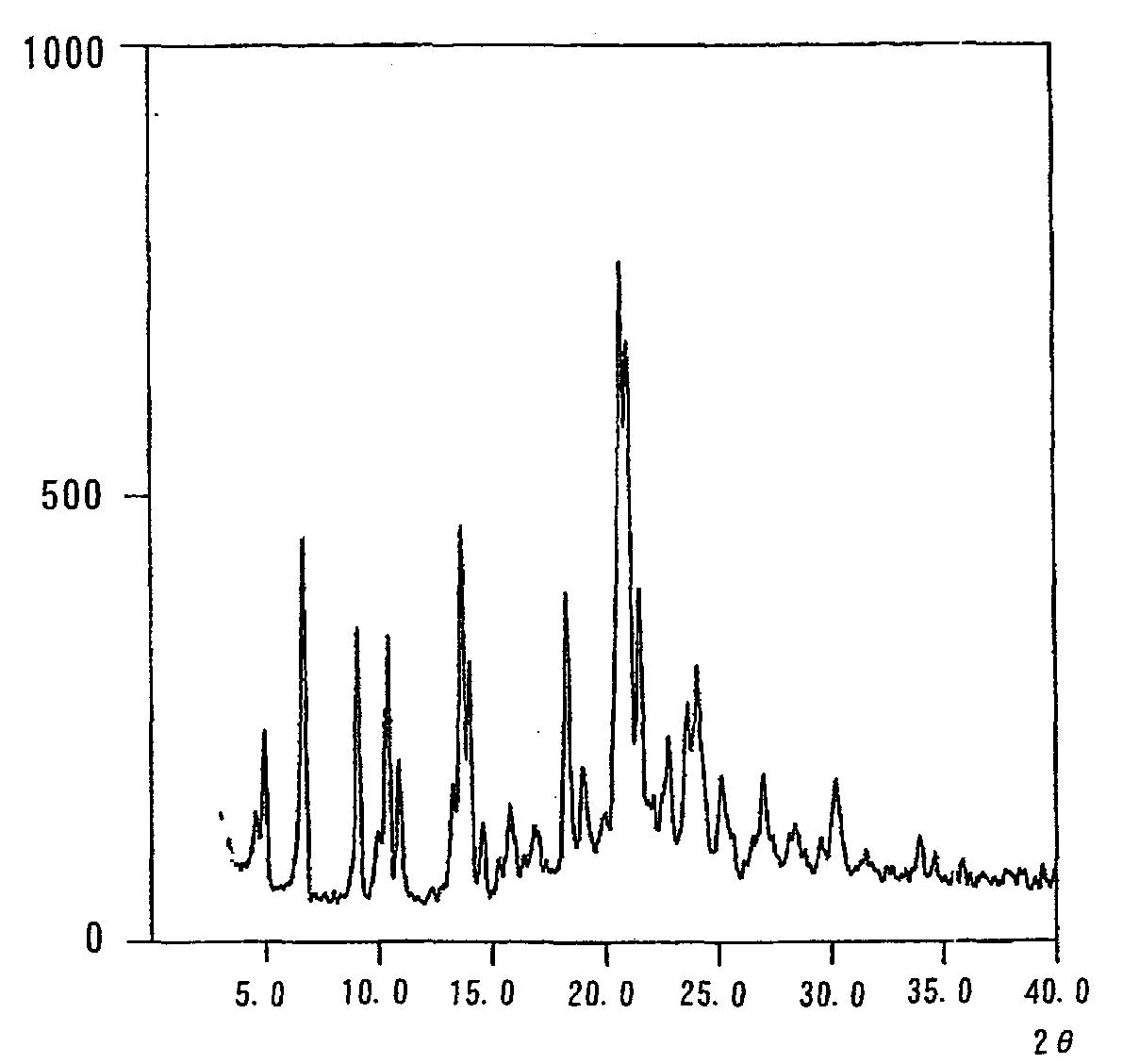
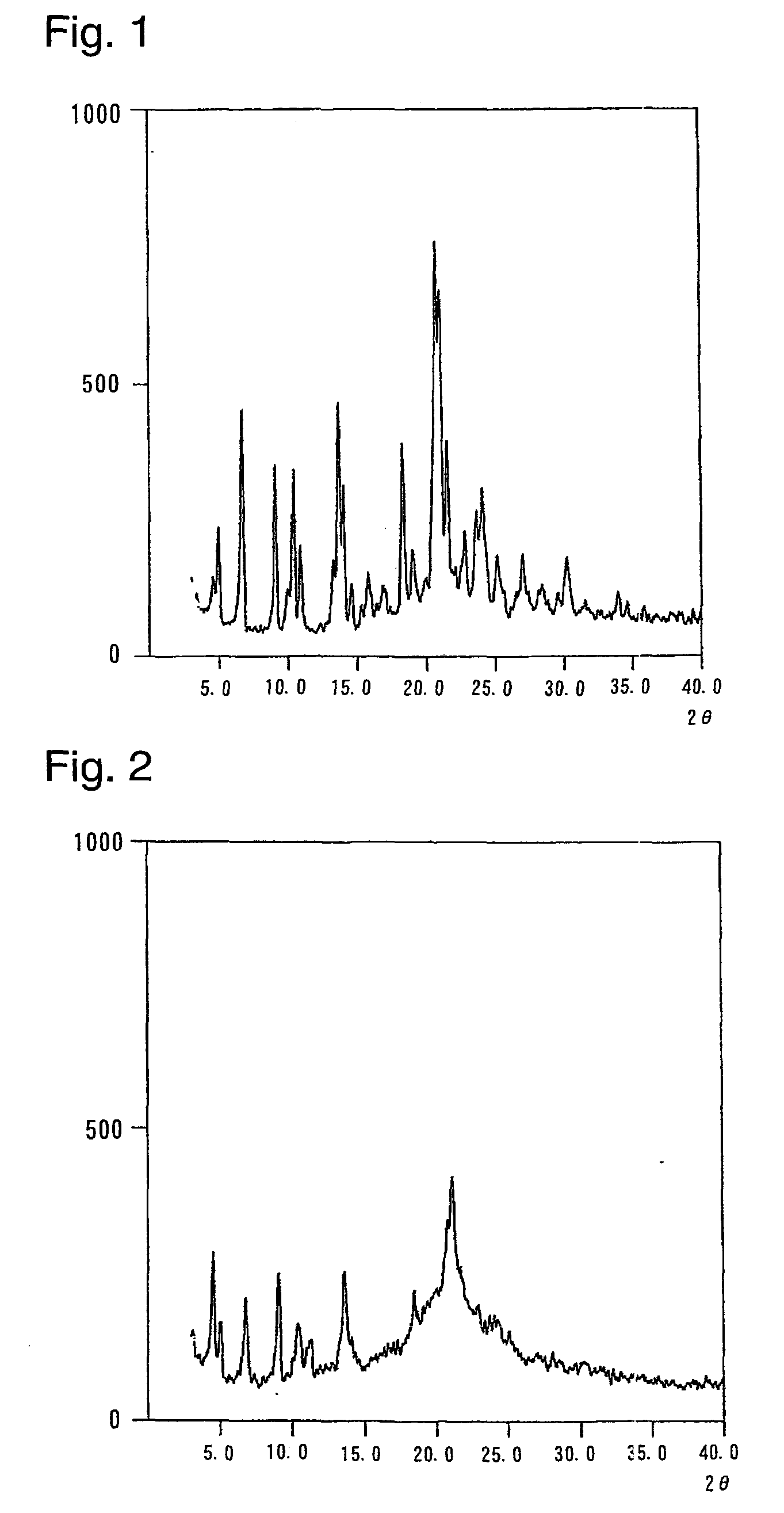
Crystal form of quinoline compound and process for its production
InactiveUS20090176987A1Improve stabilityMetabolism disorderOrganic chemistry methodsPitavastatin calciumWater content
Owner:NISSAN CHEM IND LTD
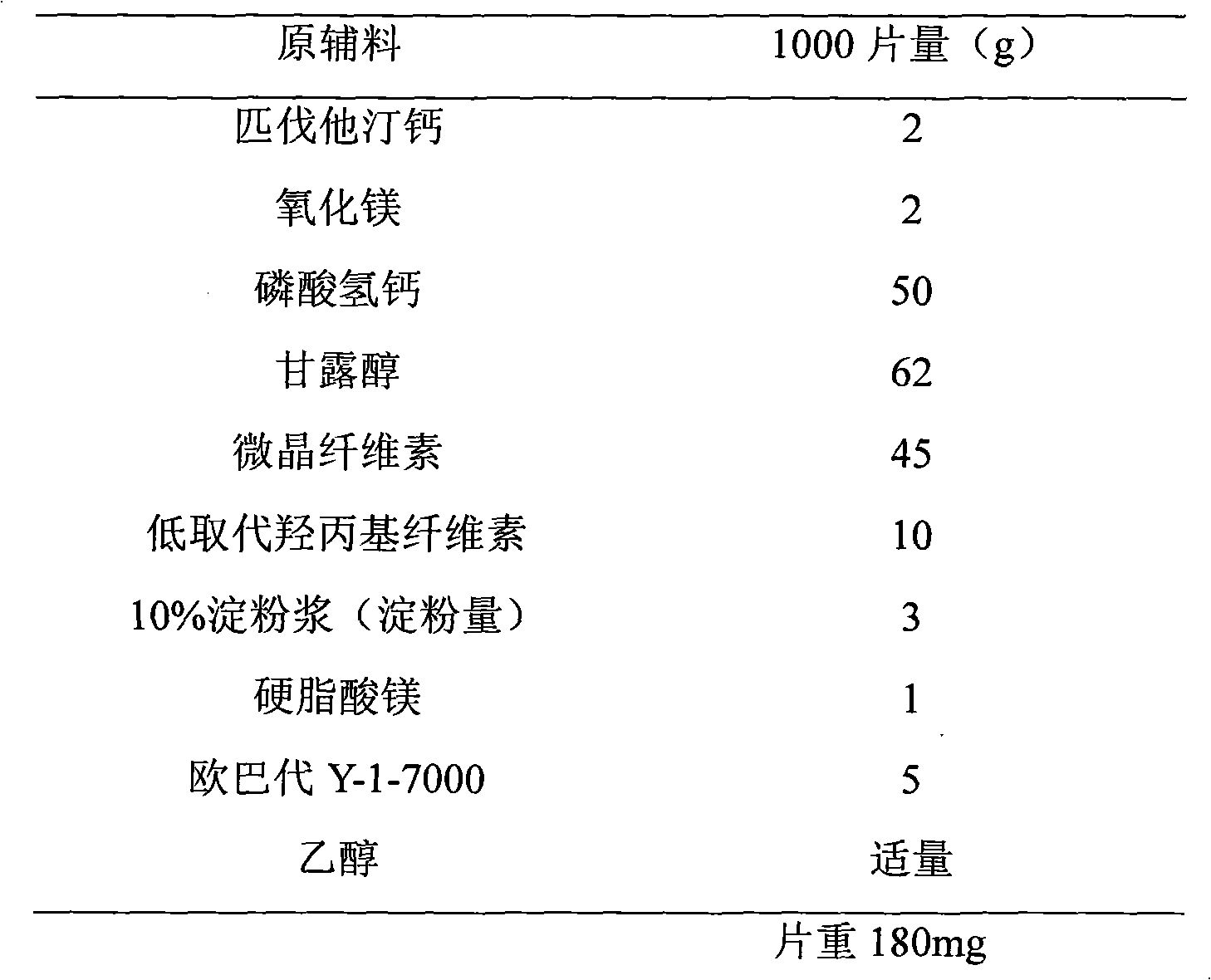
Tablet composition containing pitavastatin calcium and preparation method of tablet
InactiveCN103690508AWorkmanship is feasibleImprove practicalityMetabolism disorderPharmaceutical delivery mechanismCompression methodPitavastatin calcium
The invention discloses a tablet composition containing pitavastatin calcium and a preparation method of the tablet composition. The tablet composition contains main medicine-pitavastatin calcium, porous filler, a disintegrating agent, a lubricating agent and opadry film premix. A direct powder compression method is adopted; the stability of a product is remarkably improved.
