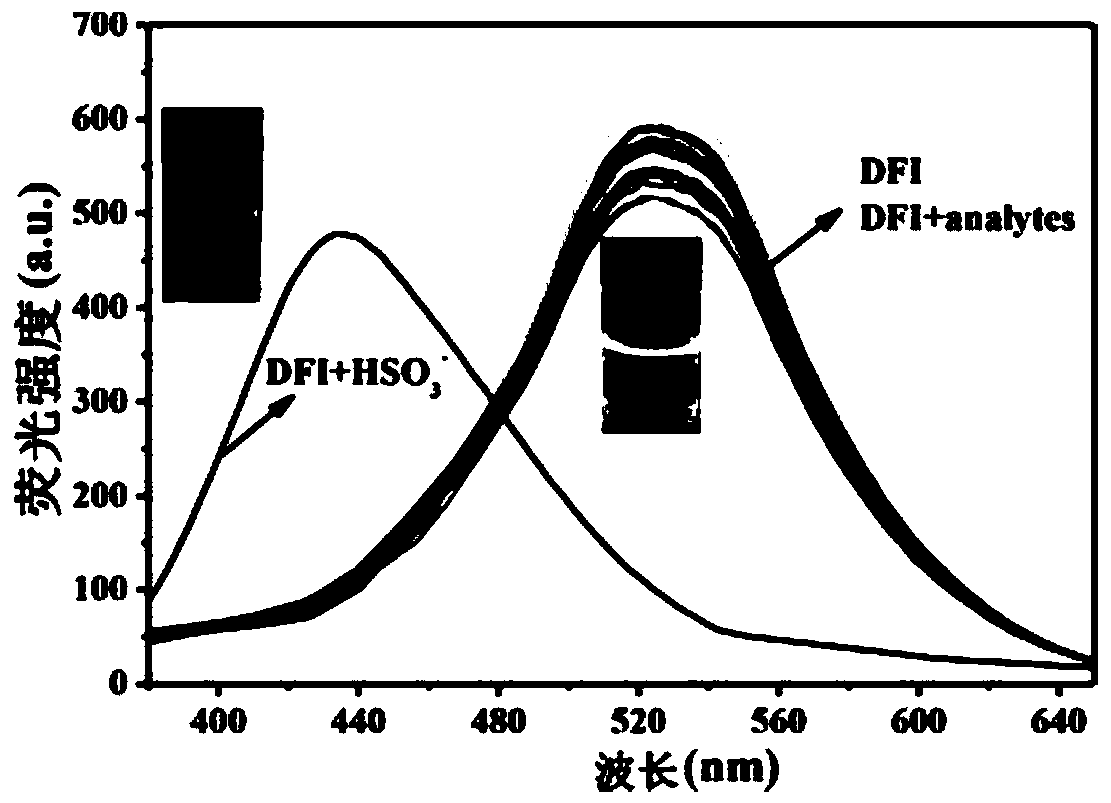
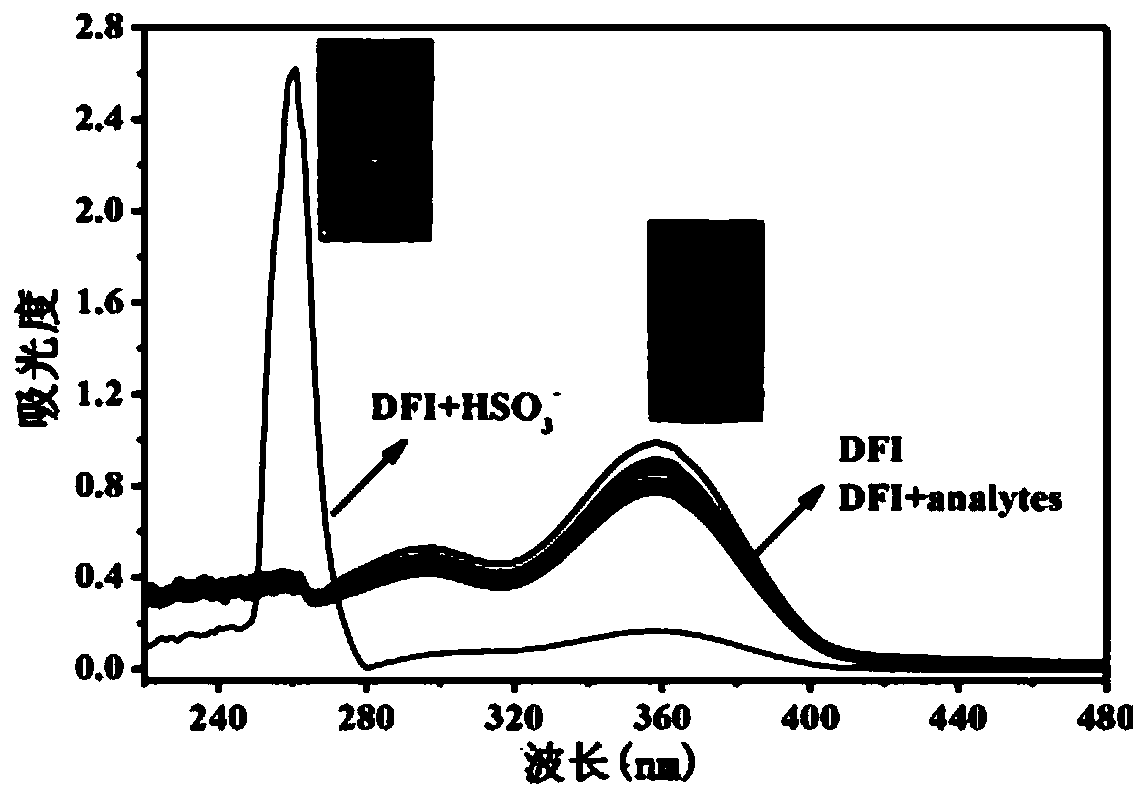
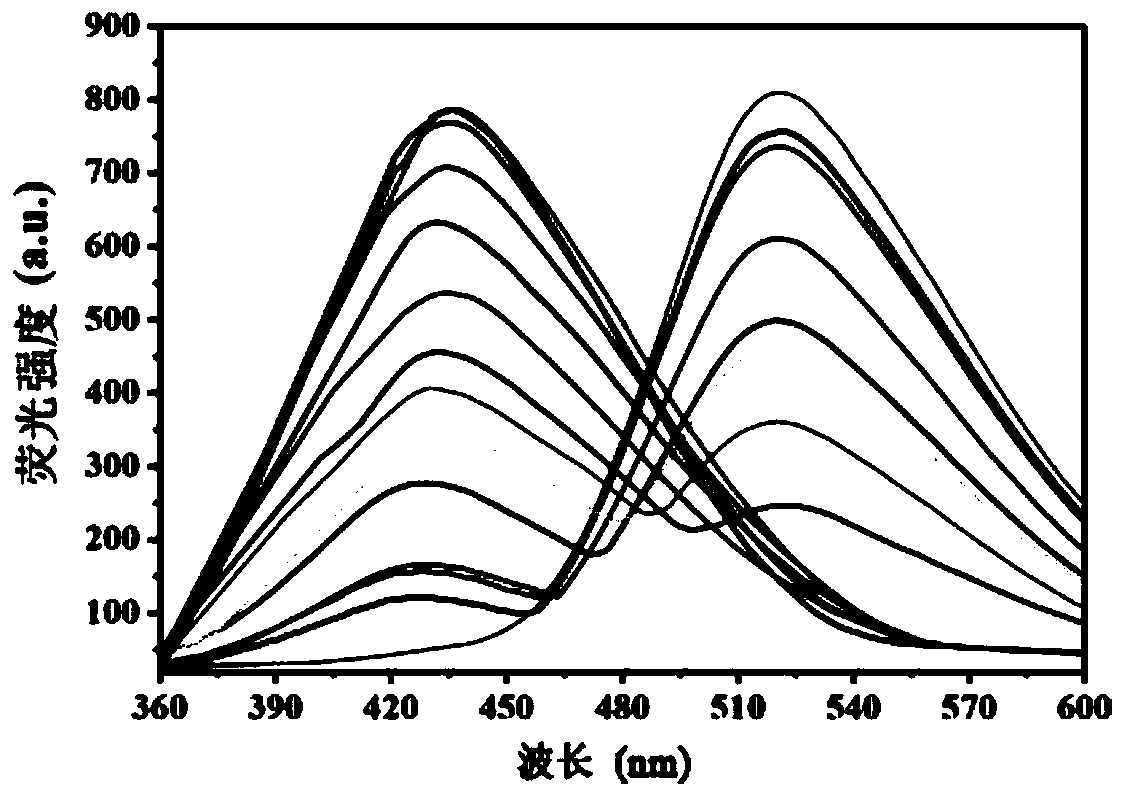
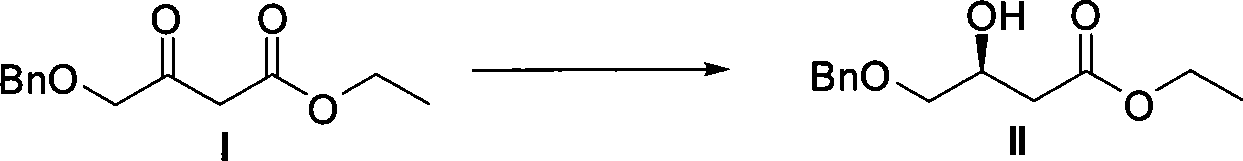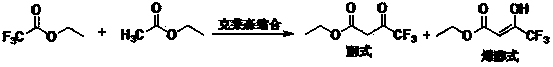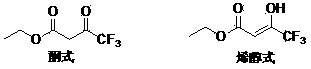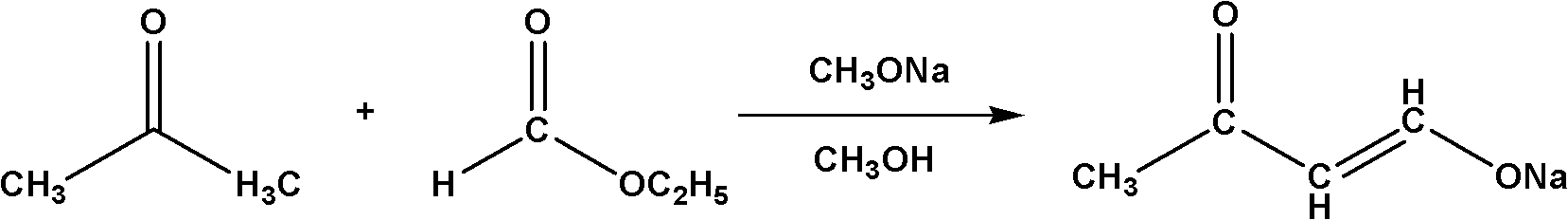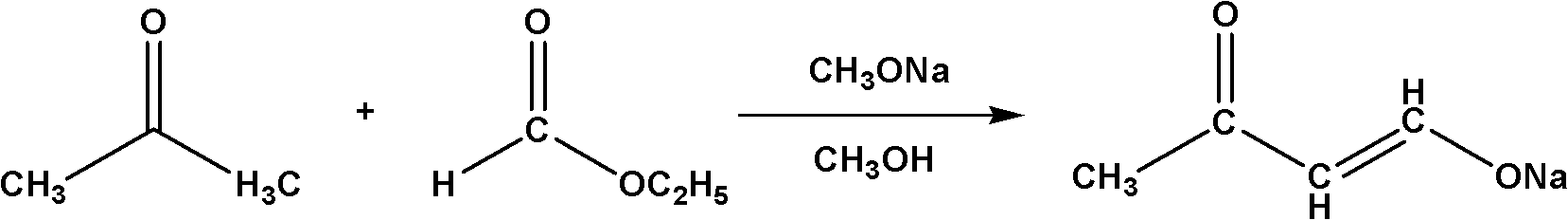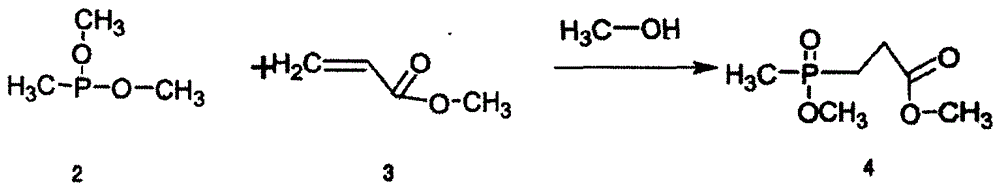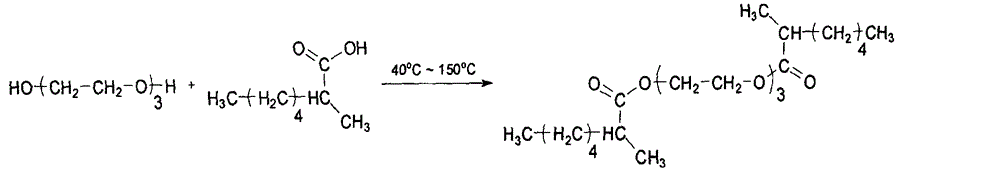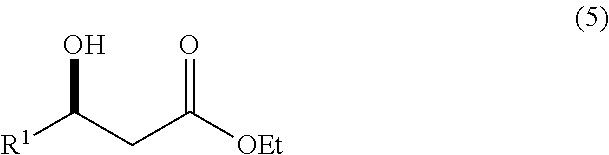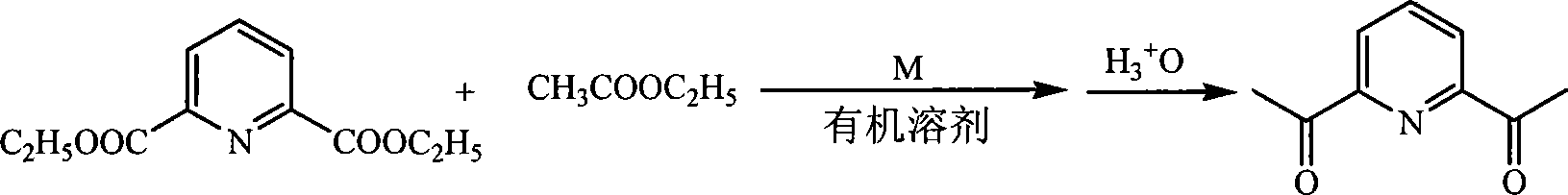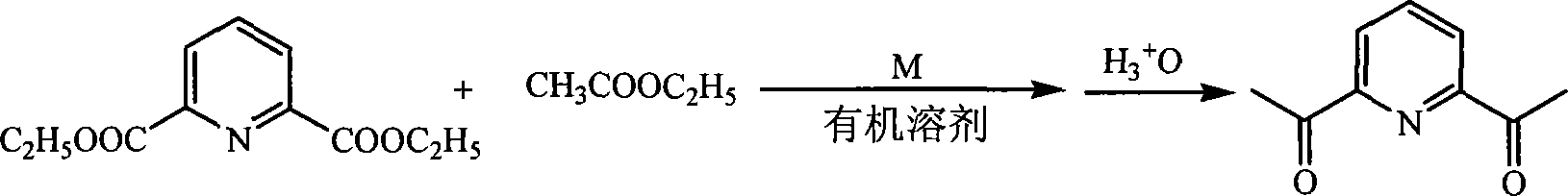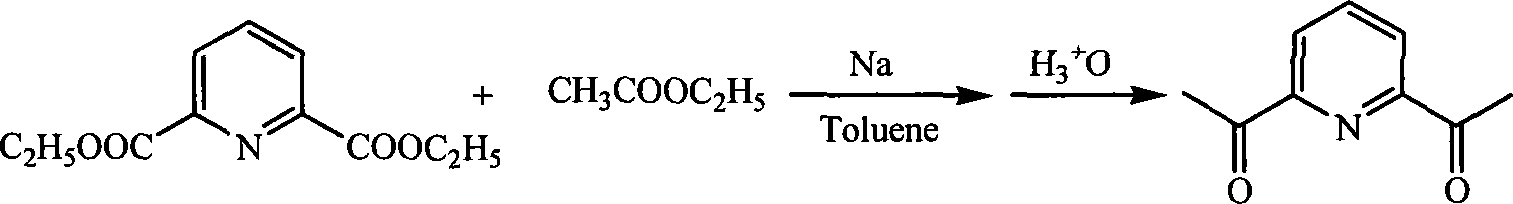Patents
Literature
64 results about "Claisen condensation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887.
