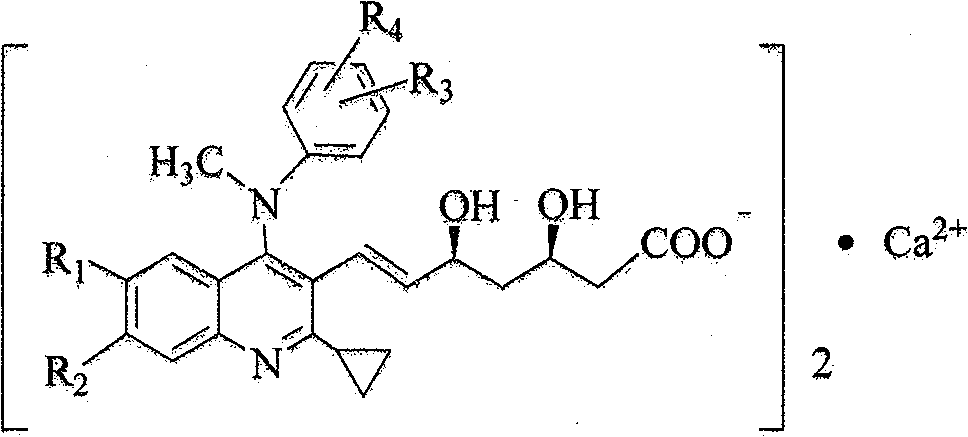
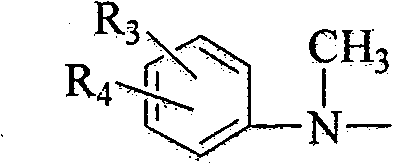
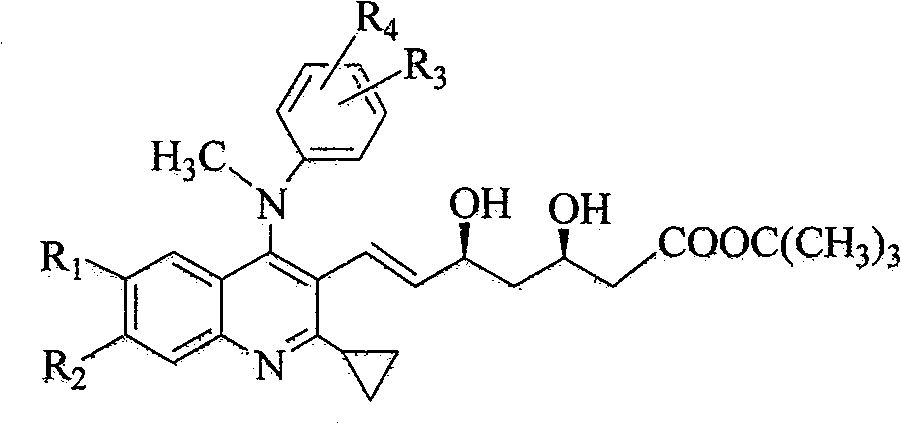
2-cyclopropyl-4-(N-methyl substituted anilino) quinoline compound as well as intermediate, preparation method and application thereof
A technology of methyl anilino group and methyl substitution, which is applied in the fields of metabolic diseases, organic chemistry, drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 (4-fluoroanilino-cyclopropyl-methylene) diethyl malonate (T2)
[0061] (Chloro-cyclopropyl-methylene)diethyl malonate (4.93g, 0.02mol), 4-fluoroaniline (2.66g, 0.024mol), potassium carbonate (2.76g, 0.02mol) were added to DMF (60ml ), stirred and heated to 100°C for 11 h. Cooled, poured into ice water (100ml), extracted with ethyl acetate, concentrated, and obtained 5.79g (4-fluoroanilino-cyclopropyl-methylene) diethyl malonate crude product yellow oil through column chromatography ( T2), yield 90.2%.
[0062] Wherein, the synthesis of (chloro-cyclopropyl-methylene) diethyl malonate is synthesized with reference to the method in the document Org syn, 1993, Coll.Vol.8, 247: diethyl malonate (150ml, 1mol) was dissolved in absolute ethanol (300ml), slowly dripped into a reaction flask equipped with magnesium chips (25.0g, 1.04mol) and carbon tetrachloride (1ml), and stirred vigorously to keep the system under stable reflux. After the addition is complete, h...
Embodiment 2
[0065] Example 2 2-cyclopropyl-6-fluoro-4-hydroxyquinoline-3-carboxylic acid ethyl ester (L2)
[0066] T2 (5.79g, 0.018mol), add heat conduction oil (60ml), heat up to 200°C, and stir vigorously for 0.5h. After cooling and suction filtration, washing with toluene, and recrystallization from ethanol, 4.58 g of white needle-like crystal L2 was obtained, with a yield of 92.3%.
[0067] According to the above method, T2 was replaced by T1 and T3 respectively to obtain L1-3 respectively. Yields, physical properties and 1 H-NMR identification results are shown in Table 2.
Embodiment 3
[0068] Example 3 2-cyclopropyl-4-chloro-6-fluoroquinoline-3-carboxylic acid ethyl ester (M2)
[0069] L2 (4.58g, 16.6mmol) was added to toluene (50ml) and POCl was added dropwise 3(5.10g, 3.1ml, 33.3mmol), reflux for 4h, pour into saturated sodium bicarbonate solution, separate the organic layer, wash with water and saturated brine respectively, dry over anhydrous sodium sulfate, and concentrate to obtain 4.57g 2-cyclo Propyl-4-chloro-6-fluoroquinoline-3-carboxylic acid ethyl ester solid (M2), yield 93.5%.
[0070] According to the above method, L2 was replaced by L1 and L3 respectively to obtain M1-3 respectively. Yields, physical properties and 1 H-NMR identification results are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com