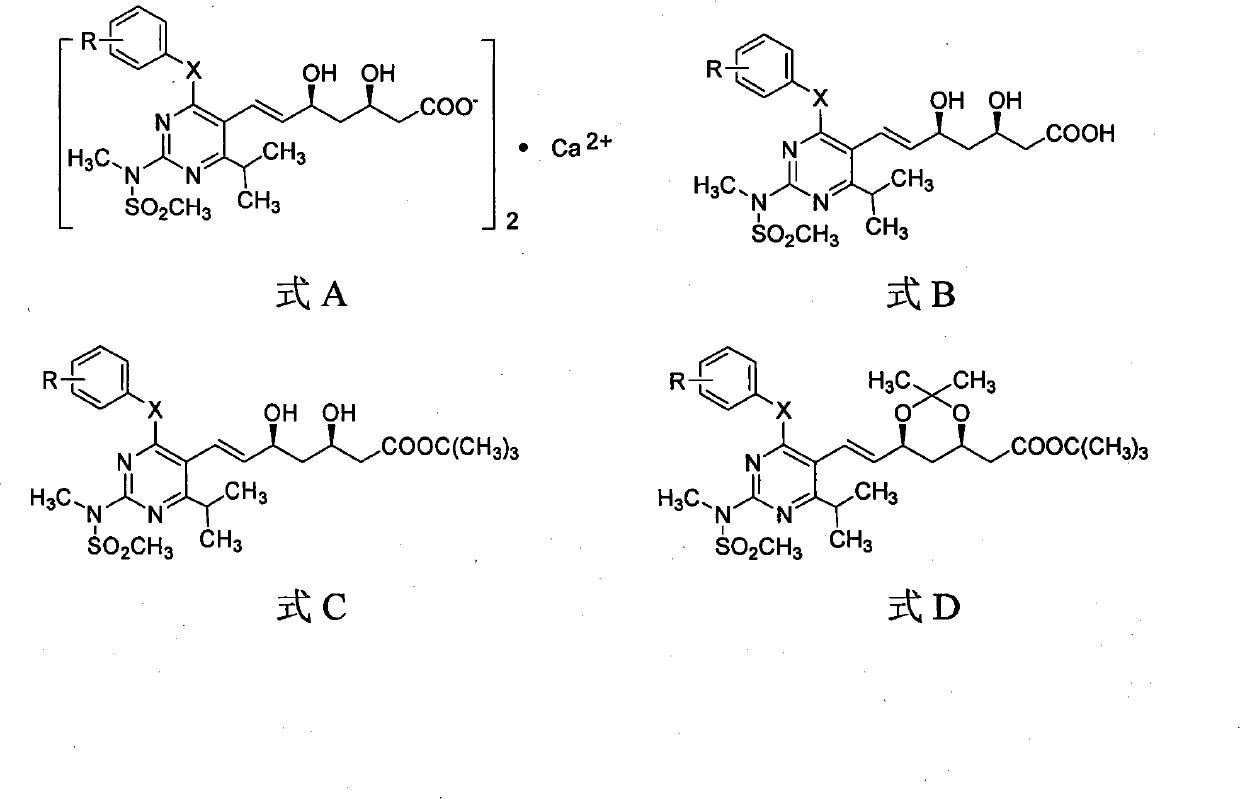
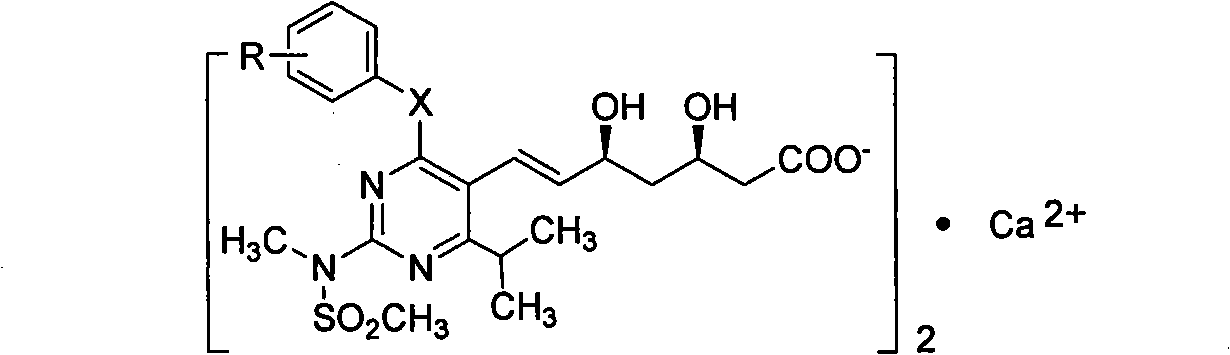
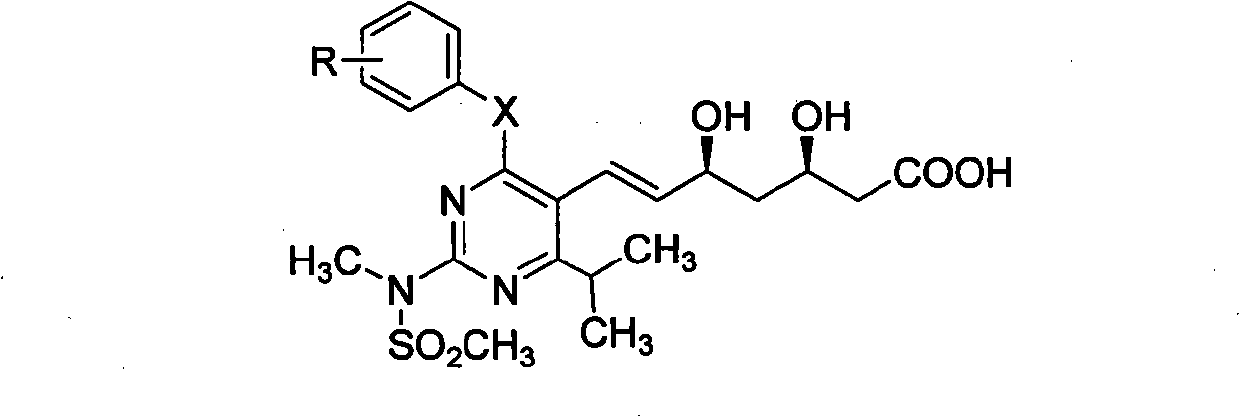
Miazine compounds, intermediates of miazine compounds, preparation method of intermediates and miazine compounds as well as application of miazine compound
A technology of compounds, pyrimidines, applied in the field of pharmaceutical chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1 2-ethoxycarbonyl-4-methyl-2-pentenoic acid ethyl ester (F)
[0103] In a 500ml three-necked flask equipped with a water separator, add diethyl malonate (15.4g, 0.096mol), isobutyraldehyde (10.6g, 0.153mol), piperidine (0.33g, 0.004mol) and acetic acid (1.14g, 0.02mol), benzene (9.3ml), stirred and refluxed for 15h. Washed with water to neutral, washed with saturated brine, anhydrous Na 2 SO 4 Dry, filter and concentrate. Distillation under reduced pressure gave 19.5 g of a colorless oily substance, yield 94.7%, bp: 107-109° C. (3 mmHg). Literature bp: 135-137°C (27mmHg). (Cope AC, Hofmamn MC, Wyckoff C, et al. Condensation reactions. II. Alkylidene cyanoacetic and malonic esters [J]. J Am Chem Soc, 1941, 63(12): 3452-3456).
Embodiment 2
[0104] Example 2 Ethyl 6-isopropyl-2-methylthio-4-hydroxy-5-6-dihydropyrimidin-5-ylcarboxylate (G)