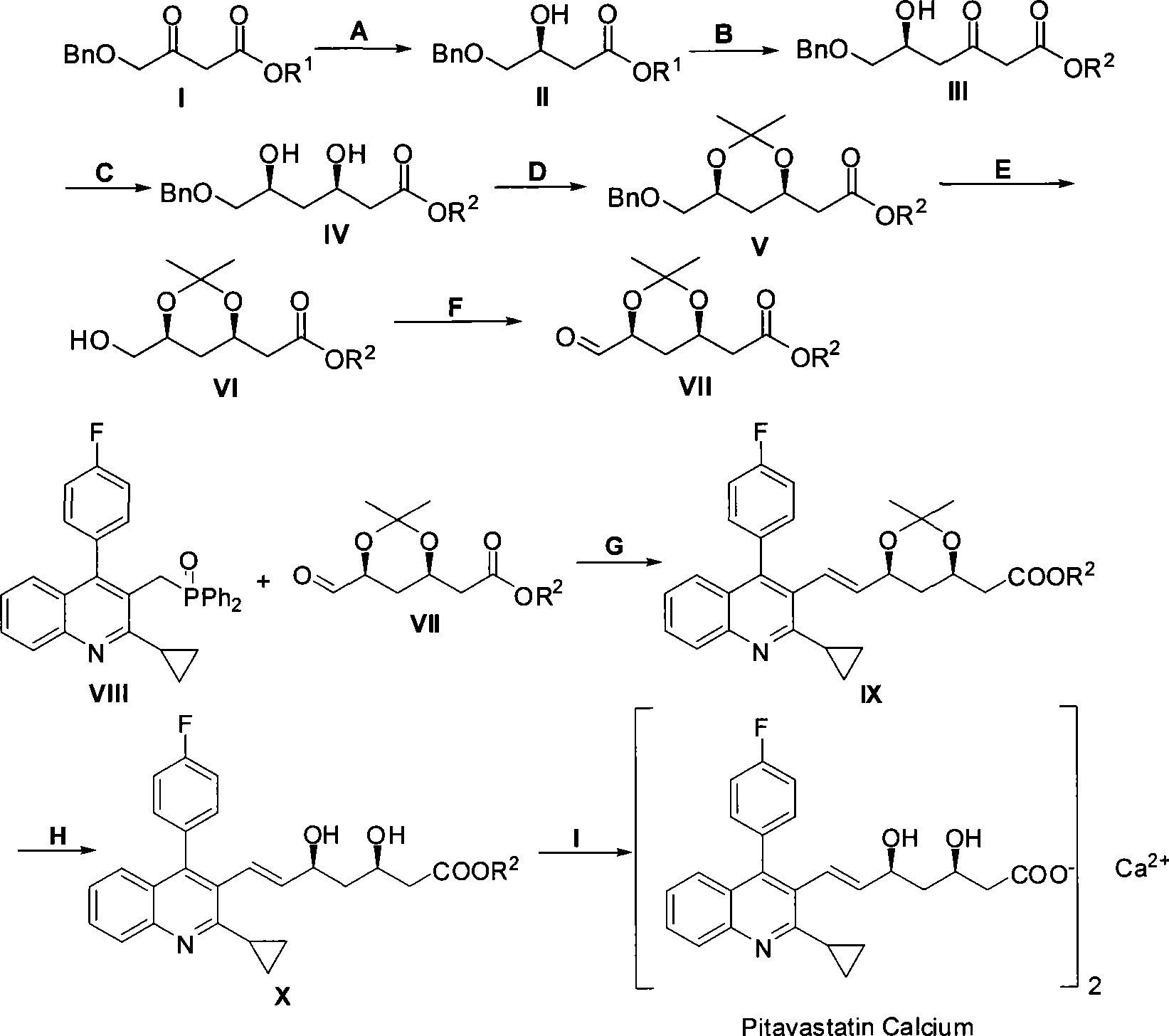
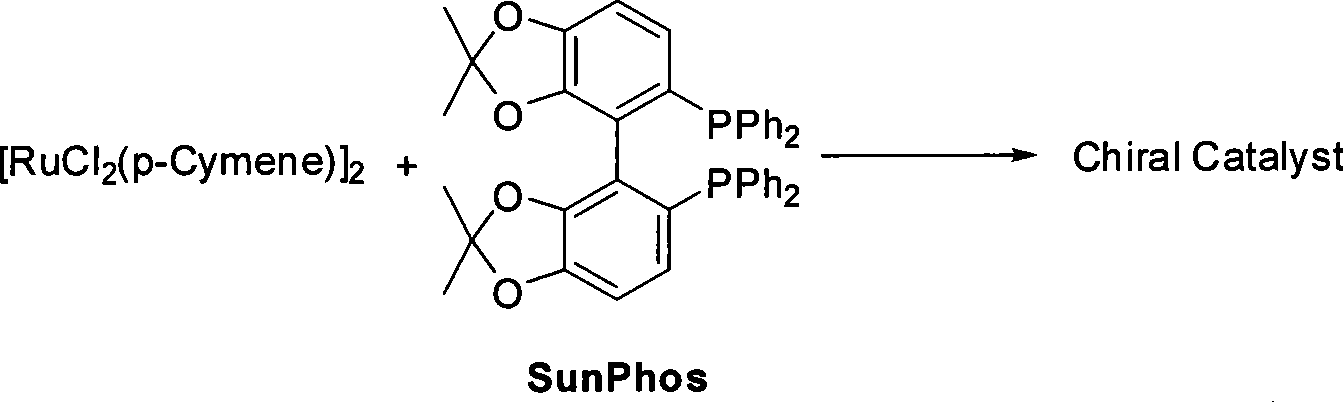
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
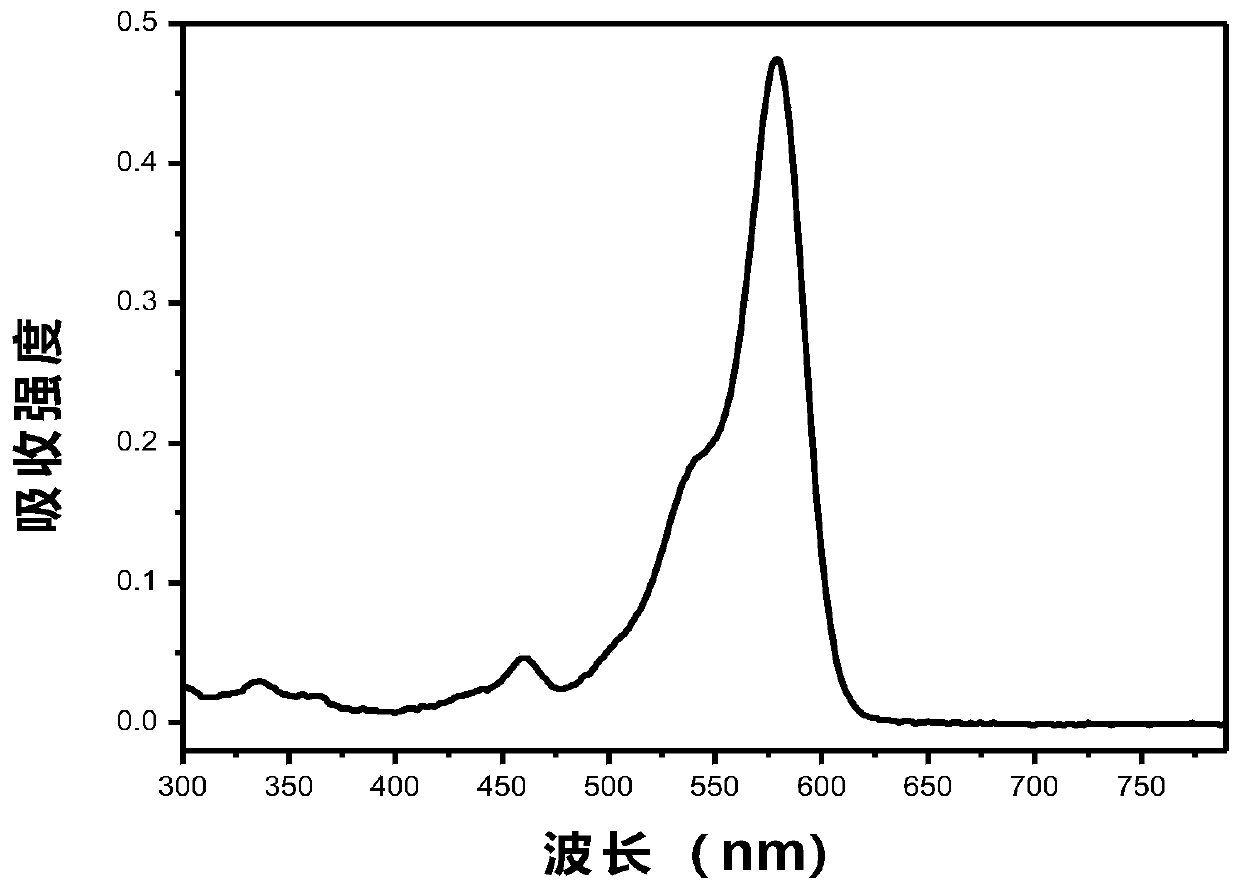
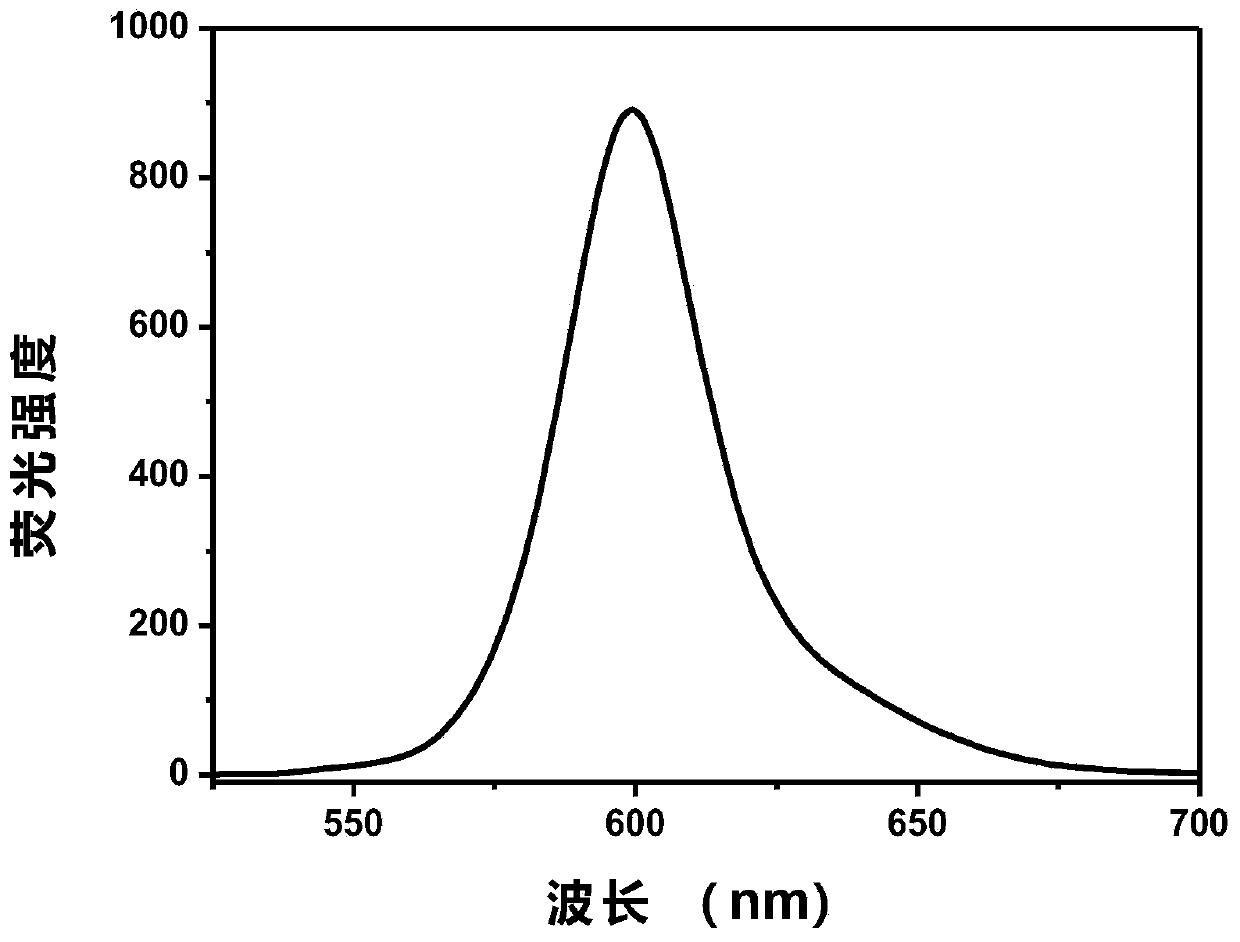
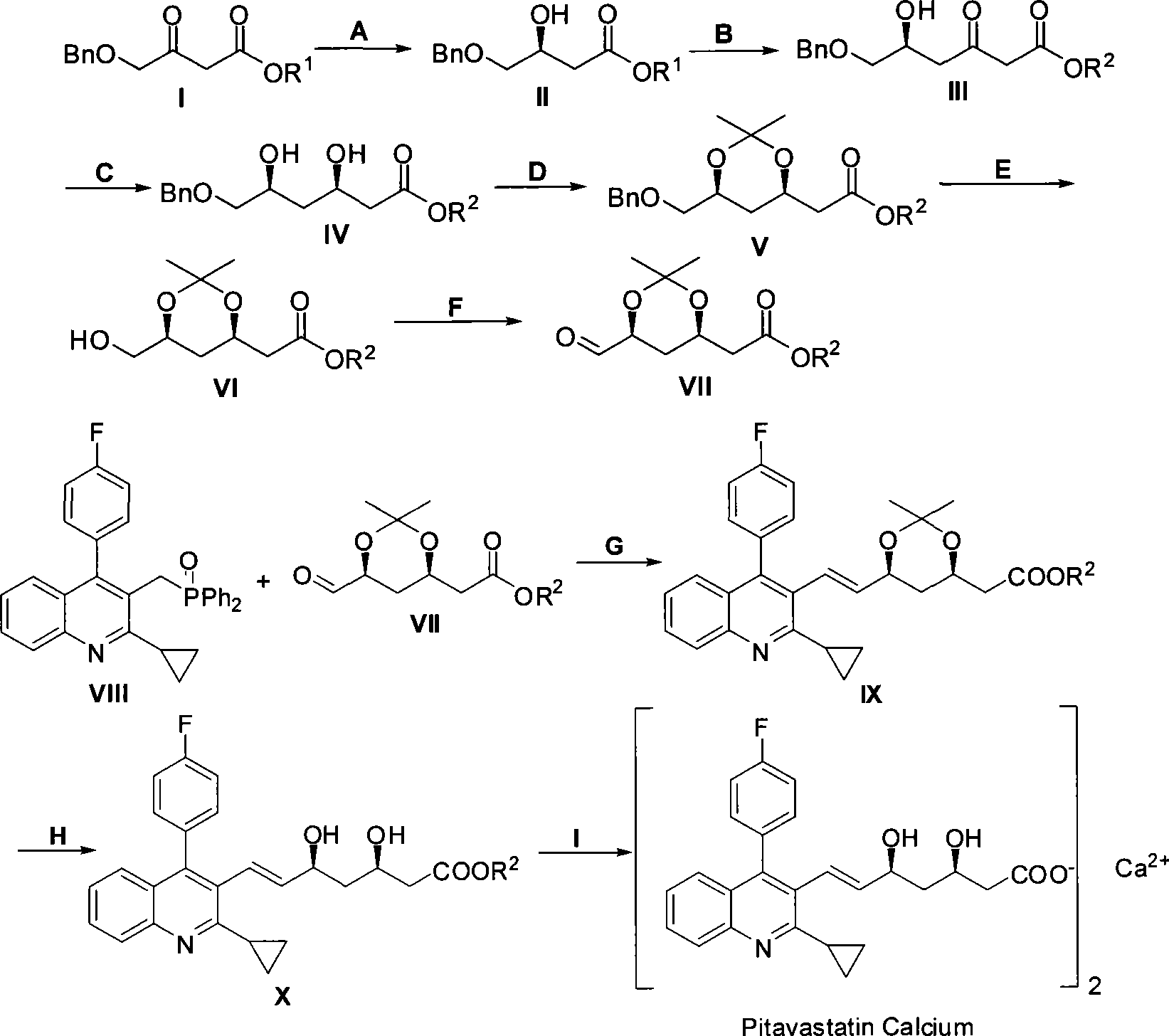
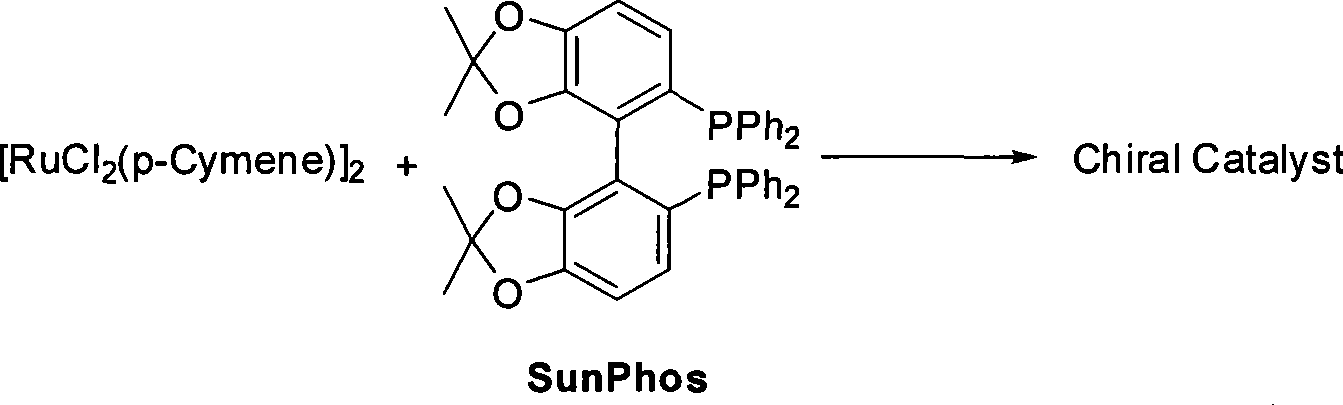
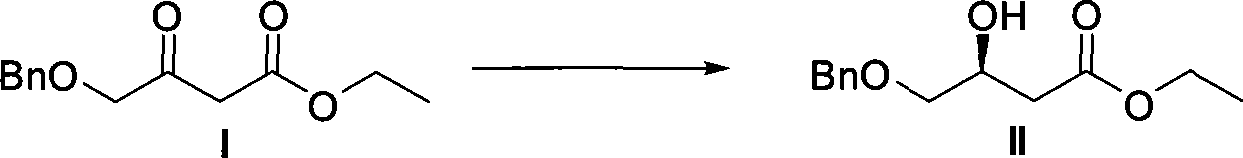
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
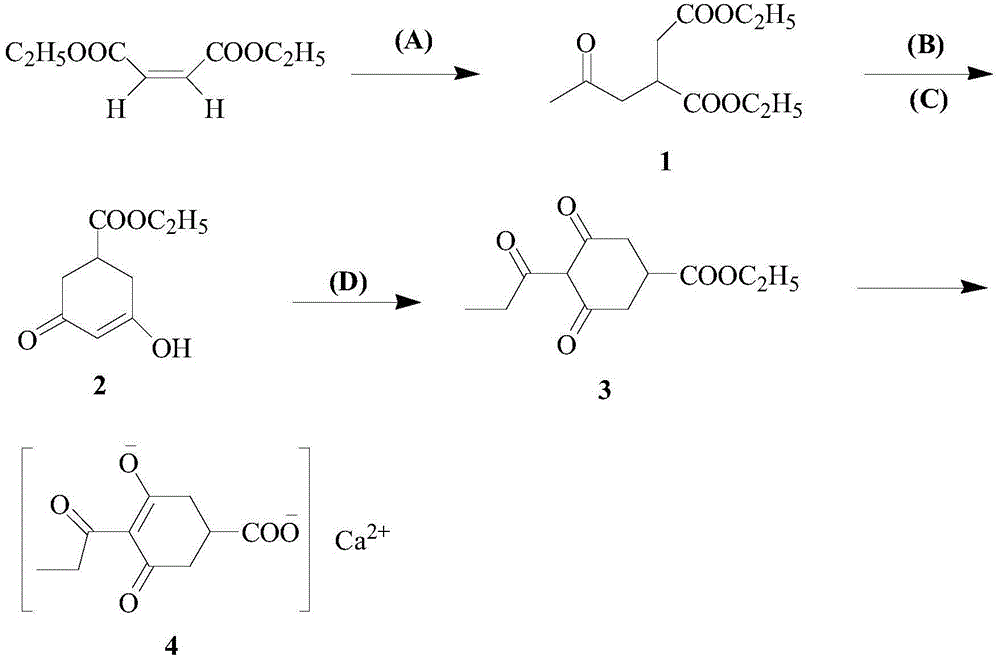
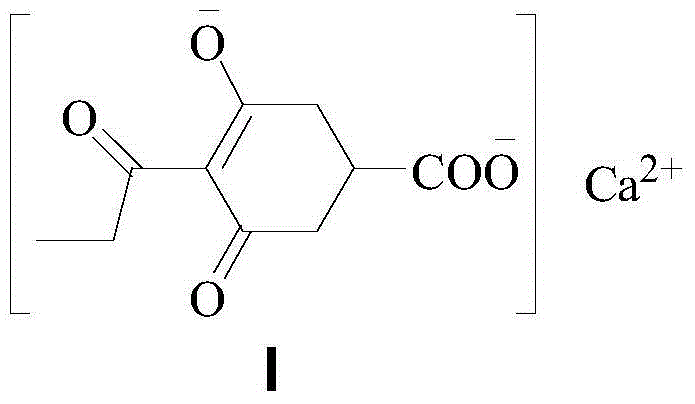
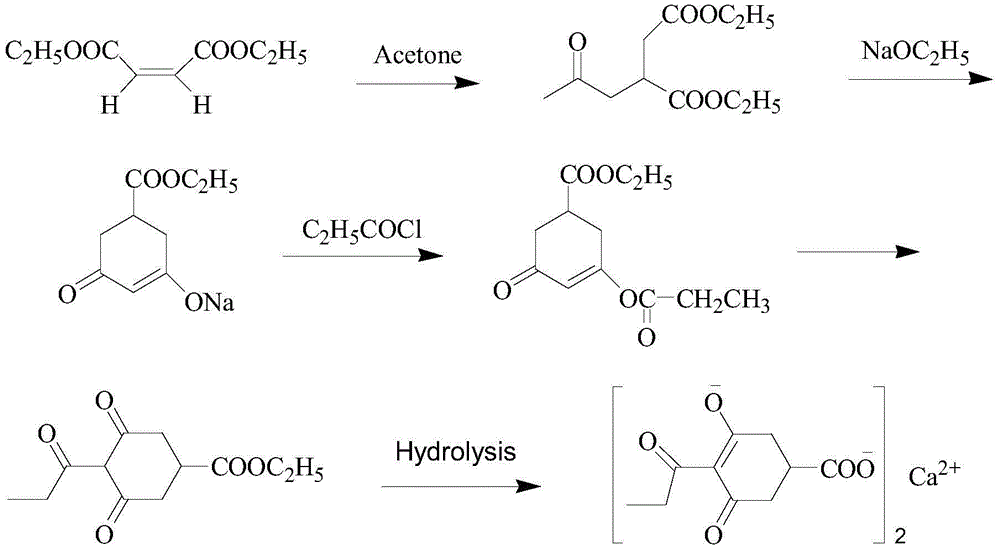
Method for preparing calcium3-oxido-5-oxo-4-propionyl cyclohex-3-enecarboxylate
ActiveCN104140368AReduce manufacturing costSuitable for industrial productionOrganic compound preparationCarboxylic acid esters preparationClaisen condensationOrganic base
Owner:JIANGXI AGRICULTURAL UNIVERSITY
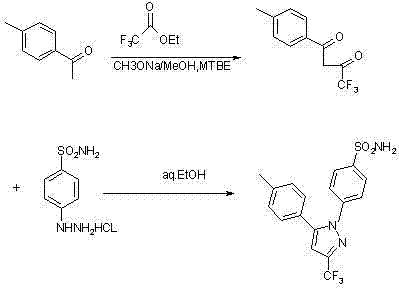
Synthesis method of celecoxib
InactiveCN102391184AHigh purityHigh yieldOrganic chemistryPhenylhydrazine hydrochlorideClaisen condensation
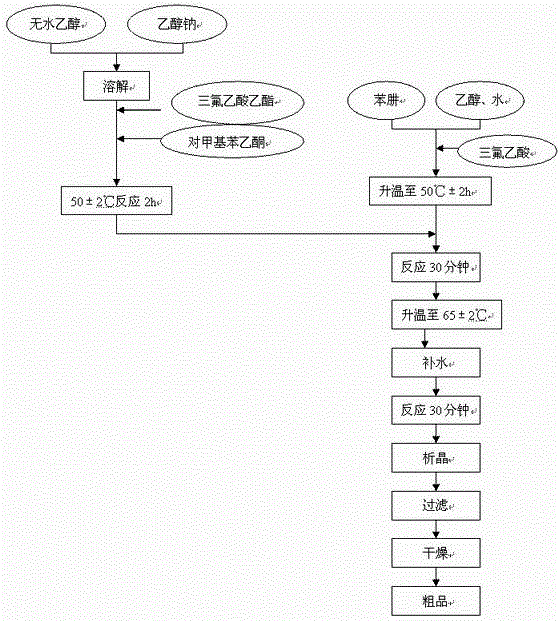
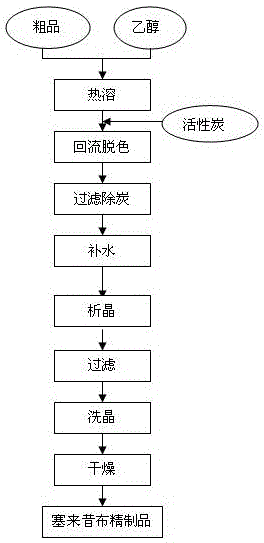
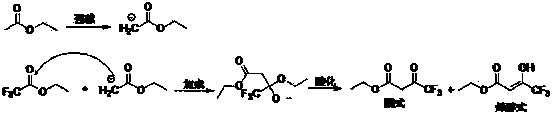
The invention relates to a synthesis method of celecoxib, which comprises the following specific steps of: 1, carrying out claisen condensation on p-methylacetophenone and trifluoroacetic acid ethyl esters in an aprotic organic solvent under the catalysis of alkali to obtain 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione; and 2, reacting the obtained 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione with sulfonamide-phenylhydrazine hydrochloride to obtain celecoxib, wherein in the step 1, the alkali for catalysis is selected from one or more of sodium hydride, potassium hydride, lithium hydride and calcium hydride. The synthesis method of celecoxib provided by the invention is easy to operate, high in yield, high in product purity and easy for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Celecoxib preparation method
InactiveCN105130901AFine granularitySuitable for productionOrganic chemistryClaisen condensationAcetophenone