Owner:北京华禧联合科技发展有限公司
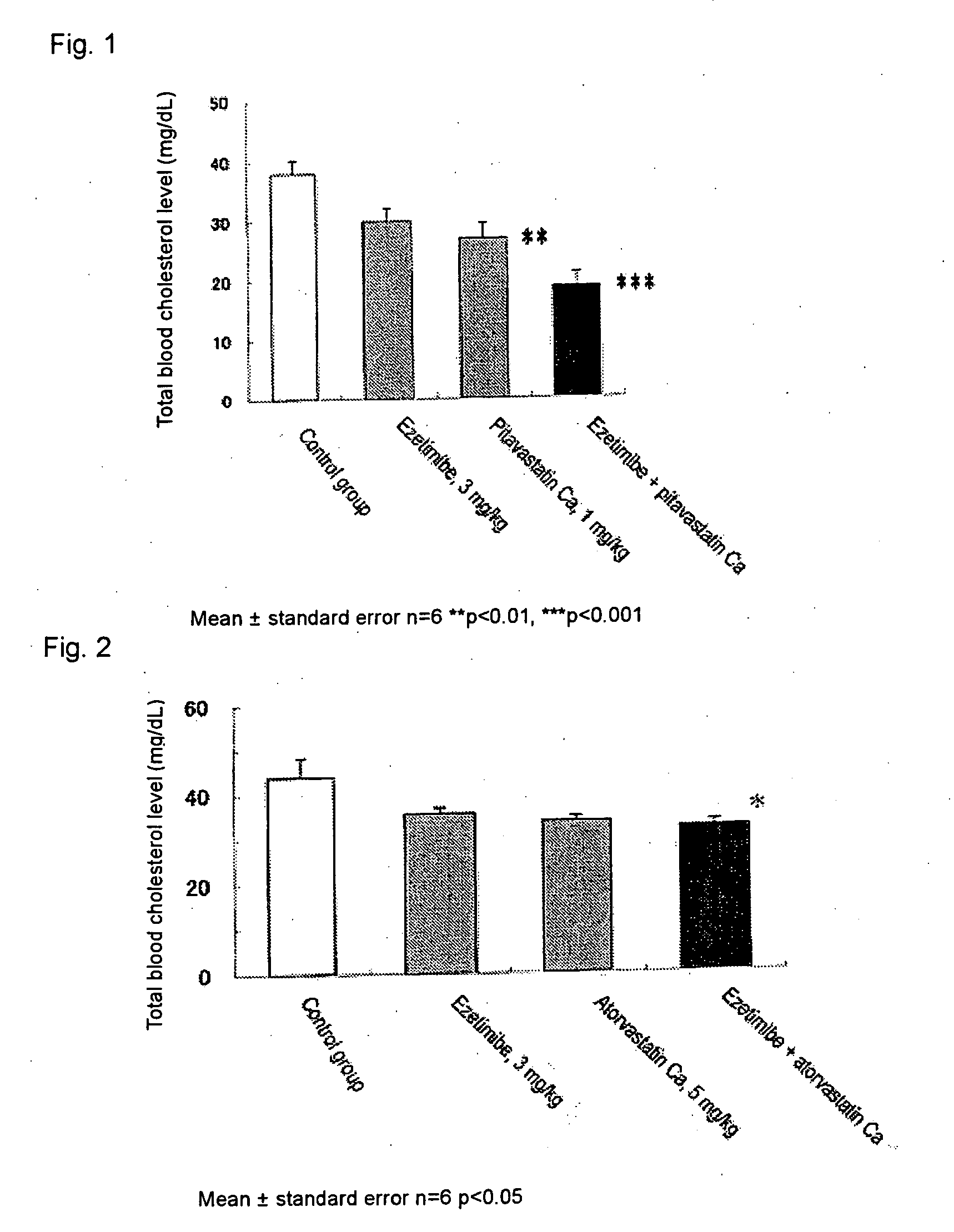
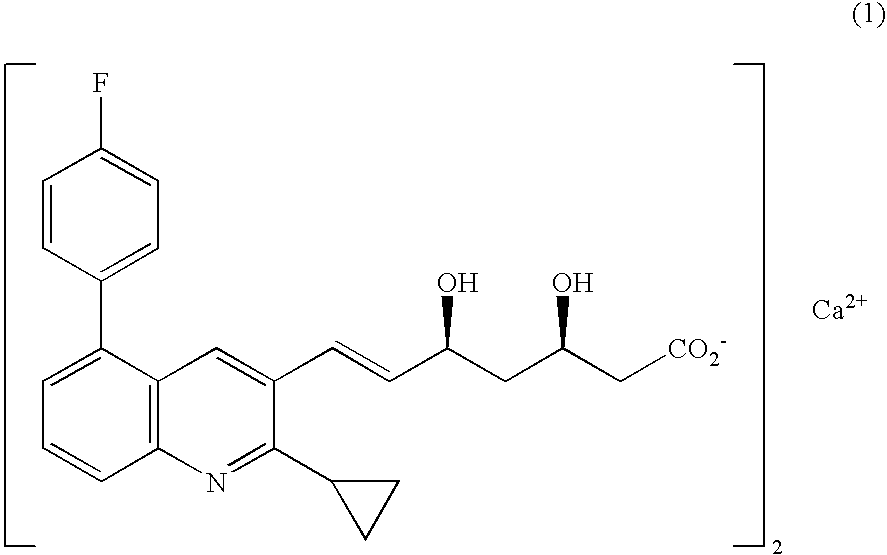
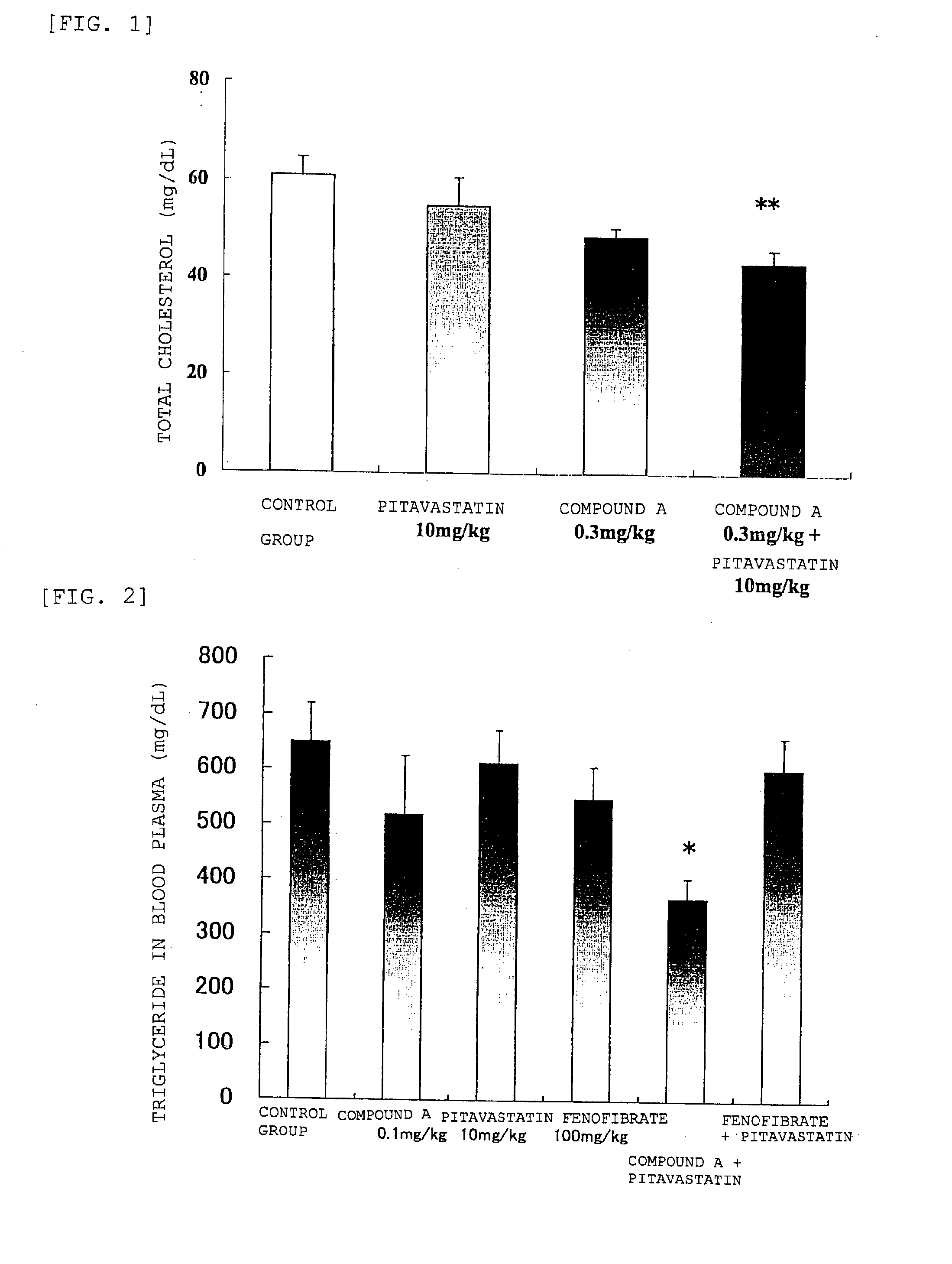
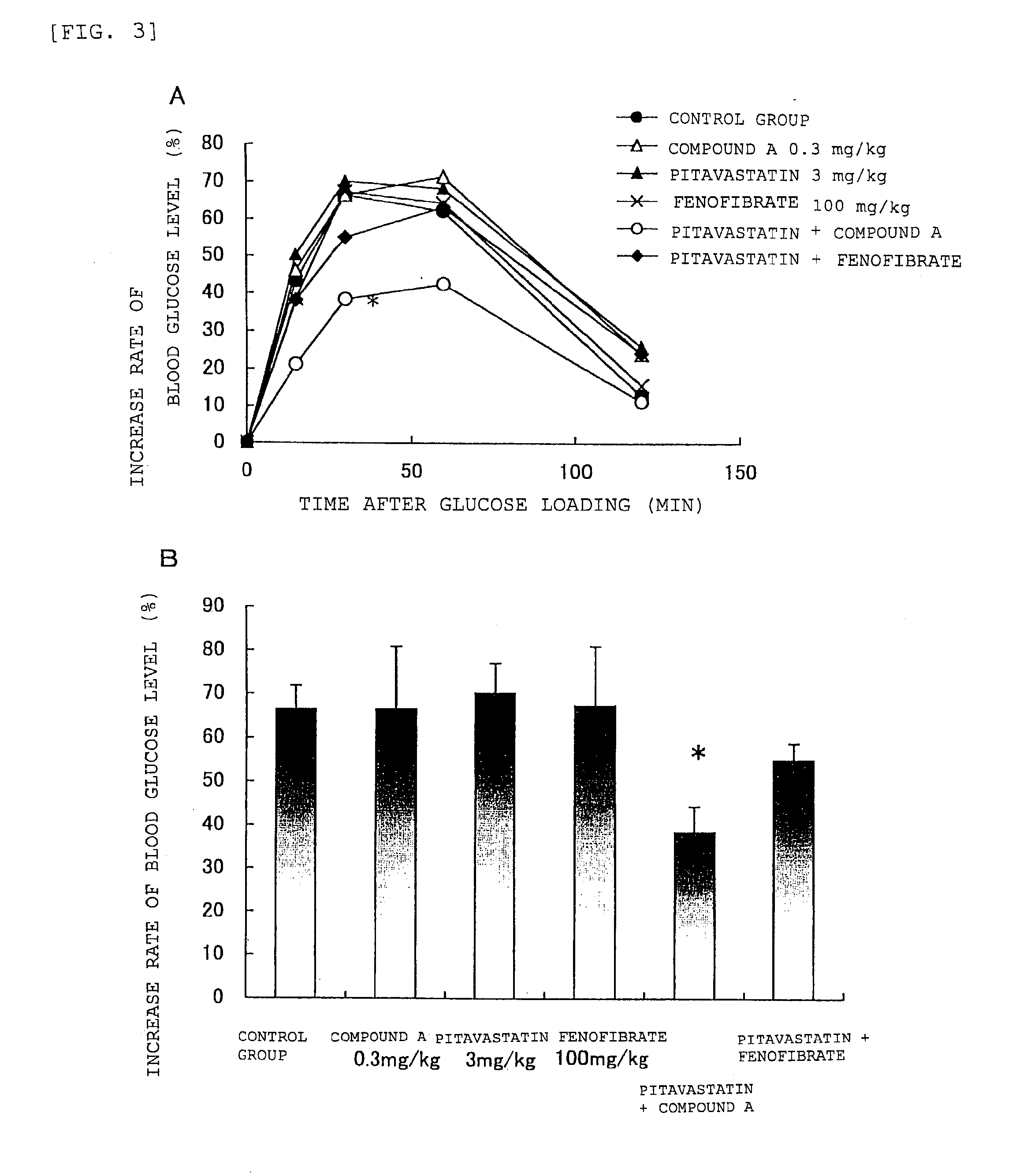
Prophylactic and/or therapeutic agent for hyperlipidemia
ActiveUS20100069433A1Improve concentrationGood effectBiocideOrganic chemistryEthyl groupBlood plasma
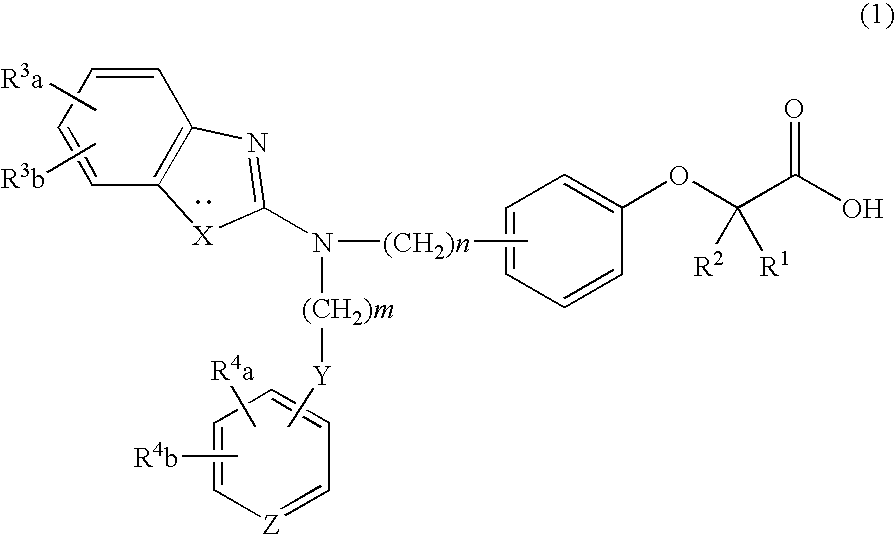
The present invention provides a therapeutic agent for hyperlipidemia having an excellent effect of lowering the cholesterol and triglyceride level in blood plasma.The present invention relates to a prophylactic and / or therapeutic agent for hyperlipidemia, a prophylactic and / or therapeutic agent for obesity or diabetes mellitus, and a prophylactic and / or therapeutic agent for metabolic syndrome, each agent including a compound represented by the following formula (1):, wherein:R1 and R2, which may be identical or different, each represent a