[0105] In a 1L four-necked flask, add F (10.0g, 0.047mol), S-methylisothiourea sulfate (10.0g, 0.072mol), triethylamine (23.5ml, 0.17mol) and tetrahydrofuran (50ml), Stirring and reflux for 24h. The insolubles were filtered off, the mother liquor was concentrated, diluted with water (60ml), extracted with ethyl acetate, washed with saturated brine, anhydrous Na 2 SO 4 After drying, concentration and washing with petroleum ether, 7.0 g of white solid was obtained, with a yield of 58.0%. mp: 114-116°C. 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 7.76 (s, 1H), 4.27-4.21 (m, 2H), 3.87 (q, 1H, J = 9.6, 4.0Hz), 3.36 (d, 1H, J = 9.2Hz), 2.42 ( s, 3H), 1.82-1.78 (m, 1H), 1.28 (t, 3H, J = 7.2Hz), 1.06 (d, 3H, J = 6.8Hz), 0.92 (d, 3H, J = 6.8Hz).
Embodiment 3
[0106] Example 3 Ethyl 6-isopropyl-2-methylthio-4-hydroxy-pyrimidin-5-ylcarboxylate (H)
[0107] In a 2L four-necked flask, add G (12.0g, 0.046mol), DDQ (14.0g, 0.062mol) and 1,4-dioxane (100ml), stir at room temperature for 24h, concentrate the reaction solution, and weigh Crystallized to obtain 8.2 g of white crystals, with a yield of 68.9%. mp: 152-154°C. 1 H-NMR (400MHz, CDCl 3 )δ(ppm): 12.4(brs, 1H), 4.39(q, 2H, J=6.8Hz), 3.18(t, 1H, J=6.8Hz), 2.58(s, 3H), 1.38(t, 3H, J=6.8Hz), 1.21 (d, 6H, J=6.4Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com