The present invention relates to a celecoxib preparation method, wherein 4'-methylacetophenone and ethyl trifluoroacetate are adopted as raw materials, Claisen condensation is performed to obtain a beta-diketone intermediate, the beta-diketone intermediate and p-hydrazinobenzenesulfonamide hydrochloride are subjected to condensation cyclization in ethanol to obtain celecoxib, and refining and crystallization are performed to obtain the celecoxib crystal. According to the present invention, with the preparation method, the celecoxib can be obtained in the high yield manner, the purity of the obtained product is high, the single impurity can be controlled to be less than or equal to 0.5%, and the obtained celecoxib crystal has characteristics of fine particle size and uniform distribution, and is suitable for bulk drug production.
Owner:SUZHOU ERYE PHARMA CO LTD
Preparation of beta-dione
ActiveCN101462930ASolve the problem of acylationReduce the impactOrganic compound preparationCarbonyl compound preparation by condensationDiketoneClaisen condensation
The invention relates to a preparation method of beta-diketone; Claisen condensation reaction is adopted; the chemical general expression of the beta-diketone is R1COCH2COR2, wherein, R1 is C6-C12 alkyl, and R2 is phenyl or alkylphenyl; the method comprises the following steps: ester, phenyl ketone and partial solvent are pre-mixed for spare; a condensating agent and remaining solvent are added in a reactor, and the temperature is raised under nitrogen atmosphere; during the period when the temperature of the reactor is raised to the needed temperature of the condensation reaction, the pre-mixed material is dropwise added in the reactor for carrying out the condensation reaction with a system which is composed of the condensating agent and the remaining solvent under an infinite reflux condition; after the pre-mixed material is dropwise added, heat-preserving condensation reaction is carried out continuously under the infinite reflux condition. The ester and the condensating agent in the raw material are contacted when the reacting condition is achieved, and acylation reaction is slow reaction, relative to the Claisen condensation reaction, thus being capable of better solving the acidylation problem of the ester, and reducing the effect of the acylation reaction to the minimum; the yield of the beta-diketone is more than or equal to 90 percent.