hydrogen atom, a methyl group or an ethyl group; R3a, R3b, R4a and R4b, which may be identical or different, each represent a hydrogen atom, a halogen atom, a nitro group, a hydroxyl group, a C1-4 alkyl group, a trifluoromethyl group, a C1-4 alkoxy group, a C1-4 alkylcarbonyloxy group, a di-C1-4 alkylamino group, a C1-4 alkylsulfonyloxy group, a C1-4 alkylsulfonyl group, a C1-4 alkylsulfinyl group, or a C1-4 alkylthio group, or R3a and R3b, or R4a and R4b are joined to represent an alkylenedioxy group; X represents an oxygen atom, a sulfur atom or N—R5 (wherein R5 represents a hydrogen atom, a C1-4 alkyl group, a C1-4 alkylsulfonyl group, or a C1-4 alkyloxycarbonyl group); Y represents an oxygen atom, a S(O)l group (l represents a number from 0 to 2), a carbonyl group, a carbonylamino group, an aminocarbonyl group, a sulfonylamino group, an aminosulfonyl group, or an NH group; Z represents CH or N; n represents a number from 1 to 6; and m represents a number from 2 to 6,or a salt thereof, and a statin, particularly pitavastatin, in combination.
Owner:KOWA CO LTD
Anhydrous amorphous form of fluvastatin sodium
The present invention relates to novel anhydrous amorphous forms of bis[(E)[4-(4-fluorophenyl)isopropyl[methyl(methylsulfonyl)amino]pyrimidinyl](3R,5S)-3,5-dihydroxyhept enoic acid]calcium salt (rosuvastatin calcium), (±)7-(3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl)3,5-dihydroxy heptenoic acid monosodium salt (fluvastatin sodium) and bis[(E)-3,5-dihydroxy-7-[4′-(4″-fluorophenyl)-2′-cyclopropyl-quinolin-3′-hept-6-enoic acid]calcium salt (pitavastatin calcium), to processes for their preparation, to pharmaceutical compositions containing them and to methods of treatment using the same. The rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium obtained are known valuable agents useful in treating hyperlipidemia and hypercholestrolemia.
