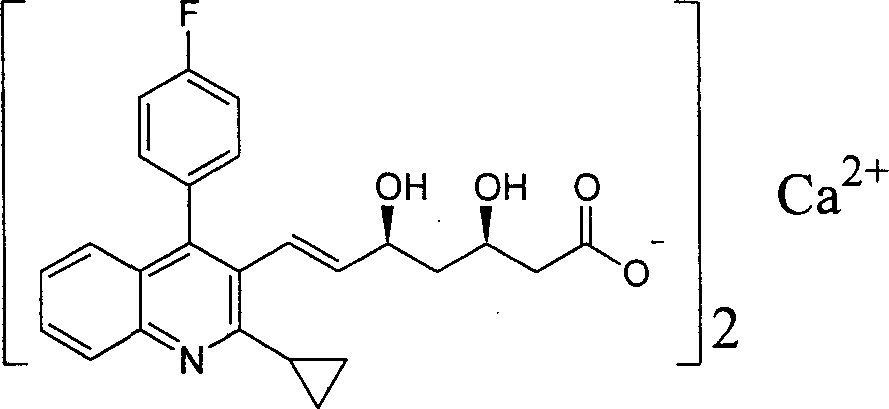
Stable pharmaceutical composition containing pitavastatin calcium and preparation process thereof
A technology of pitavastatin calcium and its composition, which is applied in the field of pharmaceutical compositions, can solve problems such as the influence of the stability of the main drug, and achieve the effects of improving general properties, reducing process steps, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Group ratio one
[0030] Pitavastatin Calcium 1mg
[0031] Lactose 15mg
[0032] Microcrystalline Cellulose 10mg
[0034] Sodium carboxymethyl starch 5mg
[0036] Total 82mg
[0037] Wet granulation process: Mix lactose, microcrystalline cellulose, calcium phosphate and sodium carboxymethyl starch evenly according to the prescription, and then fully mix the prescribed amount of pitavastatin calcium and the mixed powder according to the method of equal incremental dilution; add An appropriate amount of 5% hypromellose solution was stirred to form wet granules; the wet granules were placed in a ventilated drying oven at 50° C. for drying, then taken out, added with magnesium stearate, and mixed evenly (the moisture content of the dry granules was 0.73%). Tablets of suitable diameter are compressed on a tablet machine.
[0038] Binder-free dry direct compression process: mix lactose, microcrystalline ce...
Embodiment 2
[0042] First, mix the auxiliary materials in the following component ratio 2 uniformly to obtain a mixed powder; then fully mix 1 mg of pitavastatin calcium and the mixed powder according to the method of equal incremental dilution, or mix uniformly by a high-speed mixing mixer, that is Get pitavastatin calcium mixed powder.
[0043] Then put the mixed powder into a dry press and press it into thin slices with suitable hardness; then pass the thin slices through a swing granulator and pulverize them into granules; at the same time, add a lubricant and fully mix them with a high-efficiency mixer to obtain pitavastatin calcium pharmaceutical composition particles.
[0044] Then the pharmaceutical composition granules are compressed into tablets by a tablet machine, that is, they are compressed into tablets with suitable diameters on the tablet machine.
[0045] Finally, the Opadry is made into an aqueous dispersion of film-coating liquid, and the plain tablet is coated with a f...
Embodiment 3
[0059] Preparation of Pitavastatin Calcium Film-Coated Tablets: After mixing lactose, microcrystalline cellulose and calcium hydrogen phosphate in three proportions according to the composition ratio, mix uniformly by equal-volume incremental dilution method according to the proportion of pitavastatin calcium, and mix the mixed powder Putting it into a dry press machine to make flakes; then put the flakes into a swinging granulator to pulverize into granules, add a lubricant at the same time, and mix them uniformly in a mixer to obtain the pharmaceutical composition granules of pitavastatin calcium.
[0060] Then the pharmaceutical composition granules are compressed into tablets by a tablet machine, that is, they are compressed into tablets with suitable diameters on the tablet machine.
[0061] Finally, the Opadry is made into an aqueous dispersion of film-coating liquid, and the plain tablet is coated with a film-coat by a high-efficiency coating machine to obtain pitavastat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com