Owner:KUNSHAN CITY BANMING ELECTRONICS SCI & TECH
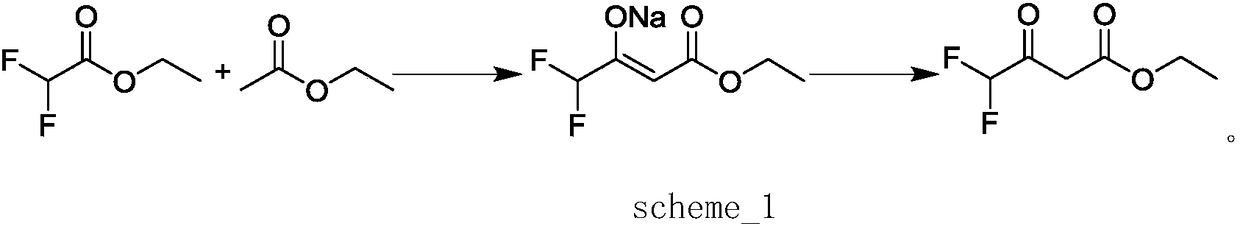
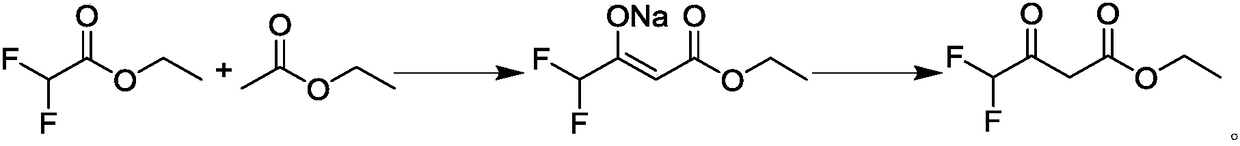
Preparation method of ethyl 4,4,4-trifluoroacetoacetate
InactiveCN103694119AThorough responseHigh yieldOrganic compound preparationCarboxylic acid esters preparationClaisen condensationTrifluoroacetic acid
The invention discloses a preparation method of ethyl 4,4,4-trifluoroacetoacetate. With an ethanol solution of sodium ethoxide as a catalyst and in the presence of an organic solvent, ethyl trifluoroacetate and ethyl acetate are subjected to a Claisen condensation reaction, and ethyl 4,4,4-trifluoroacetoacetate is synthesized. The method provided by the invention has the advantages of mild conditions, simple operation, relatively high conversion rate of raw materials, relatively high product selectivity, easy separation of the product, low energy consumption and the like, and is suitable for industrialized production. The prepared ethyl 4,4,4-trifluoroacetoacetate is an important pesticide and medicine intermediate, can be used for preparation of thifluzamide, fluacrypyrim, thiazopyr and other pesticides, and also can be used for preparation of befloxatone and other medicines.
Owner:SINOCHEM LANTIAN +1
Fluorescent probe capable of quickly detecting hydrogen sulfite ions and preparation method and application thereof
InactiveCN109734738ASpecific complexationHigh selectivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluorescenceClaisen condensation
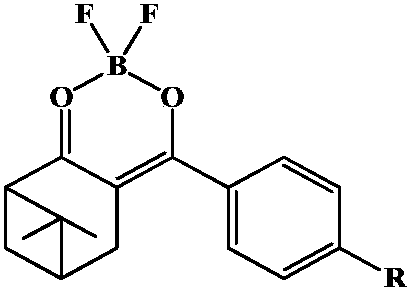
The invention discloses a fluorescent probe capable of quickly detecting hydrogen sulfite ions and a preparation method and application thereof. The preparation method includes: taking beta-pinene oxidation derivative nopinone which is one of main ingredients of natural renewable natural resource turpentine as a raw material, and allowing Claisen condensation reaction with 4-methyl bromobenzoate to obtain 3-(4'-bromobenzoyl) nopinone; allowing 3-(4'-bromobenzoyl) nopinone to be in coupling reaction with 4-formylbenzeneboronic acid to obtain 3-(4''-formyl-1', 1''-biphenyl-4'-carbonyl) nopinone;allowing 3-(4''-formyl-1', 1''-biphenyl-4'-carbonyl) nopinone to react with boron trifluoride etherate to obtain a 3-(4''-formyl-1', 1''-biphenyl-4'-carbonyl) nopinone based fluoroboron complex. Thecompound serving as a high-selectivity high-sensitivity color ratio type fluorescent probe has good application in the aspect of detecting the hydrogen sulfite ions.
Owner:NANJING FORESTRY UNIV
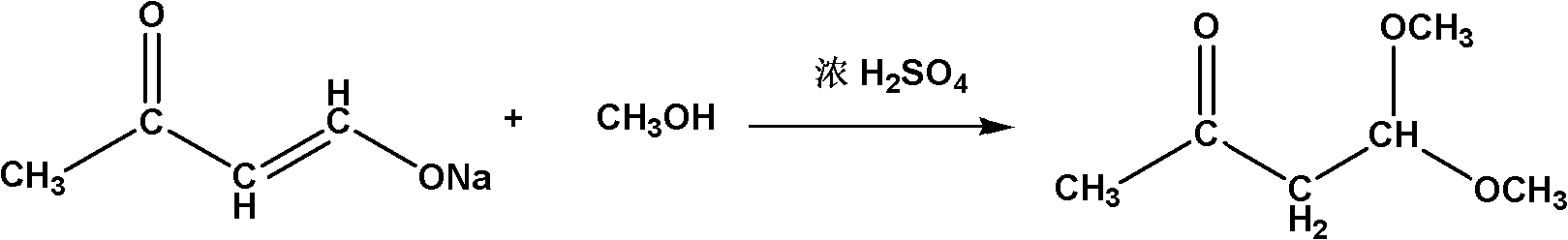
Method for synthesizing 4,4-dimethoxy-2-butanone
InactiveCN102010311AHigh boiling pointEasy to storeOrganic compound preparationCarbonyl compound preparationSodium methoxideClaisen condensation
The invention relates to a method for synthesizing a 4,4-dimethoxy-2-butanone fine chemical engineering raw material. The method comprises the following steps of: performing a Claisen condensation reaction of ethyl formate, liquid sodium methoxide, acetone and concentrated sulfuric acid, which serve as raw materials, to prepare butanone sodium enolate, dripping solution of the treated butanone sodium enolate into solution of methanol containing the concentrated sulfuric acid and reacting to obtain a crude product of the 4,4-dimethoxy-2-butanone, and distilling under normal pressure and reduced pressure to obtain the 4,4-dimethoxy-2-butanone. In the invention, the raw materials are cheap, and the method and process are simple, convenient to operate and suitable for large-scale industrial production and have a high industrial application value.
Owner:SOUTHEAST UNIV
Synthesizing method for glufosinate-ammonium ammonium salt
ActiveCN106632467AEasy to separate and purifyLow costGroup 5/15 element organic compoundsPhosphateClaisen condensation
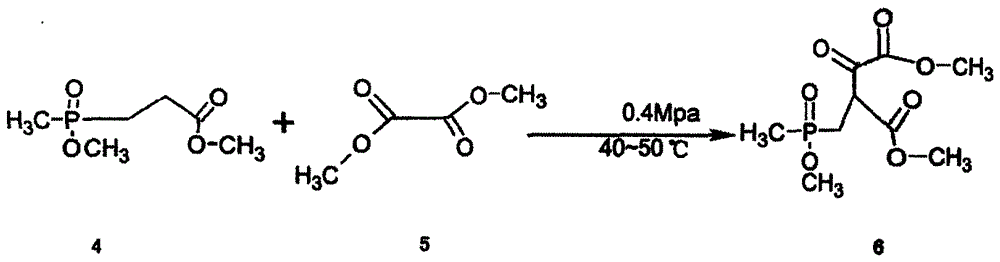
The invention relates to a synthesizing method for glufosinate-ammonium ammonium salt. The synthesizing method comprises the following steps: 1) synthesizing methyl methoxyphosphoryl propionate by the reaction of methyl phosphite dimethyl phosphate with methyl acrylate and methyl alcohol; 2) synthesizing 2-[(methyl methoxy)phosphoryl]methyl-3-oxo-butyrate dimethyl ester by the Claisen condensation of methyl methoxyphosphoryl propionate and dimethyl oxalate; 3) under the acidic condition, carrying out the hydrolysis reaction to the 2-[(methyl methoxy)phosphoryl]methyl-3-oxo-butyrate dimethyl ester so as to generate 4-hydroxymethylphosphonic oxide butyric acid; and 4) under the catalyst function, successively reacting with ammonia gas and hydrogen by the 4-hydroxymethylphosphonic oxide butyric acid, and preparing the glufosinate-ammonium ammonium salt. The synthesizing method is capable of avoiding using cyanide poisonous materials. The format reaction is not used, the amount of the solvent used in the production process is small, the reaction condition is moderate and easily controlled, the synthesizing method is suitable for industrial production, the production is safe and convenient to operate, the reaction process is short, and the yield is high.
Owner:石家庄瑞凯化工有限公司
Method for continuous production of dimethyl succinylo succinate
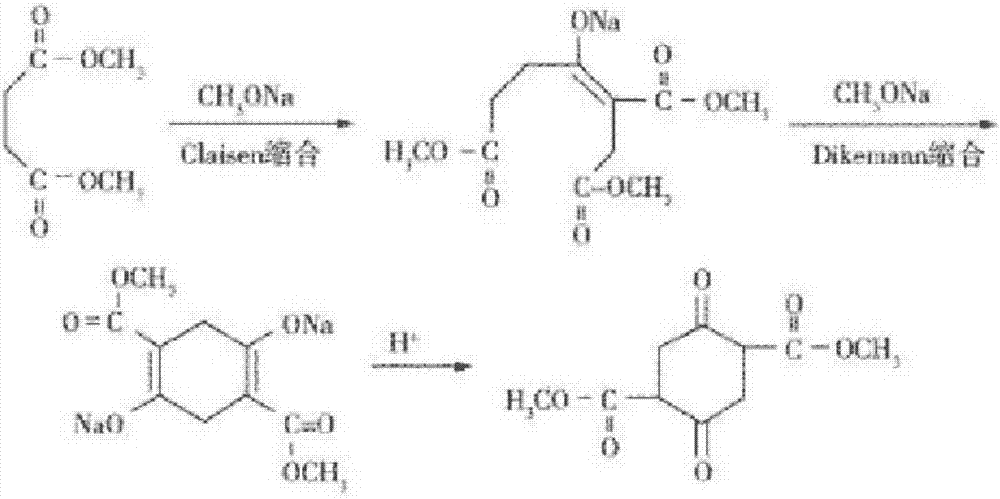
InactiveCN106986771AOvercome the problem of large quality differencesShort reaction timeOrganic compound preparationCarboxylic acid esters preparationSodium methoxideClaisen condensation
The invention provides a method for continuous production of dimethyl succinylo succinate. The technical scheme employs continuous drip adding to carry out Claisen condensation reaction on dimethyl succinate and sodium methoxide and Dieckmann condensation reaction, and can realize continuous discharge, after a certain product is collected, and then dilute sulfuric acid acidification is carried out to obtain dimethyl succinylo succinate. The method provided by the invention is designed based on classical experimental principles, and the operation mode is improved to form a brand-new technological process. The technical scheme shortens the reaction time, also reduces energy consumption, significantly improves the production efficiency, and at the same time overcomes the problem of large quality difference between different batches in batch production. The method provided by the invention takes innovative technological improvement and achieves good technical effect, simultaneously has the characteristics of low cost and easy realization, thus having good popularization prospect.
Owner:淄博鸿润新材料有限公司
Preparation method of triethylene glycol di-2-ethylhexoate
InactiveCN104529760ASimple methodEasy to implementOrganic compound preparationCarboxylic acid esters preparationClaisen condensationDistillation
The invention discloses a preparation method of triethylene glycol di-2-ethylhexoate and relates to a method for preparing a plasticizer from triethylene glycol and isocaprylic acid serving as initial raw materials through processes such as Claisen condensation reaction, neutralization and distillation. The preparation method comprises the steps of adding isocaprylic acid and triethylene glycol serving as the raw materials into a reactor, stirring while heating to dissolve isocaprylic acid and triethylene glycol, keeping the temperature at 40-80 DEG C, and adding an adsorbent and a catalyst; reacting under the conditions that the temperature is 80-110 DEG C and the vacuum degree is 1-15mmHg, then, cooling, and dropwise adding a sodium hydroxide water solution with the concentration of 1-2.5%; and then, adding a decolorizing agent, distilling under the conditions that the temperature is 80-150 DEG C and the vacuum degree is 0.5-1mmHg, and filtering to obtain triethylene glycol di-2-ethylhexoate. The preparation method disclosed by the invention is simple and is easily implemented. The preparation method has the characteristics that raw materials are available, and negative pressure and low-temperature reaction is adopted, so that the production cost is greatly reduced. In addition, an adsorbent is added to inhibit side reaction and adsorb colored impurities, so that the quality of triethylene glycol di-2-ethylhexoate is improved, and the heat resistance of triethylene glycol di-2-ethylhexoate is greatly improved. The prepared triethylene glycol di-2-ethylhexoate has the chroma of less than 30 which is superior to the chroma of a domestic product (more than 50) if being detected after being heated at the temperature 180 DEG C for 2 hours. Problems existing in the existing production method are solved.
Owner:天元航材(营口)科技股份有限公司
Nopinic alkyl beta-dione boron difluoride complexes and preparation method and application thereof
InactiveCN108409765AIncrease productionLow priceSolid-state devicesSemiconductor/solid-state device manufacturingQuantum yieldClaisen condensation
The invention discloses nopinic alkyl beta-dione boron difluoride complexes and a preparation method and application thereof. The natural reusable resource beta-pinene oxidation product, namely nopinone, is used as a raw material to prepare novel nopinic alkyl beta-dione boron difluoride complexes 3a and 3b; nopinone is subjected to Claisen condensation with methyl p-methoxybenzoate 1a and methyl4-bromobenzoate 1b to separately obtain nopinic alkyl beta-dione compounds 2a and 2b; the compounds 2a and 2 b are subjected to complexing with boron trifluoride etherate to separately obtain nopinicalkyl beta-dione boron difluoride complexes 3a and 3b. The nopinic alkyl beta-dione boron difluoride complexes 3a and 3b have good fluorescent performance and high fluorescent quantum yield; an electroluminescent device made with the nopinic alkyl beta-dione boron difluoride complexes can emit blue-green light. It is shown that the nopinic alkyl beta-dione boron difluoride complexes as light-emitting materials have good application value in the preparation of electroluminescent devices.