Owner:MAI DE
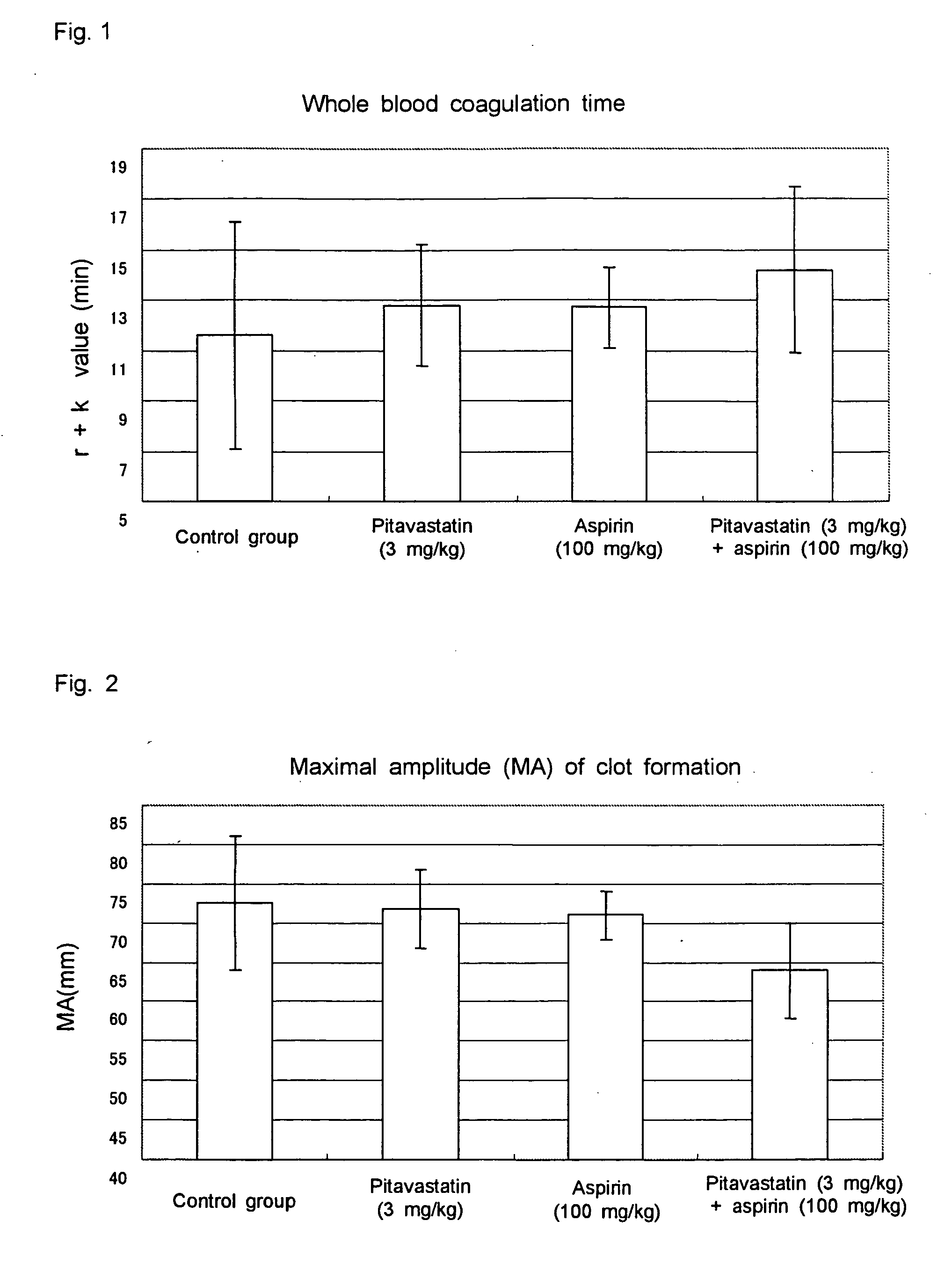
Method for treating thrombosis
InactiveUS20060217352A1Without causing severe side effectsSuppressing blood coagulationSalicyclic acid active ingredientsBiocideAspirinThrombus
This invention relates to a method for treating thrombosis by combined administration of a pitavastatin with aspirin.
Owner:KOWA CO LTD +1
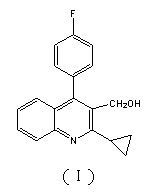
Preparation method of pitavastatin calcium intermediate compound
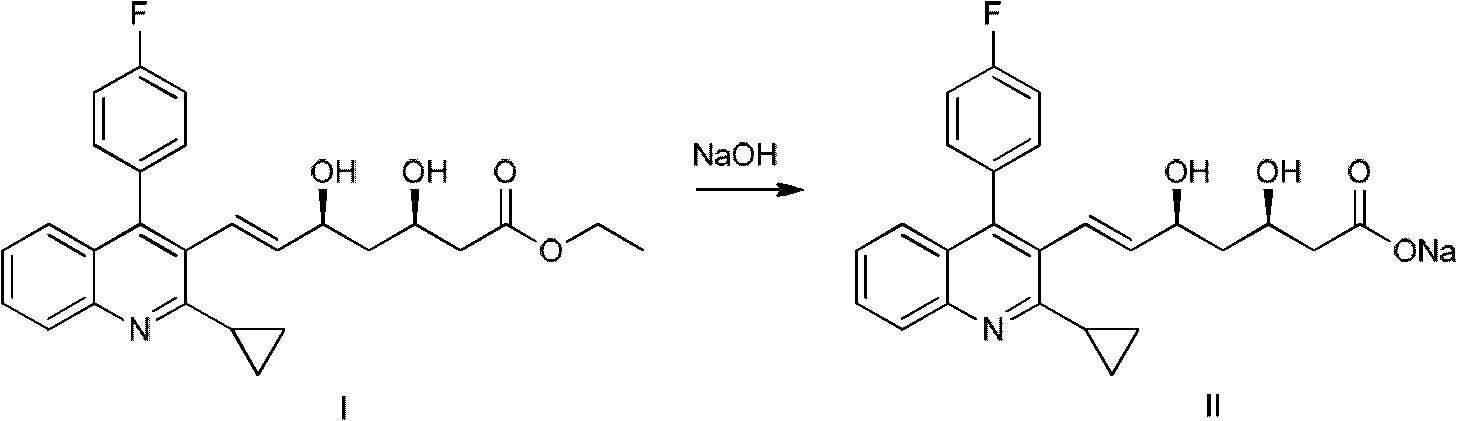
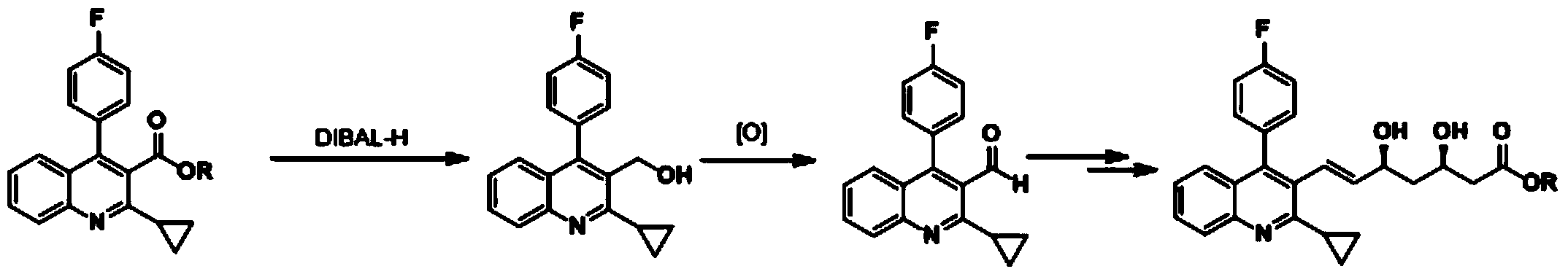
The invention relates to a preparation method of a pitavastatin calcium intermediate compound which has a chemical name of 2-cyclopropyl-3-hydroxymethyl-4-(4-fluorophenyl)-quinoline and is shown in Formula (I). The pitavastatin calcium intermediate compound is prepared by hydrolyzing and reducing an ester compound shown in Formula (II) under specific conditions.
Owner:湖北丰融医药有限公司
Preparation method of pitavastatin calcium by recrystallization
InactiveCN102653523AGood crystal formImprove solubilityOrganic chemistrySolubilityPitavastatin calcium
The invention relates to the technical field of medicine, particularly a preparation method of pitavastatin calcium by recrystallization. The method is implemented in a way that: pitavastatin calcium is dissolved in a tetrahydrofuran / water mixed solvent, wherein the solubility is high; and after gradually removing the tetrahydrofuran by volatilization, since the solubility of the pitavastatin calcium in water is very low, the pitavastatin calcium gradually crystallizes and precipitates, thereby obtaining the pitavastatin calcium solid with favorable crystal form and also refining and purifying the pitavastatin calcium.