Owner:NANJING FORESTRY UNIV
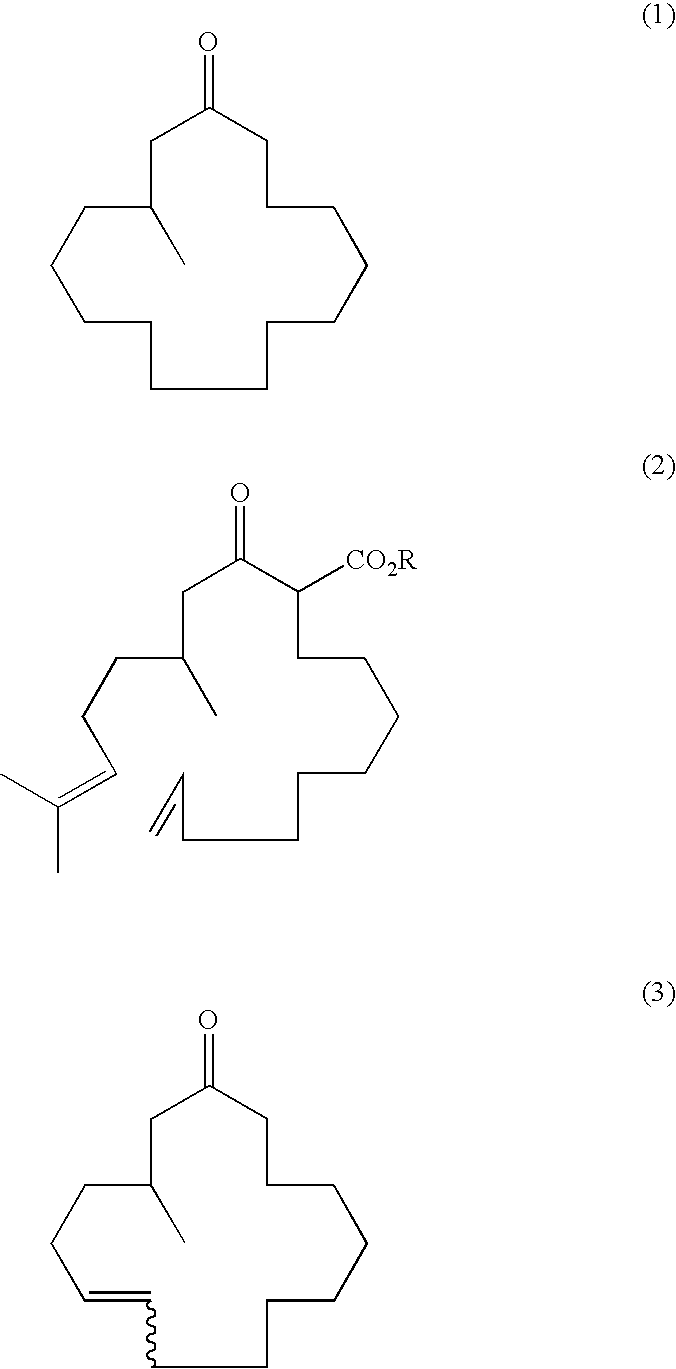
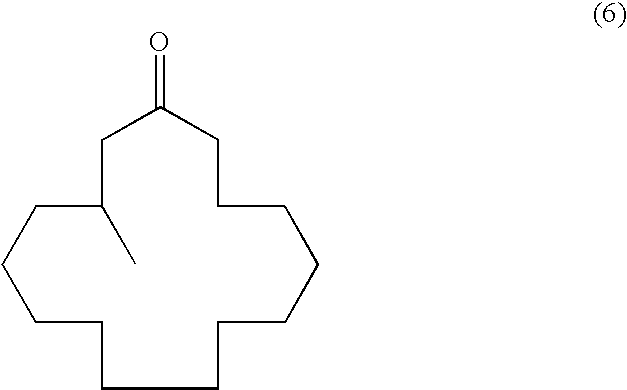
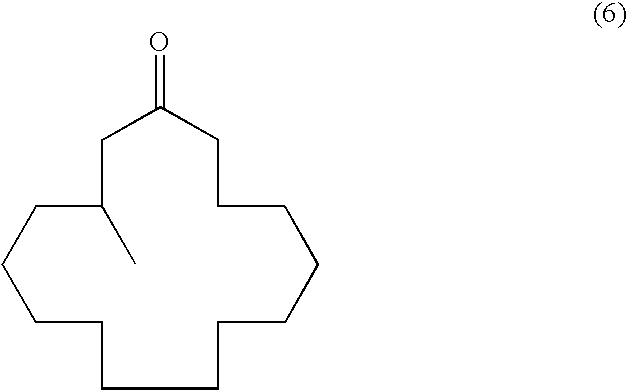
Continuous process for the production of beta-keto esters by claisen condensation
InactiveUS20140051869A1Carboxylic acid nitrile preparationOrganic compound preparationAlkaline earth metalHalogen
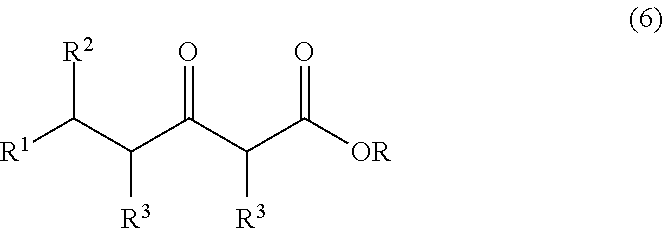
A continuous process for producing compounds having the general formula (6) is provided:wherein R is a straight or branched chain alkyl group, R1 is a straight or branched chain alkyl group substituted with a nitrile group, a hydroxy group or a halogen atom, R2 is a hydroxy group or a keto group, and each R3 is, independently, hydrogen or a straight or branched chain alkyl group, comprising providing a continuous stream of an alkyl acetate and a continuous stream of an alkali metal or alkaline earth metal amide base and contacting the continuous streams to yield an enolate compound, providing a continuous stream of a compound of formula (5):wherein R1 is as previously defined, and contacting the continuous stream of compound (5) with a continuous stream of the enolate above 20° C. to yield an intermediate compound and treating the intermediate compound with an acid.
Owner:PHOENIX CHEM LTD (GB)
Synthesis method for 1,5-ketonic ester compound under catalyzing of inorganic base
InactiveCN105061125AEasy to getMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationAlcoholSynthesis methods
The invention discloses a synthesis method for a 1,5-ketonic ester compound under catalyzing of inorganic base. The synthesis method comprises the following steps: taking a compound of the general formula I as a raw material, taking inorganic base as a catalyst, controlling the temperature of an alcohol solvent to be 60 to 100 DEG C, and adding a compound of the general formula II for tertiary series reaction of Michael addition, reverse Claisen condensation and ester interchange, so as to obtain the 1,5-ketonic ester compound of the general formula III. The synthesis method has the advantages that the synthesis cost of the 1,5-ketonic ester compound is greatly reduced; the reaction conditions are mild; use of precious and / or poisonous metal and storge oxidant are avoided; the yield of 1,5-ketonic ester compound is greatly improved; industrial synthesis of the 1,5-ketonic ester compound can be realized.
Owner:SOUTHWEST UNIV
Synthesis method of 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-formate
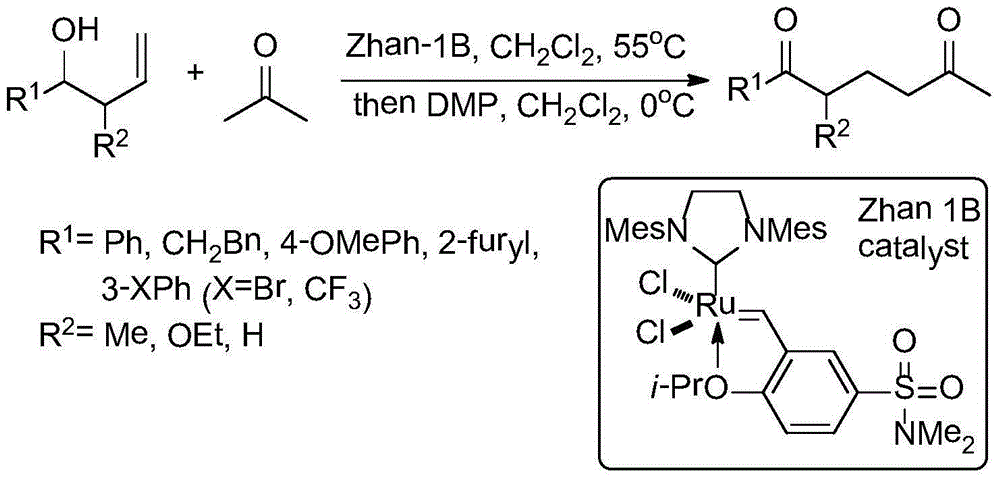
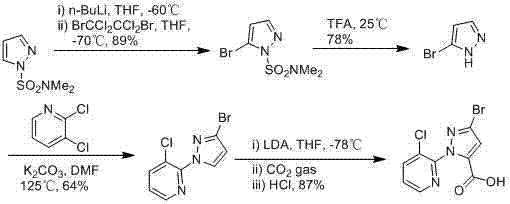
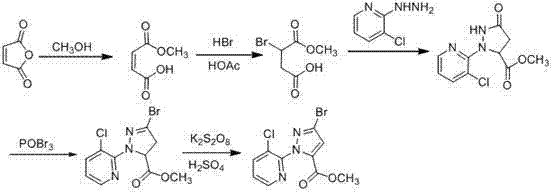
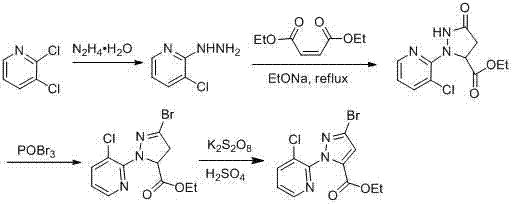
ActiveCN103044393AComponents are inexpensive and readily availableShort processOrganic chemistryClaisen condensationSynthesis methods
The invention discloses a synthesis method of 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-formate. According to the synthesis method, the 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-formate is prepared by condensation of 2,4-dioxobutanoate and 2-hydrazyl-3-chloropyridine, and the 2,4-dioxobutanoate is prepared by Claisen condensation of pyruvate and formate. The synthesis method disclosed by the invention has the characteristics of cheap and available raw materials, short technological process, simple operation steps, mild reaction condition, low energy consumption and low cost; besides, the synthesis method avoids poisonous raw materials adopted by a traditional method, reduces the corrosion to equipment and the danger to the health of operators; moreover, the synthesis method has little discharge of the three wastes and is beneficial to realizing the industrial application; therefore, the synthesis method disclosed by the invention is an environmentally friendly, economical and effective synthesis route.
Owner:HUAIAN WAN BANG SPICE IND CO LTD +1
Method for preparing ethyl 4,4-difluoroacetoacetate
InactiveCN108218703AHigh yieldHigh selectivityOrganic compound preparationCarboxylic acid esters preparationAcetic acidOrganic solvent
The invention discloses a method for preparing ethyl 4,4-difluoroacetoacetate. The method comprises the following step: by taking ethyl difluoroacetate and ethyl acetate as raw materials, and an ethanol solution of sodium ethoxide as a catalyst, in the presence of an organic solvent, carrying out a Claisen condensation reaction, thereby obtaining the ethyl 4,4-difluoroacetoacetate. The method disclosed by the invention has the characteristics of being gentle in reaction condition, high in security, good in reaction selectivity, easy in product separation and purification, applicable to industrial production, and the like.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Process for producing muscone and its intermediate
InactiveUS20060135819A1High yieldProduct can be usedOrganic compound preparationOrganic chemistry methodsClaisen condensationKetone
The present invention provides a process for producing a muscone represented by (1) by subjecting a citronellic acid derivative and an undecenoate to a Claisen condensation reaction to produce a keto ester compound represented by (2) decarboxylating the keto ester compound to produce 2,6-dimethyl-8-oxy-2,17-heptadecadiene, cyclizing the heptadecadiene using a metathesis catalyst to produce a 6-dehydromuscone represented by (3) and then hydrogenating the double bond. The present invention also provides the keto ester compound.
Owner:TAKASAGO INTERNATIONAL CORPORATION
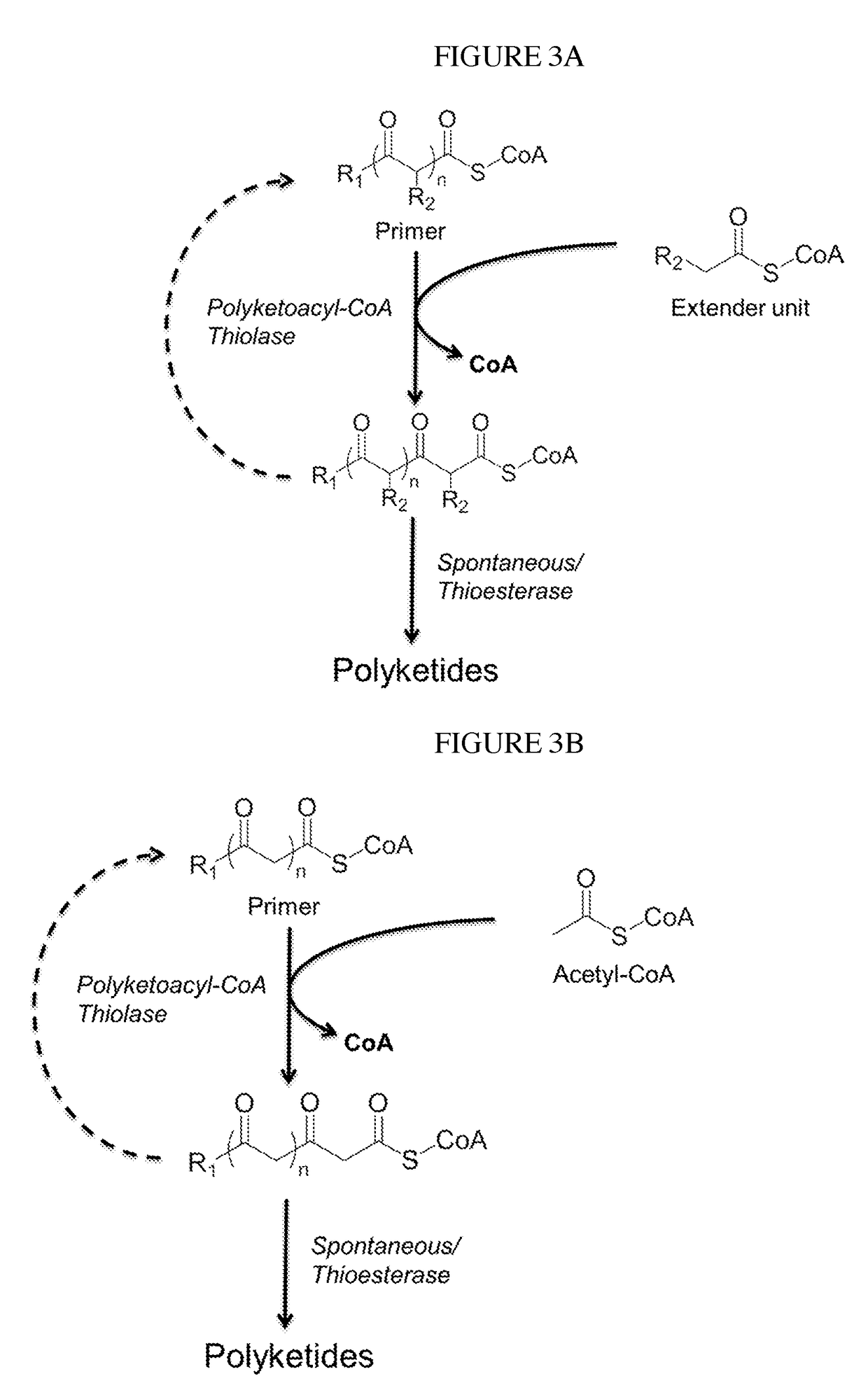
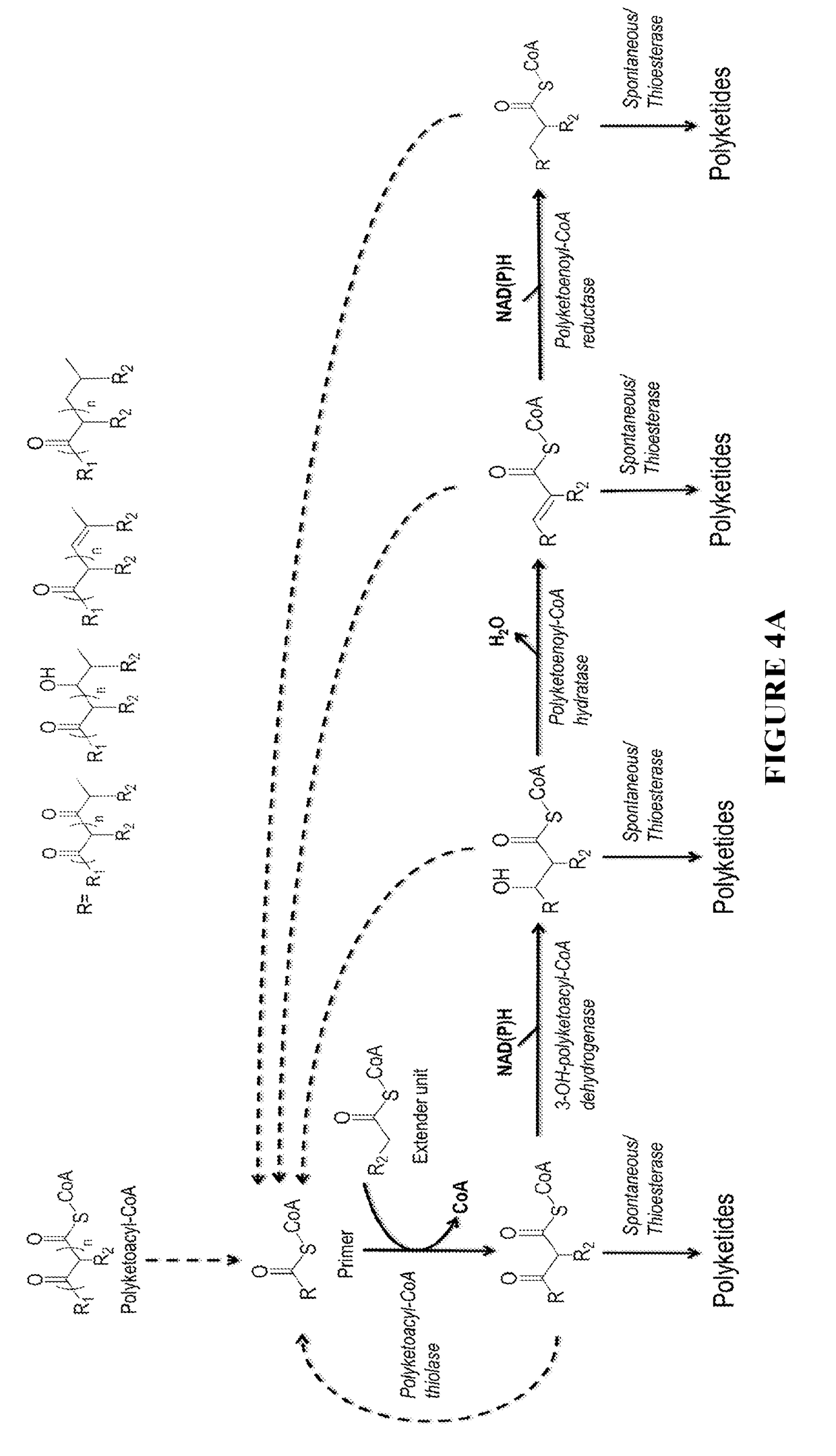
Biosynthesis of polyketides
InactiveUS20190002848A1Increase diversityWide-ranging product diversityAcyltransferasesFermentationClaisen condensationPolyketide biosynthesis
This disclosure generally relates to the use of microorganisms to make various functionalized polyketides through polyketoacyl-CoA thiolase-catalyzed non-decarboxylative condensation reactions instead of decarboxylative reactions catalyzed by polyketide synthases. Native or engineered polyketoacyl-CoA thiolases catalyze the non-decarboxylative Claisen condensation in an iterative manner (i.e. multiple rounds) between two either unsubstituted or functionalized ketoacyl-CoAs (and polyketoacyl-CoAs) serving as the primers and acyl-CoAs serving as the extender unit to generate (and elongate) polyketoacyl-CoAs. Before the next round of polyketoacyl-CoA thiolase reaction, the β-keto group of the polyketide chain of polyketoacyl-CoA can be reduced and modified step-wise by 3-OH-polyketoacyl-CoA dehydrogenase or polyketoenoyl-CoA hydratase or polyketoenoyl-CoA reductase. Dehydrogenase converts the β-keto group to β-hydroxy group. Hydratase converts the β-hydroxy group to α-β-double-bond. Reductase converts the α-β-double-bond to single bond. Spontaneous or thioesterase catalyzed termination reaction terminates the elongation of polyketide chain of polyketoacyl-CoA at any point through CoA removal and spontaneous reactions rearrange the structure, generating the final functional polyketide products.
Owner:GONZALEZ RAMON
Method for preparing scutellarin
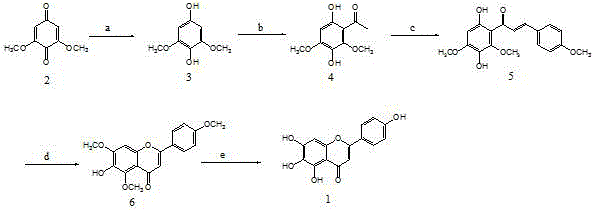
InactiveCN105906600AHigh medicinal valueHigh yieldOrganic chemistryClaisen condensationDehydrogenation
The invention relates to a method for preparing scutellarin. According to the method, the scutellarin can be efficiently prepared from 2,6-dimethoxy benzoquinone as a starting raw material through five reactions of reduction, Friedel-Crafts acetylation, Claisen condensation, dehydrogenation oxidation cyclization and demethylation. The starting raw material and a reagent are cheap and easily available, synthetic steps are few, the operation is simple and convenient, the production control is simple, the product yield is high, and the purity is high. The method is applicable to large-scale preparation and production application of the scutellarin.
Owner:KUNMING UNIV OF SCI & TECH
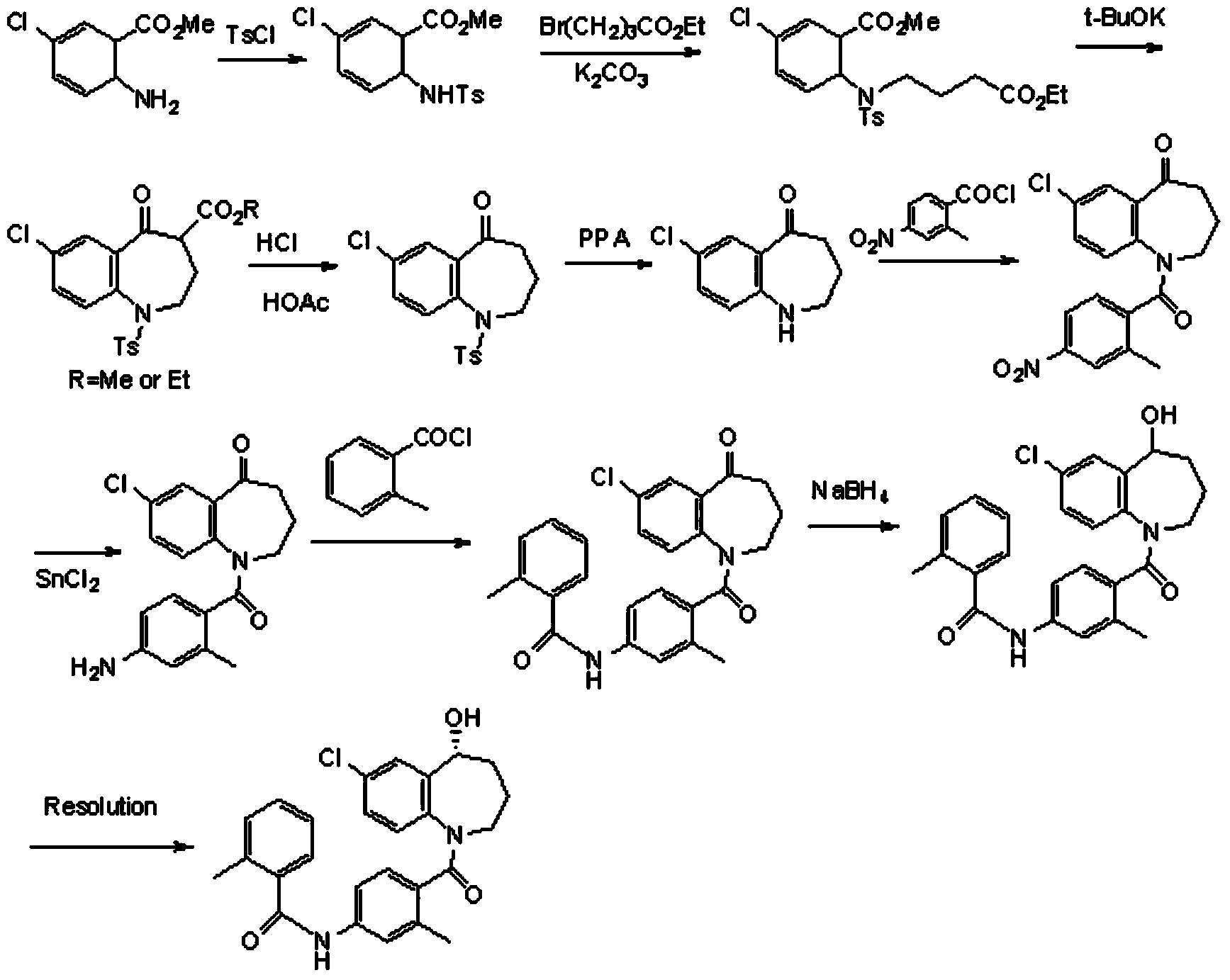
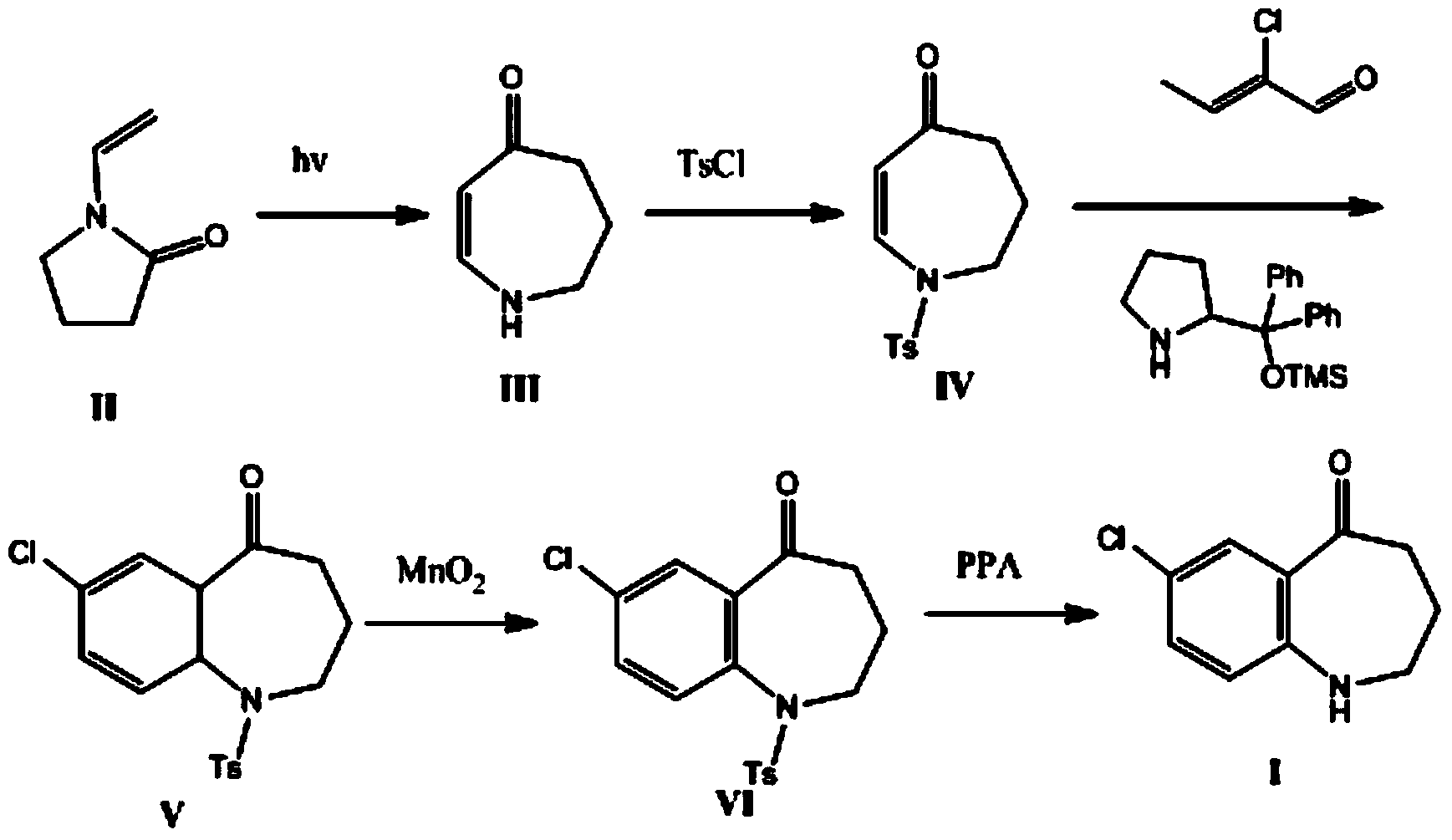
Preparation method of tolvaptan intermediate
ActiveCN103896842AMild conditionsRaw materials are easy to getOrganic chemistryBenzodiazepineSynthesis methods
The invention belongs to the field of drug synthesis, and particularly relates to a preparation method of a tolvaptan intermediate 7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzodiazepines-5-one. The method comprises the following steps: by taking N-vinylpyrrolidone as an active ingredient, rearranging under light to obtain 4-azecycloheptatrienone, then protecting nitrogen methyl p-toluenesulfinate, reacting with 2-chloro-2-butenal in the presence of diaryl D-prolinol derivatives to generate 7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzodiazepines-5-one, dehydrogenizing and aromatizing in the presence of manganese dioxide, and finally removing protection groups to obtain the 7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzodiazepines-5-one. Due to no Claisen condensation or Friedle-Crafts reaction in the conventional synthesis method, the potassium tert-butanol and aluminum trichloride are omitted, the preparation method is mild in reaction conditions, easily available in raw materials, capable of lowering the cost, and more suitable for industrial production.