Owner:FOSHAN DAYI TECH LTD
Method for preparing high optical purity pitavastatin calcium raw material drug
The invention relates to a method for preparing a raw material drug of pitavastatin calcium with high optical purity, which mainly solves the technical problems of high difficulty in separation and purification of the raw material drug of pitavastatin calcium and low yield. The method comprises the following steps: 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinecarbaldehyde II and (3R)-3-alkylsilyloxy-5-carbonyl-6-triphenyl (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline]-5-carbonyl-(3R)- 3-Alkylsilyloxy-6-heptenoate IV, IV is deprotected with a deprotecting agent to obtain (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3- Quinoline]-5-carbonyl-(3R)-hydroxyl-6-heptenoic acid ester V, which was mixed with NaBH in a mixed solvent of an alcohol and an ether compound 4 or KBH 4 And selective reduction under the action of the ligand, the reaction temperature is -100 ° C to 0 ° C, to obtain (E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline] -(3R,5S)-dihydroxy-6-heptenoic acid ester VI, which is hydrolyzed into calcium salt with alkali to obtain pitavastatin calcium. It is mainly used in the preparation of HMG-CoA reductase inhibitors, a drug for lowering blood lipids.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Method for preparing pitavastatin calcium
InactiveCN103508948ASolve the technical bottleneck of synthesisAchieve separationOrganic chemistryAcetic acidAfter treatment
The invention discloses a method for preparing pitavastatin calcium. The method comprises the following step: performing seven-step synthesis on 3-cyclopropyl-oxopropionate (comprising methyl ester and ethyl ester), (2-aminophenyl)(4-fluorophenyl) ketone and (4R-cis)-6-[(acetoxy)methyl]2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate which are used as initial raw materials so as to obtain the product. The reaction in each step is a conventional reaction and is suitable for large-scale production. The process is stable, the reaction conditions are mild, the after-treatment operation is simple, the intermediate is easy to separate, and the technical bottleneck in the existing pitavastatin calcium synthesis is solved.
Owner:ASYMCHEM LAB TIANJIN +4
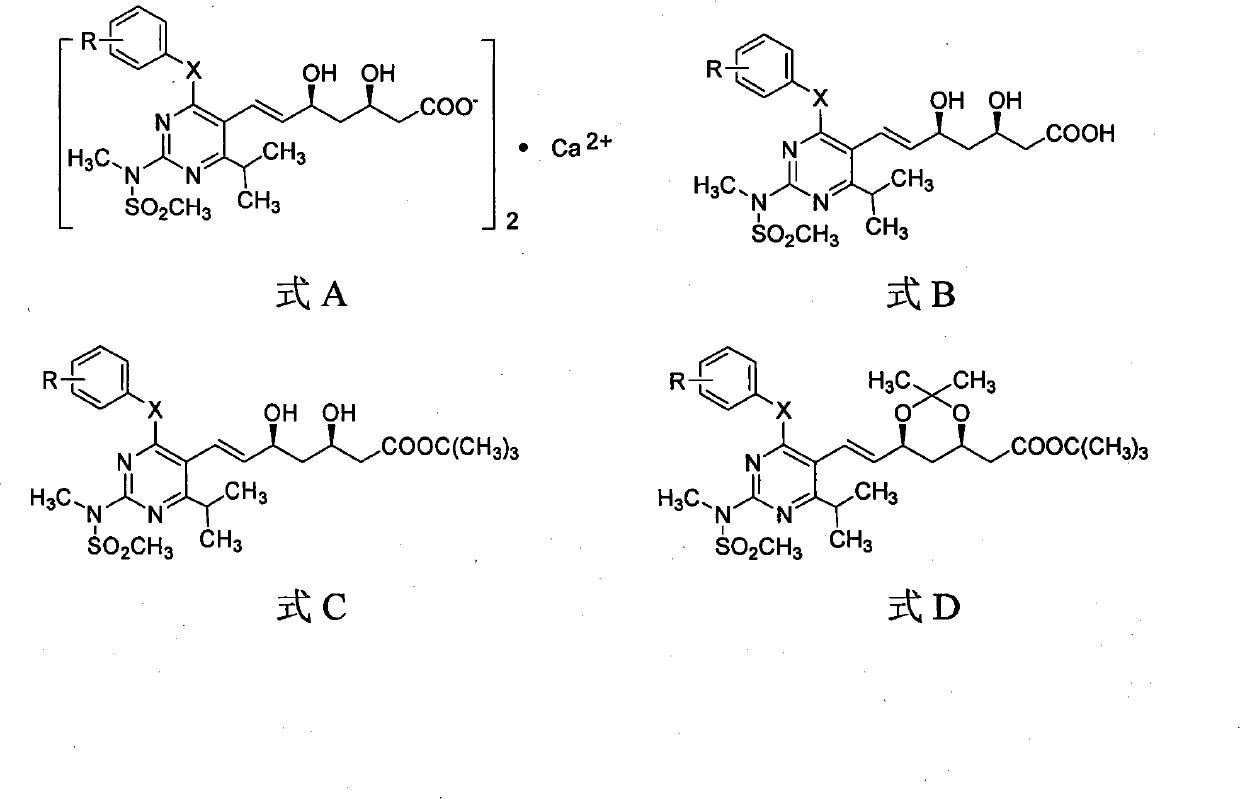
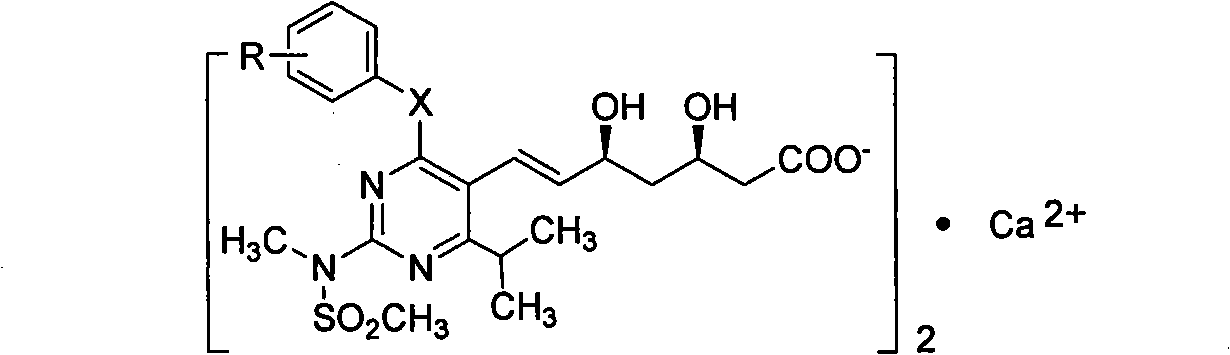
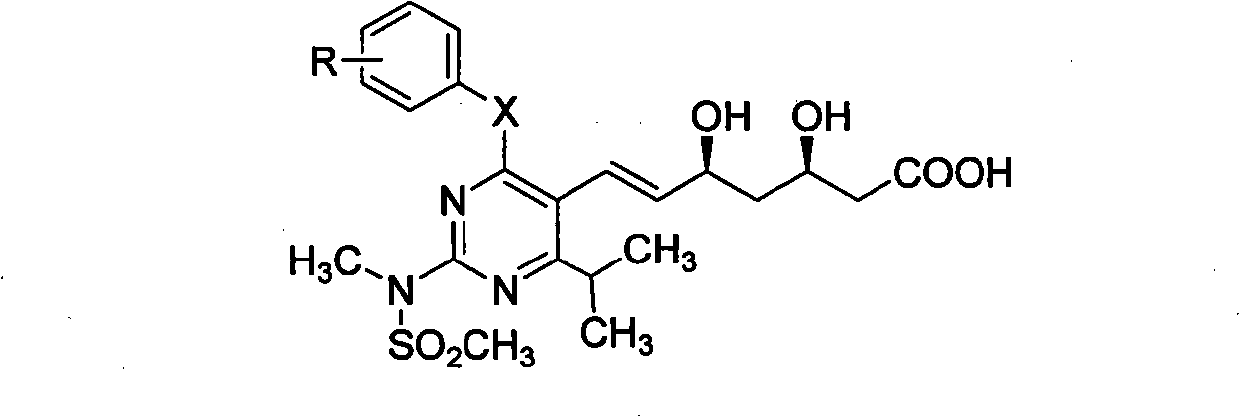
Miazine compounds, intermediates of miazine compounds, preparation method of intermediates and miazine compounds as well as application of miazine compound
InactiveCN102079726AInhibits reductase activityOrganic active ingredientsOrganic chemistryHMG-CoA reductaseSecondary hyperlipidemia
The invention discloses miazine compounds as shown in a formula A which is disclosed in the specification and reaction intermediate compounds as shown in formulas B, C and D, wherein X is O or S, R is H,F,C1-C3 alkyl or C1-C3 alkoxy. The invention also discloses preparation methods of the intermediates and miazine compounds and an application of the miazine compounds in preparing medicaments for inhibiting HMG-CoA reductase and / or treating hyperlipidemia diseases. Compared with the pitavastatin, osuvastatin and atorvastatin in the prior art, the 6-isopropyl-2-(N-methyl-N-sulfonyl) amino-4-substituted phenoxy (or thiphenyl) miazine compounds have better or at least comparative activity for inhibiting the HMG-CoA reductase, and can be used for treating the hyperlipidemia diseases.
Owner:SHANGHAI INST OF PHARMA IND
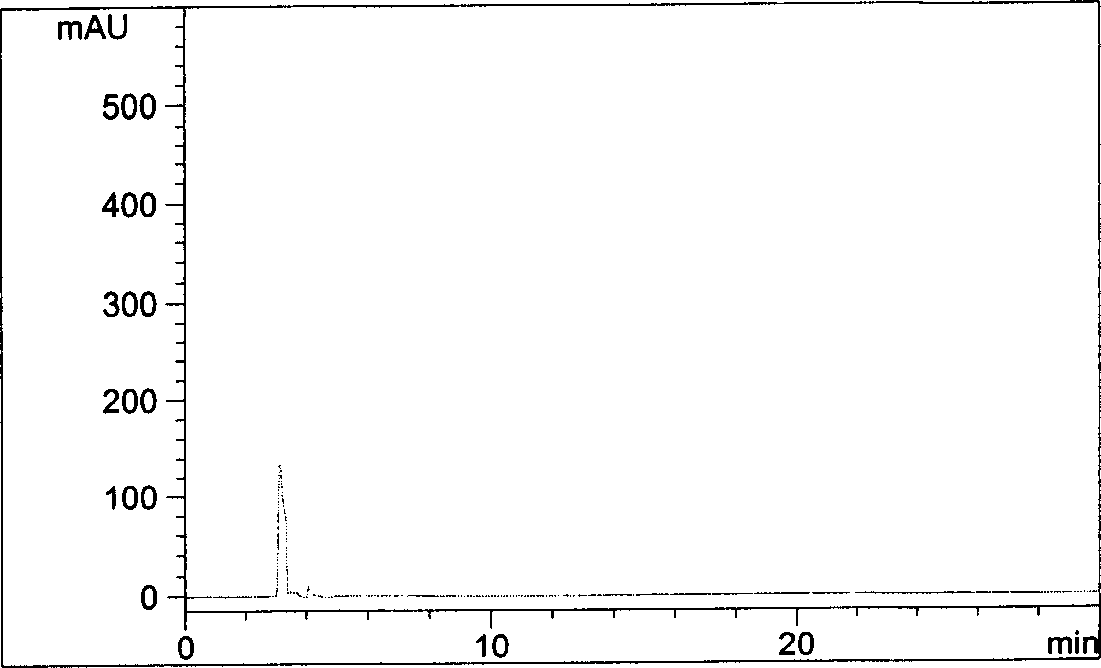
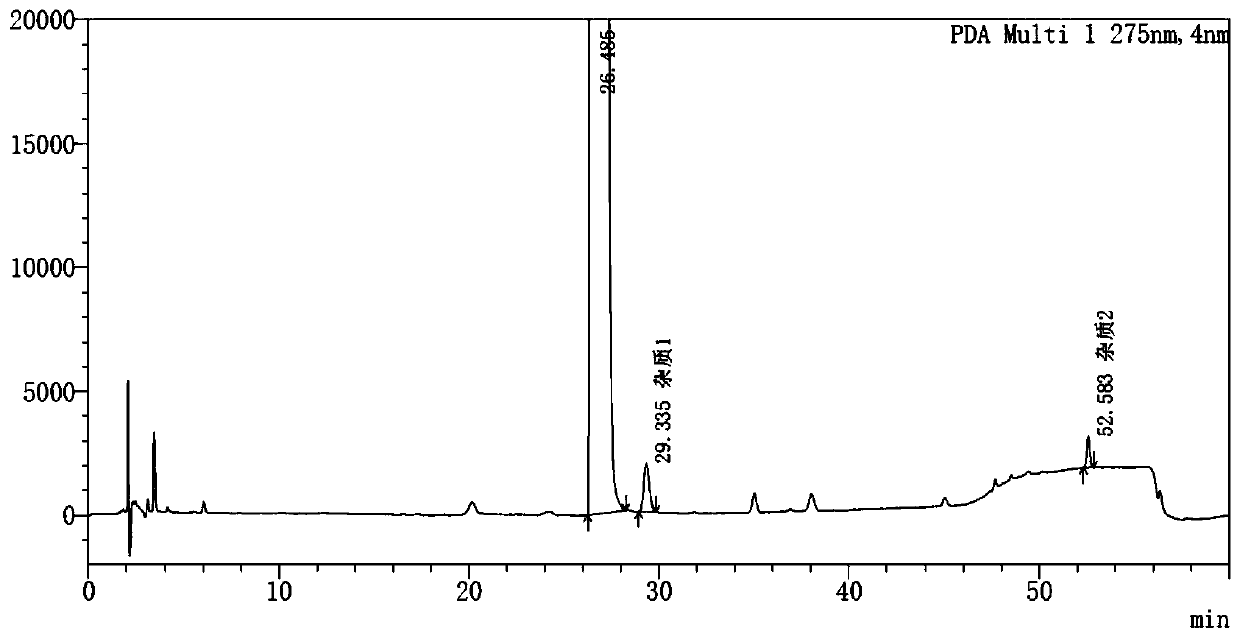
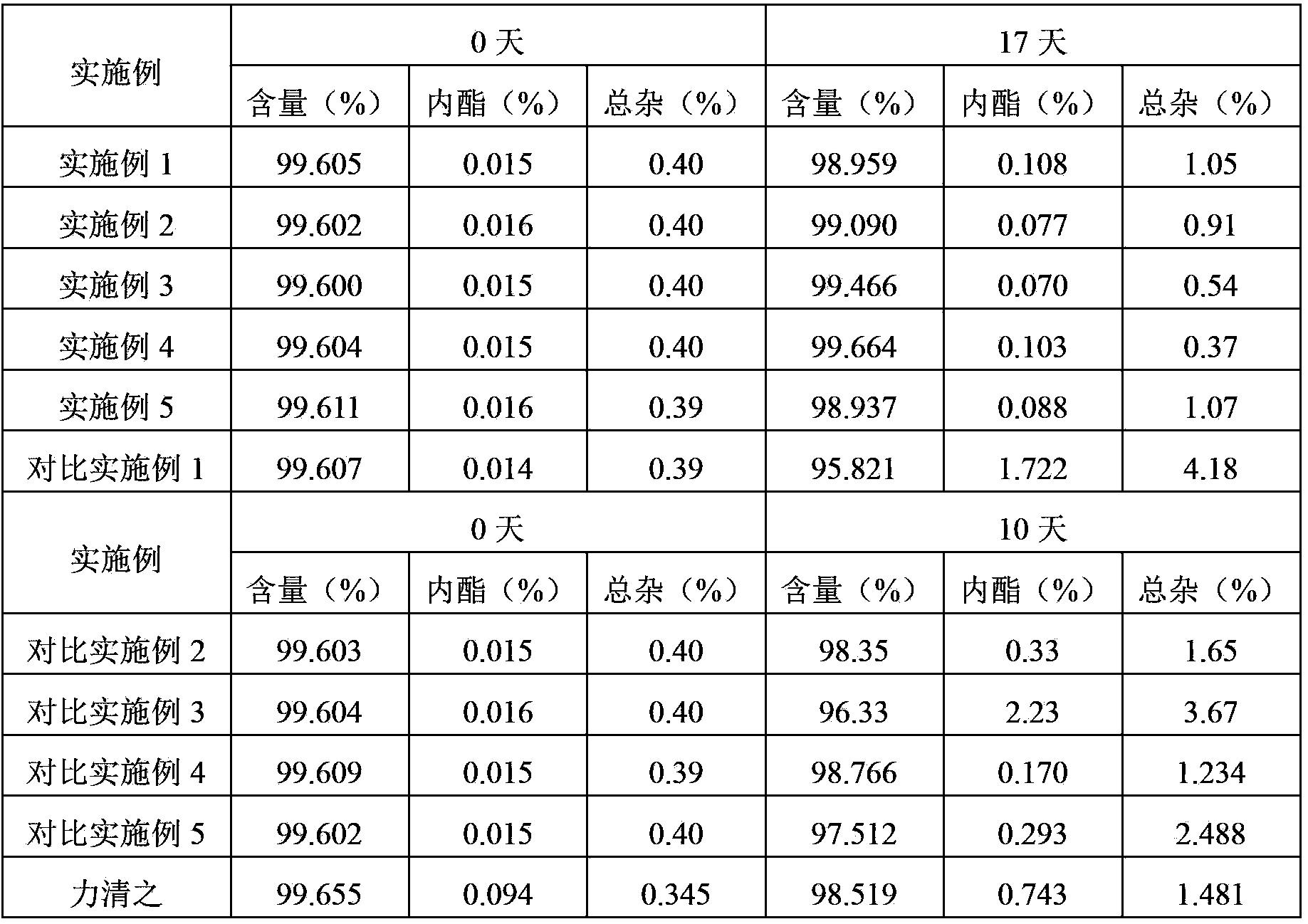
Method for detecting potential mutagenic impurities in pitavastatin calcium tablets
InactiveCN111505150AAvoid interferenceEffective monitoring of drug qualityComponent separationGradient elutionSolvent
The invention relates to the field of medicine quality detection, in particular to a method for detecting potential mutagenic impurities in pitavastatin calcium tablets. The method comprises the following steps: dissolving a to-be-detected sample by adopting a solvent, and detecting by adopting a high performance liquid chromatography under the chromatographic conditions that a mobile phase comprises an organic acid salt buffer solution and acetonitrile; the flow rate is 1.0 mL / min; the column temperature is 25-40 DEG C; a chromatographic column: octadecylsilane chemically bonded silica is used as a filler; the sample injection volume is 50 [mu] L; the detection wavelength ranges from 270 nm to 280 nm; an elution mode is gradient elution; the potential mutagenic impurities are an impurity1 and an impurity 2. According to the method disclosed by the invention, the pitavastatin calcium, the impurity 1 and the impurity 2 can be effectively separated; meanwhile, interference of other related impurities of pitavastatin calcium on detection of the impurity 1 and the impurity 2 is avoided, the content of potential mutagenic impurities 1 and 2 of the pitavastatin calcium tablets can be accurately detected, the quality of the pitavastatin calcium tablets is effectively monitored, and the medication safety is improved.
Owner:SHANDONG QIDU PHARMA
Process for the preparation of HMG-COA reductase inhibitors and intermediates thereof
ActiveUS8476432B2Improve isolationReduce stepsOrganic active ingredientsOrganic chemistryHMG-CoA reductasePitavastatin
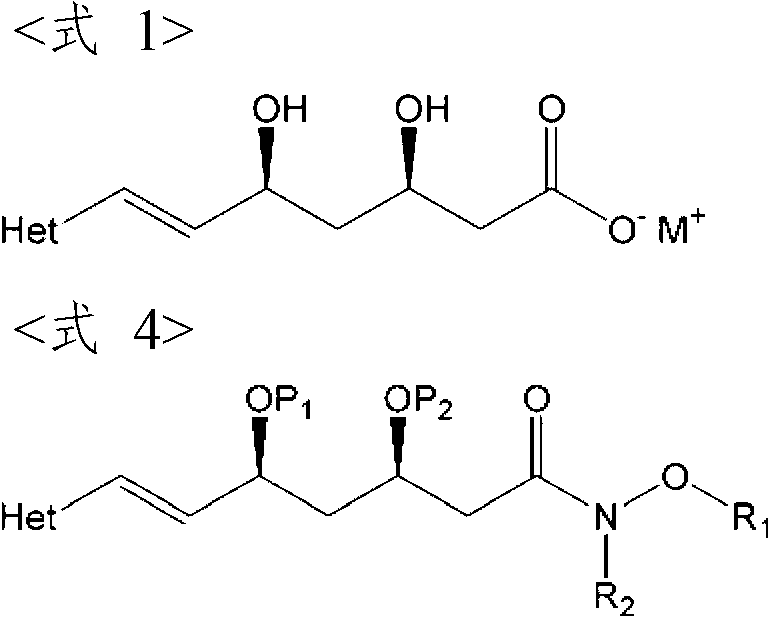
The present invention provides an improved process for preparing HMG-CoA reductase inhibitors such as rosuvastatin calcium, fluvastatin sodium, and pitavastatin calcium under a mild condition, using a novel amide-bond-containing compound having R2—N—O—R1 moiety as a key intermediate. And also, the present invention provides the novel compound, an intermediate useful for the preparation thereof, and a process for the preparation thereof.
Owner:YUHAN