Owner:HUBEI GRAND LIFE SCI & TECH CO LTD
Aluminum ion fluorescence probe, preparation method and application thereof in food detection
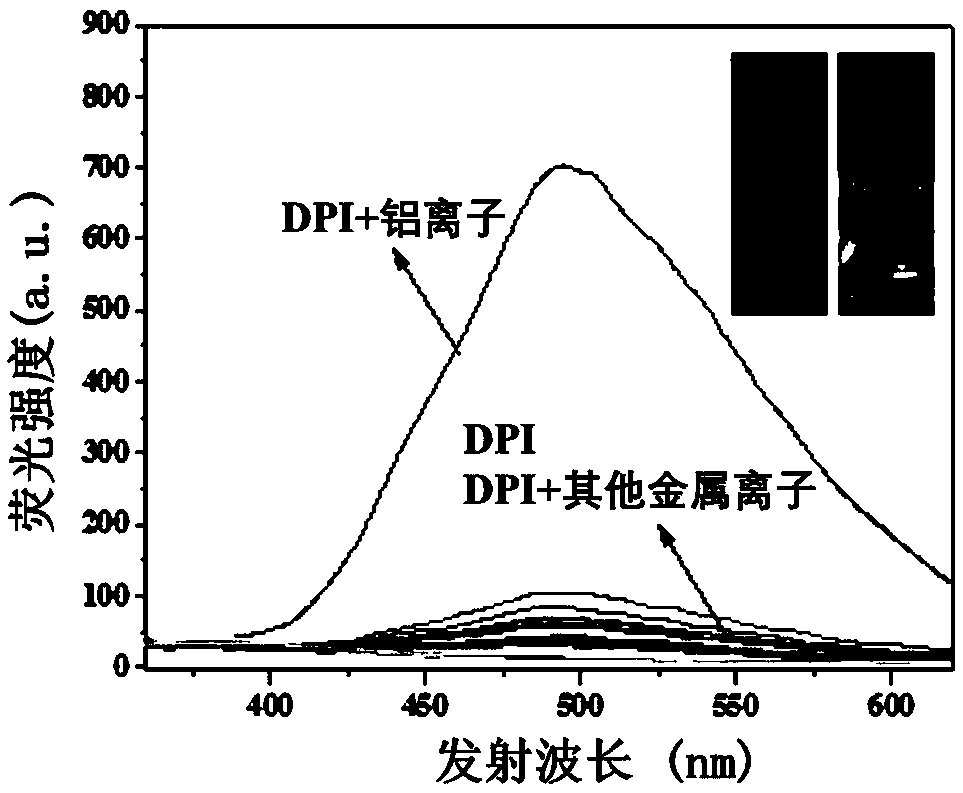
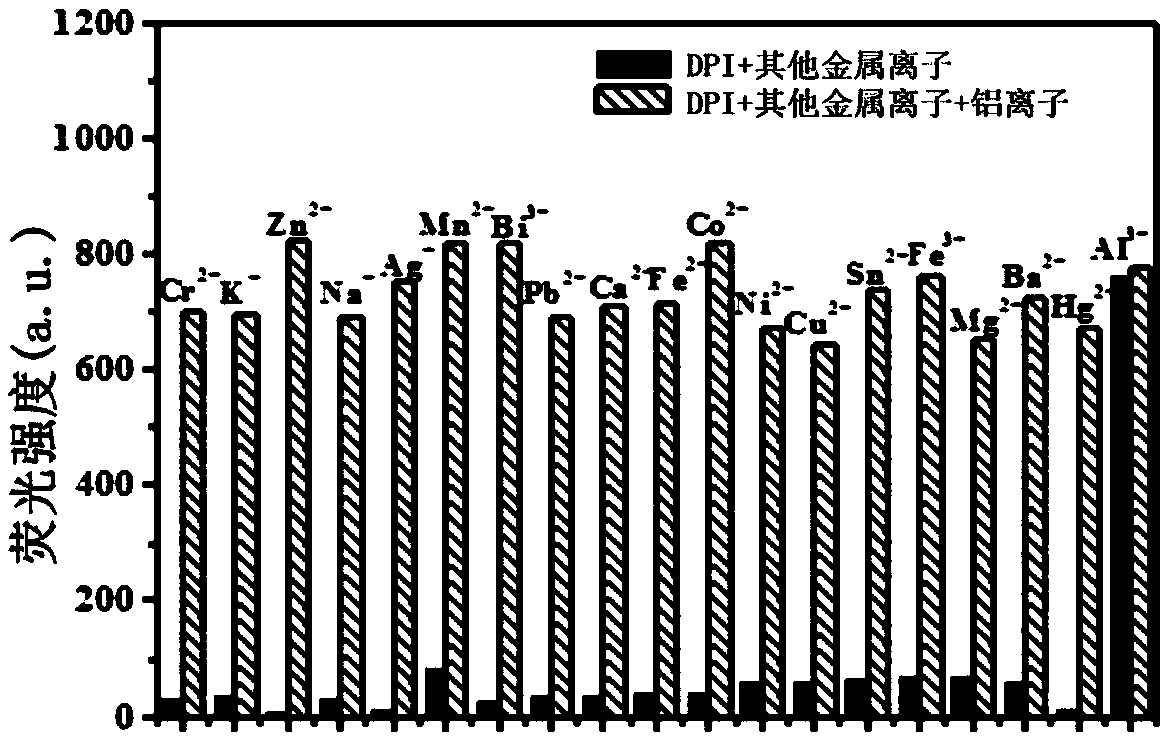
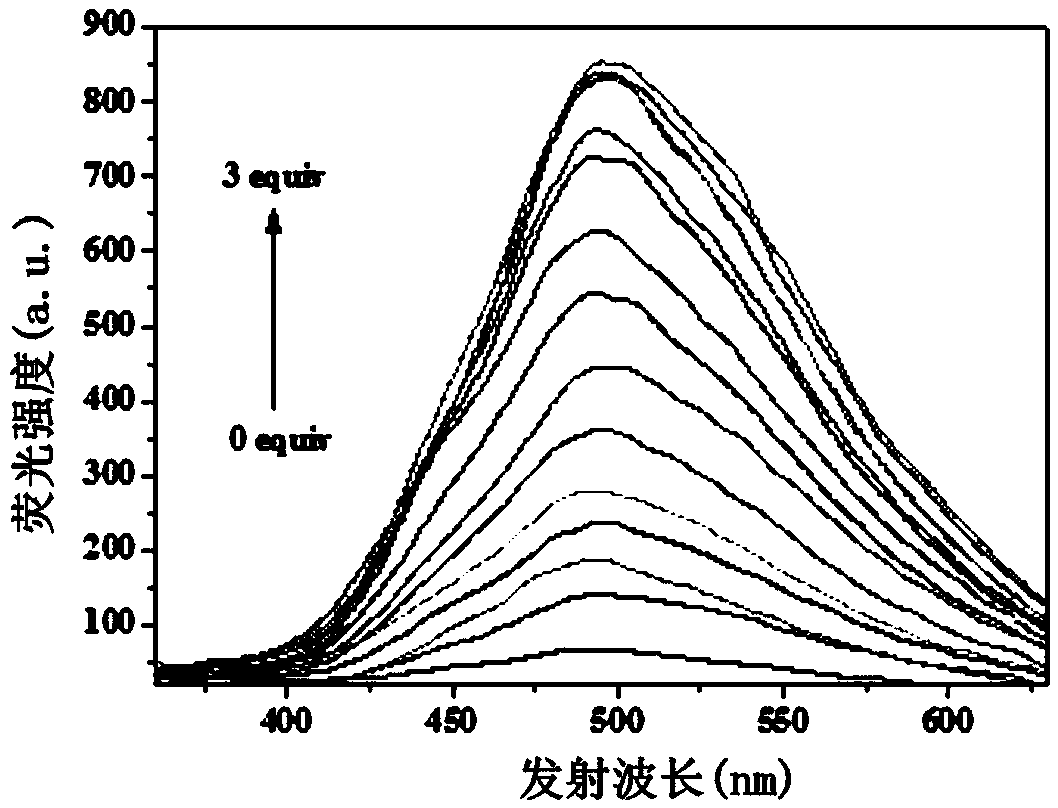
InactiveCN108947962AHas selective complexationHigh selectivityOrganic chemistryFluorescence/phosphorescenceAluminum IonFluorescence
The invention discloses a pino alkyl indazole aluminum ion fluorescence probe, a preparation method and an application thereof. The preparation method comprises the following steps: taking a natural regenerated resource beta-pinene derivative nopinone as a raw material; carrying out claisen condensation reaction between nopinone and 2-pyridine methyl formate, thereby acquiring 3-(2'-pyridine formyl) nopinone; cyclizing 3-(2'-pyridine formyl) nopinone and hydrazine hydrate, thereby acquiring a pino alkyl indazole compound 6,6-dimethyl-3-(2'-pyridyl)-4,5,6,7-tetralin-2H-5,7-bridge methylene indazole. The compound as a fluorescence probe with high selectivity and sensitivity has an excellent application in detecting aluminum ions in food.
Owner:NANJING FORESTRY UNIV
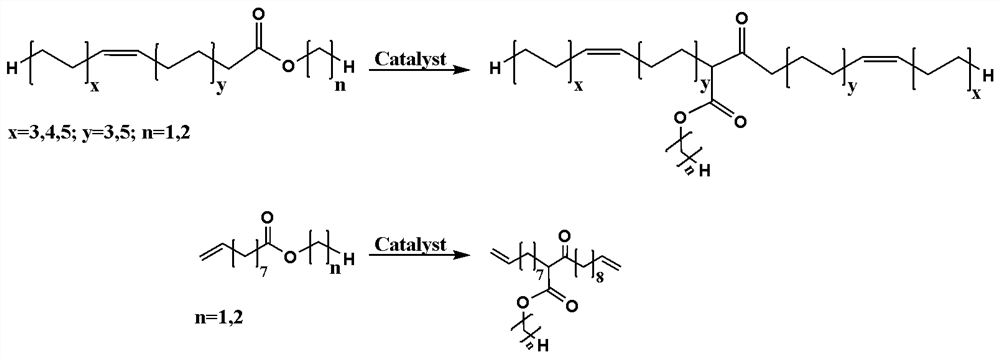
Preparation method of binary primary alcohol with adjustable molecular weight for bio-based polyurethane
ActiveCN113735749AHigh molecular weightHigh reactivityOrganic compound preparationCarboxylic acid esters preparationPolymer scienceVegetable oil
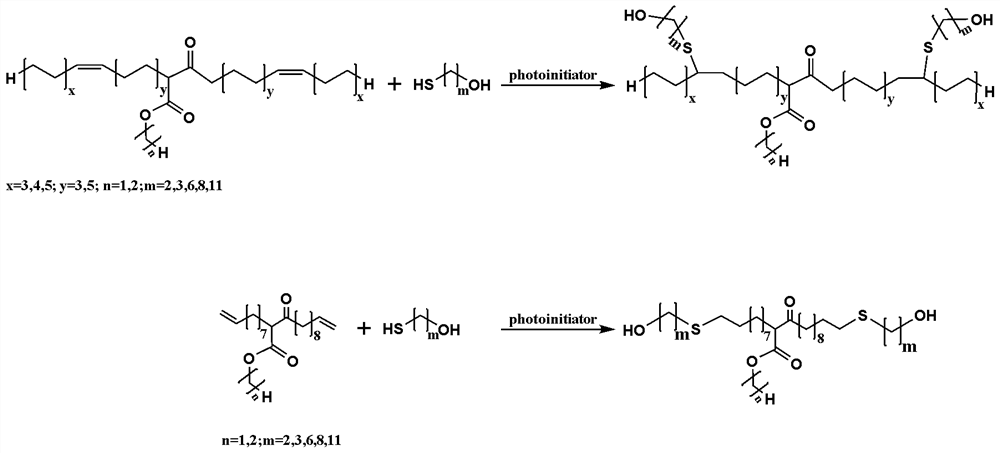
The invention discloses a preparation method of binary primary alcohol with adjustable molecular weight for bio-based polyurethane. Firstly, bio-based fatty acid ester containing alpha-hydrogen atoms is used as a raw material and subjected to Claisen condensation reaction in the presence of a basic catalyst and a solvent, and beta-keto ester with the molecular weight increased by one time is obtained. And then, by introducing the primary hydroxyl group into beta-keto ester and further prolonging a carbon chain under ultraviolet irradiation by utilizing a thiol-ene click reaction, the bio-based binary primary alcohol with adjustable molecular weight, a long main chain and a short side chain is obtained. The bio-based binary primary alcohol is prepared by using the natural vegetable oil derivative fatty acid ester instead of a petroleum-based monomer as a raw material, has the advantages of reproducibility, easiness in degradation and the like, and can be widely applied to manufacturing of polyurethane for leather, synthetic leather, textiles, plastics, rubber, building materials and coatings.
Owner:SICHUAN UNIV
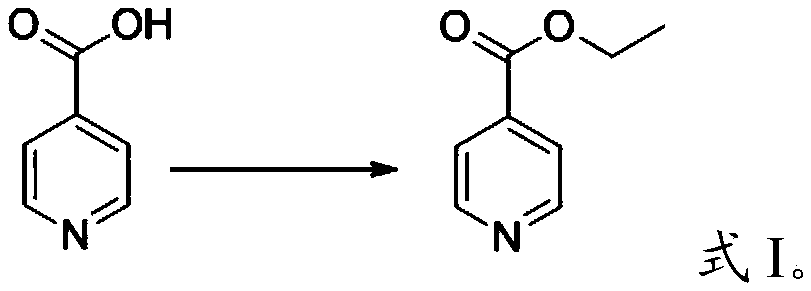
Preparation method of 4-ethylpyridine
The invention relates to the technical field of organic synthesis, in particular to a preparation method of 4-ethylpyridine. The preparation method of the 4-ethylpyridine comprises the following stepsof: mixing ethyl 4-picolinate with sodium ethoxide, heating to 90 to 110 DEG C, then dropwise adding ethyl acetate, and carrying out claisen condensation reaction, thus obtaining ethyl 3-oxo-3-(4-pyridyl) propionate; mixing the ethyl 3-oxo-3-(4-pyridyl) propionate, dimethyl sulfoxide and water, and carrying out heating treatment, thus obtaining 4-acetylpyridine; cooling after mixing glycol with potassium hydroxide, adding hydrazine hydrate, rising the temperature to 60 to 80 DEG C, then mixing with the 4-acetylpyridine, and carrying out reduction reaction, thus obtaining the 4-ethylpyridine.The 4-ethylpyridine prepared through the preparation method provided by the invention is higher in yield and purity.
Owner:郑州长宽科技有限公司 +1
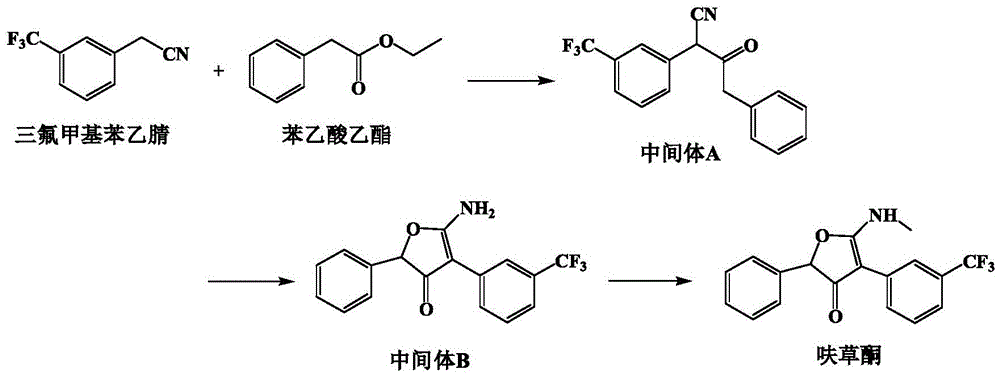
Production method of high-efficiency herbicide flurtamone
ActiveCN105669613ANot easy to generate emissionsImprove securityOrganic chemistryEthyl phenylacetatePhenylacetic acid
The invention discloses a production method of high-efficiency herbicide flurtamone. The production method comprises that a desired product flurtamone is prepared from trifluoromethyl phenylacetonitrile and ethyl phenylacetate as main raw materials through Claisen condensation, cyclization in an acid solvent and methylation. The production method has high safety, low requirements on reaction purifying conditions and product separation easiness, does not easily discharge an acid solution, realizes recovery and recycle of a solvent and has a high production yield. The production method is suitable for flurtamone industrial production and is conducive to wide application of high-efficiency herbicide flurtamone.
Owner:JIANGSU MINGCHEM CORP
Method for preparing 2,6-diacetyl pyridine
InactiveCN101157653AHigh yieldExperiment operation is simpleOrganic chemistryAlkaline earth metalClaisen condensation
The invention relates to a preparative method of 2, 6-diacetylpyridine. By adopting alkali metal or alkaline earth metal as the alkaline reagent, the method carries out the Claisen condensation to 2, 6-pyridine dicarboxylic aid diethyl ester and ethyl acetate to gain the 2, 6-diacetylpyridine. Specifying in the formulate on the right, compared with other methods, the method provided in the invention needs not to conduct special treatment for the reaction raw materials and the alkaline reagents. The minor water or ethanol in the reaction system has no distinct influence to the reaction, and the reaction system cancels drying and purifying treatment in order to simplify the experimental operation, reduce the reaction period, save cost, improve the output rate of the product and demonstrate good applicable valve.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for preparing baicalein
ActiveCN105906599ASuitable preparationSuitability for productivityOrganic chemistryBaicaleinClaisen condensation
The invention discloses a method for preparing baicalein. According to the method, 2,6-dimethoxy-p-benzoquinone used as an initial raw material is subjected to reduction, Friedel-Crafts acetylation, claisen condensation, dehydrogenation oxidation cyclization and demethylation five reactions to obtain baicalein with high yield. The method uses low-price and easily-available initial raw material, reagent and the like, has few synthesizing steps, is easy and convenient to operate and easy for production control, has high product yield and high purity, and is suitable for large-scale preparation and production application of baicalein.
Owner:KUNMING UNIV OF SCI & TECH
Complexes of metal salts of organic acids and beta-diketones and methods for producing same
InactiveUS6872854B2Low costMore efficientOrganic compound preparationCarbonyl compound preparationClaisen condensationKetone
Metal salts of organic acids complexed with β-diketone compounds are multifunctional complexes useful in the formulation of stabilizers for halogenated resins. These complexes may be used jointly with other low toxicity intermediates, such as zinc or magnesium intermediates, to form effective stabilizers that are non-toxic and exhibit better performance than other known stabilizers, including those containing toxic heavy metals such as cadmium or lead. The complex is prepared utilizing a Claisen condensation reaction and precipitation with water and heptane.
Owner:GALATA CHEM LLC
Nopinalkyl indazole silver ion fluorescence probe and preparation method thereof
ActiveCN110759890AHas selective complexationOrganic chemistryFluorescence/phosphorescenceClaisen condensationSilver ion
The invention discloses a nopinalkyl indazole silver ion fluorescence probe and a preparation method thereof. Beta-pinene derivative nopinone which is a natural renewable resource is used as a raw material, nopinone is subjected to a Claisen condensation reaction with methyl pyridine-2-carboxylate, and 3-(2'-pyridyl formyl) nopinone is prepared; and 3-(2'-pyridyl formyl) nopinone is subjected to condensation cyclization with phenylhydrazine, and a nopinalkyl indazole compound 6,6-dimethyl-2-phenyl-3-(2'-pyridyl)-4,5,6,7-tetrahydro-2H-5,7-endomethylene indazole is obtained. The compound as a specific fluorescence probe molecule has good application in the aspect of silver ion detection.
Owner:NANJING FORESTRY UNIV
Preparation and application of beta-diketone boron fluoride fluorescent dye adopting first-class coumarin as framework
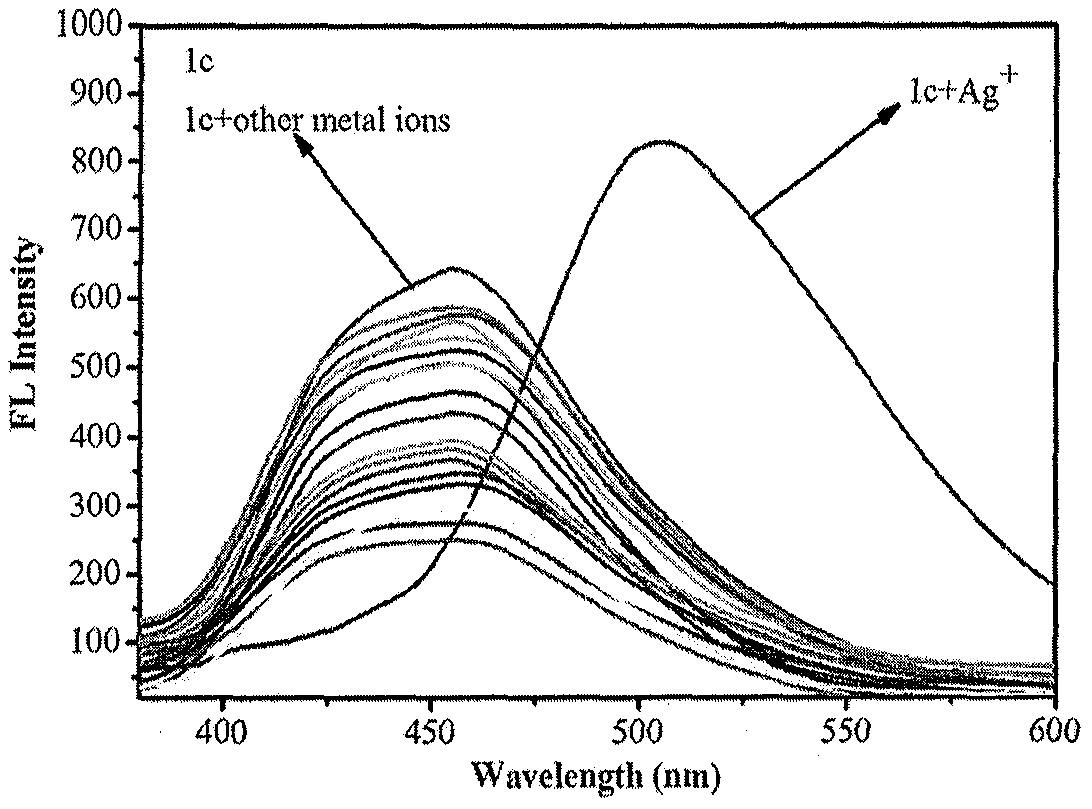
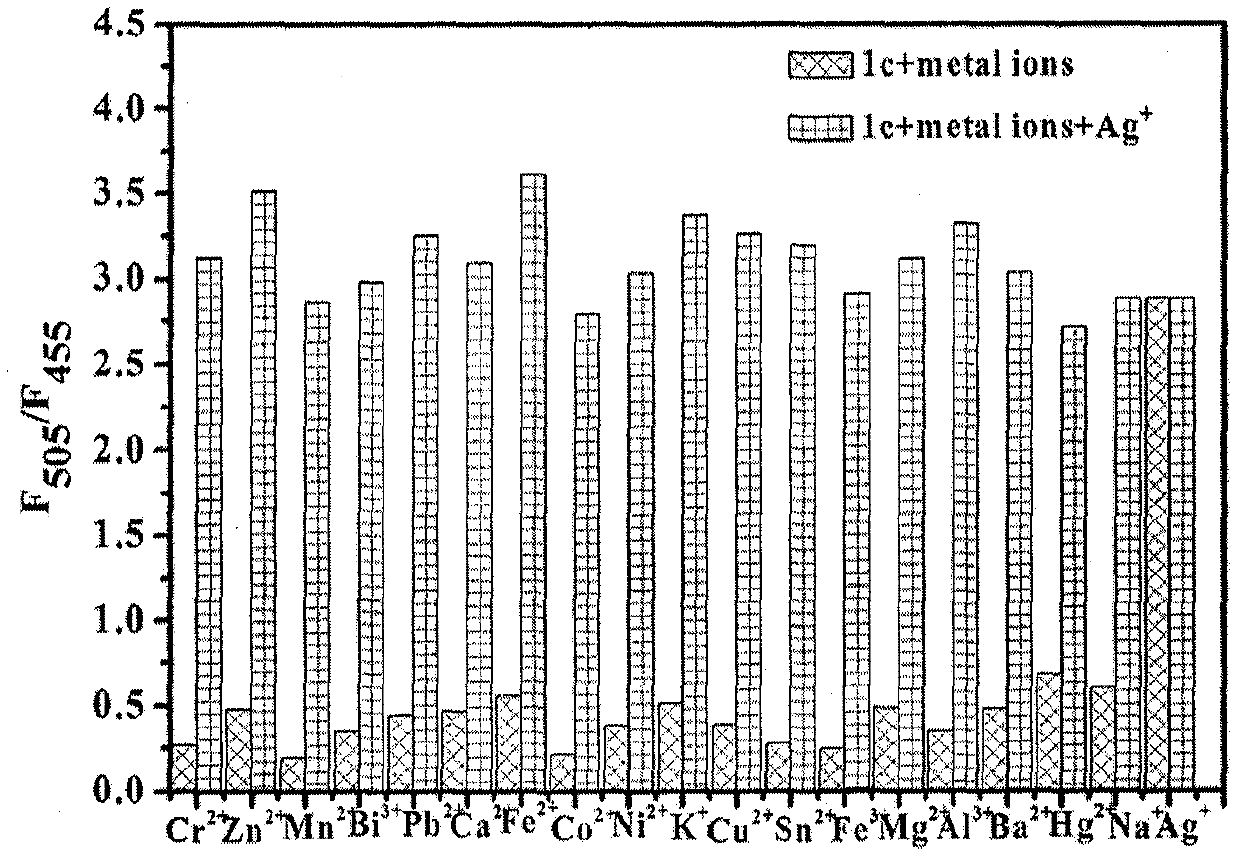
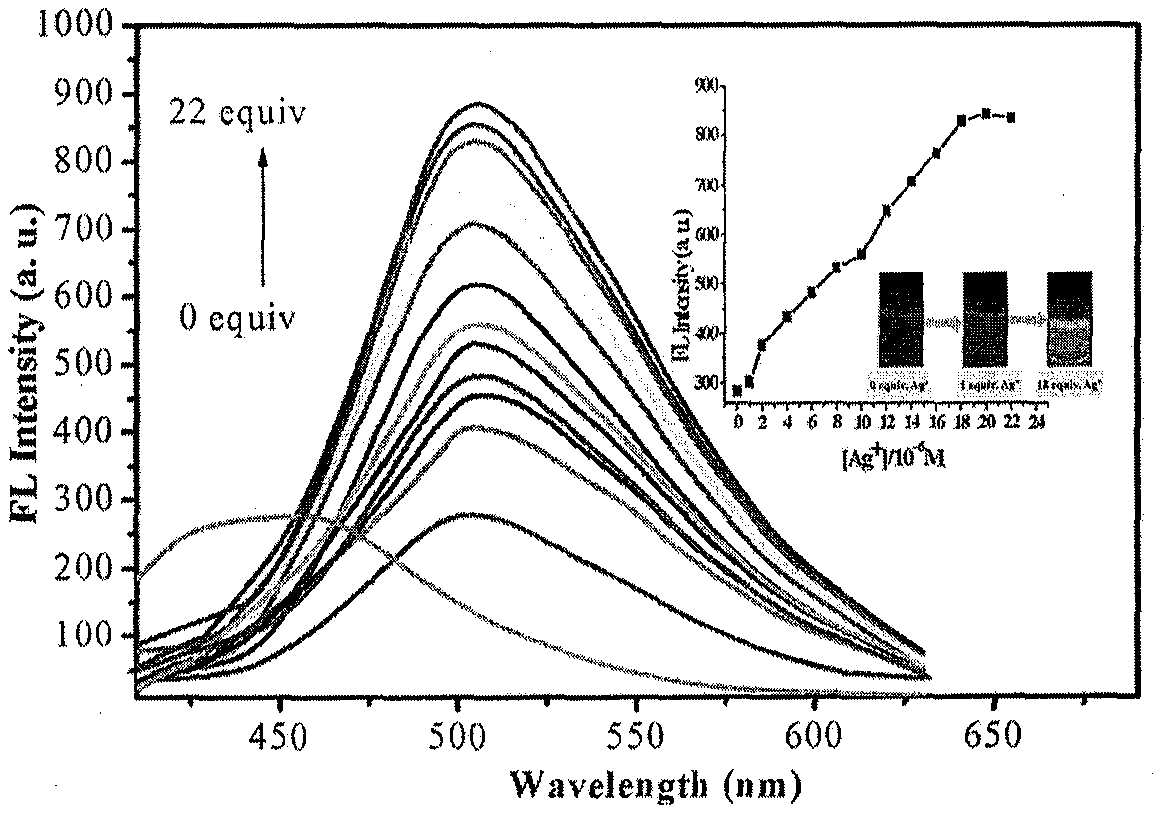
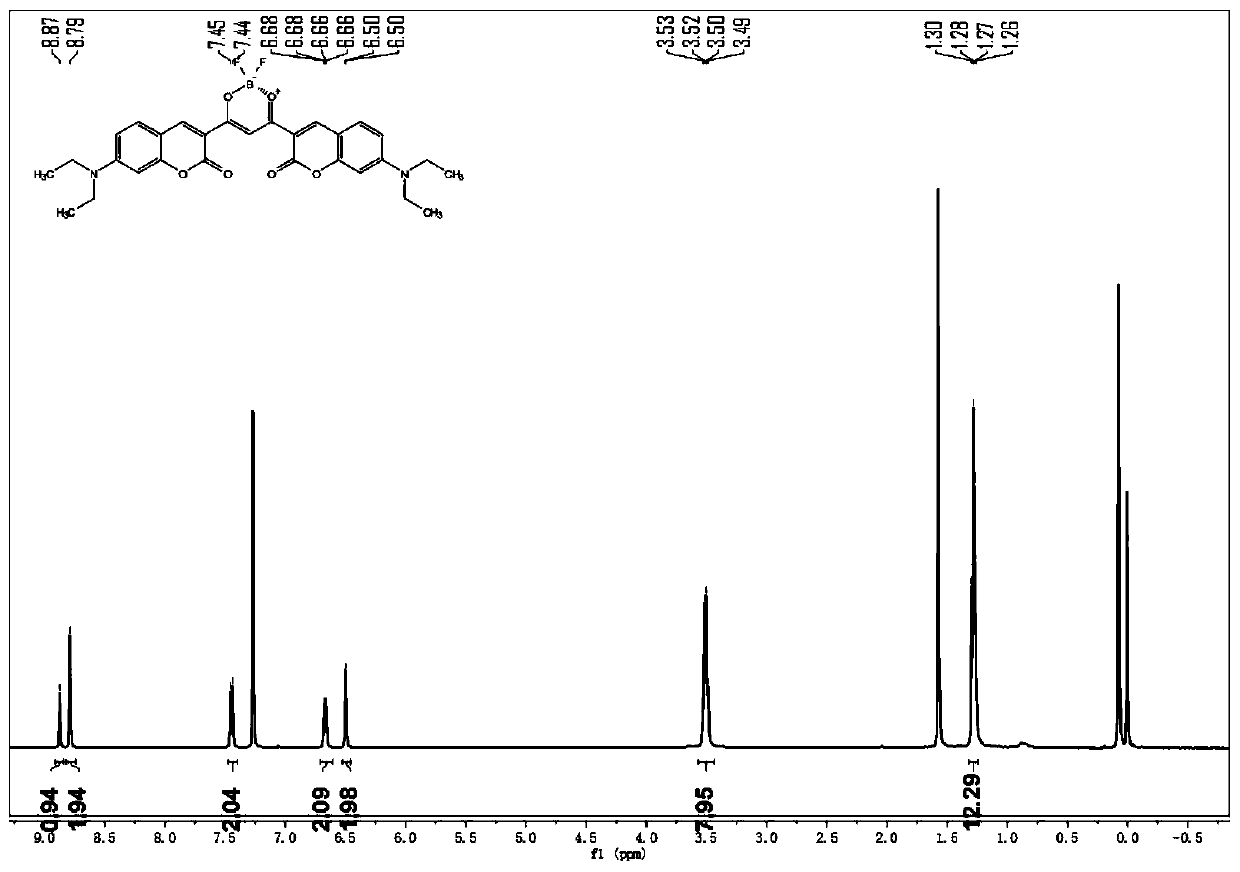
ActiveCN110105381AOptically stableAbsorption and emission wavelength longAzo dyesGroup 3/13 element organic compoundsCarbon numberClaisen condensation
The invention discloses synthesis of a beta-diketone boron fluoride fluorescent dye adopting dicoumarin as a framework. The structural general formula of the beta-diketone boron fluoride fluorescent dye is shown as a formula I or a formula II. Through the design, a series of coumarin with substituents introduced from three positions is prepared, then Claisen condensation is conducted on coumarin-containing ester and a corresponding carbonyl compound to obtain a series of compounds of beta-diketo acid ligands, the length of conjugated chains is increased, and the emission wavelength of the conjugated chains is improved. Finally, the beta-diketo acid ligands are subjected to a coordination reaction with boron under the action of boron trifluoride diethyl ether to form a series of the beta-diketone boron fluoride fluorescent dye based on coumarin. The method has the advantages that the synthesis steps of raw materials are simple, the yield is high, and the method is simple and easy to implement. The fluorescent dye has a narrow ultraviolet absorption visible spectrum, a narrow fluorescence emission spectrum and long absorption emission waves and is stable in optical property; the dyecan be used for cell imaging, fluorescent probes and the like and has a broad application prospect in the fields of chemical sensing, biological labeling, biological imaging, organic light emitting diodes (OLED), photoelectric materials, fluorescent probes and the like. In the formula I, R1, R2, R3 and R4 refer to alkyl groups with the carbon number smaller than 20.
Owner:QINGDAO UNIV OF SCI & TECH
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
ActiveCN101386592BSolve the problem of expensive chiral side chainsHigh optical purityMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
Owner:JIANGSU WANBANG BIOPHARMLS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com