Stable pitavastatin calcium pharmaceutical composition and preparation method thereof
ActiveCN104095850AImprove stabilityAvoid degradationMetabolism disorderInorganic non-active ingredientsZincPitavastatin calcium
The invention discloses a stable pitavastatin calcium pharmaceutical composition, at least one of zinc oxide, aluminium oxide and ferric oxide is taken as a stabilizing agent, and the obtained pitavastatin calcium pharmaceutical composition and a further-prepared preparation product both have good stability, and the dissolving-out property of the preparation accords with the medicinal standard.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Preparation method of pitavastatin calcium
InactiveCN104072415ALow costSuitable for mass productionOrganic chemistryPitavastatin calciumStereochemistry
The invention discloses a preparation method of pitavastatin calcium. The preparation method comprises the following steps: (1) preparing an intermediate I; (2) preparing an intermediate II; (3) preparing an intermediate III; (4) preparing an intermediate IV; and (5) preparing pitavastatin calcium. The preparation method disclosed by the invention has the beneficial effects that the synthetic route disclosed by the invention is simple and feasible and reasonable in cost and suitable for mass production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Pitavastatin calcium preparation and preparation technology
InactiveCN102861018AEasy to takeStability impactPharmaceutical non-active ingredientsDrageesMedicineAdhesive
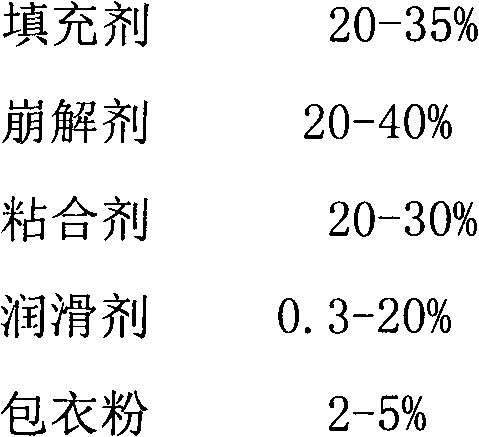
The invention provides a pitavastatin calcium preparation, which employs a direct powder tabletting technology. The weight ratio of a medicine to the auxiliary materials is 1:90-150, the pharmaceutic auxiliary materials comprise the following components by weight: 20-35% of filler, 20-40% of disintegrating agent, 20-30% of adhesive, 0.3-20% of lubricant and 2-5% of coating powder. The auxiliary materials are common pharmaceutic auxiliary materials, the medicine stability is good, the biological availability is high, the powder fluidity is good in tabletting, the compressibility is strong, and the requirements of coating and production can be satisfied.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Preparation method of pitavastatin calcium intermediate
Owner:FOSHAN DAYI TECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com