Nopinalkyl indazole silver ion fluorescence probe and preparation method thereof
A technology of nopinyl indazoles and fluorescent probes, applied in the field of fine organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation process of nopinyl indazole compound is:
[0028]
[0029] Specific steps are as follows:
[0030] 1) Preparation of 3-(2'-pyridineformyl) nopinone:
[0031] Add 0.06 mol of sodium hydride into a three-neck flask equipped with a stirrer, thermometer and reflux condenser, slowly inject 8 mL of ethylene glycol dimethyl ether under nitrogen protection, dissolve 0.02 mol of nopinone in 9 mL of ethylene glycol dimethyl ether, and then nitrogen Slowly pour into the flask under protection, raise the temperature to control the reaction temperature at 82°C, heat to reflux for 0.5h, dissolve 0.024mol methyl picolinate in 9mL of ethylene glycol dimethyl ether, slowly inject into the flask under nitrogen protection, track the reaction by thin-layer chromatography Process, reaction 7-8h. The reaction solution was extracted 3 times with 100 mL ethyl acetate, the organic phases were combined, washed with saturated brine until neutral, the organic phase was dried o...
Embodiment 2
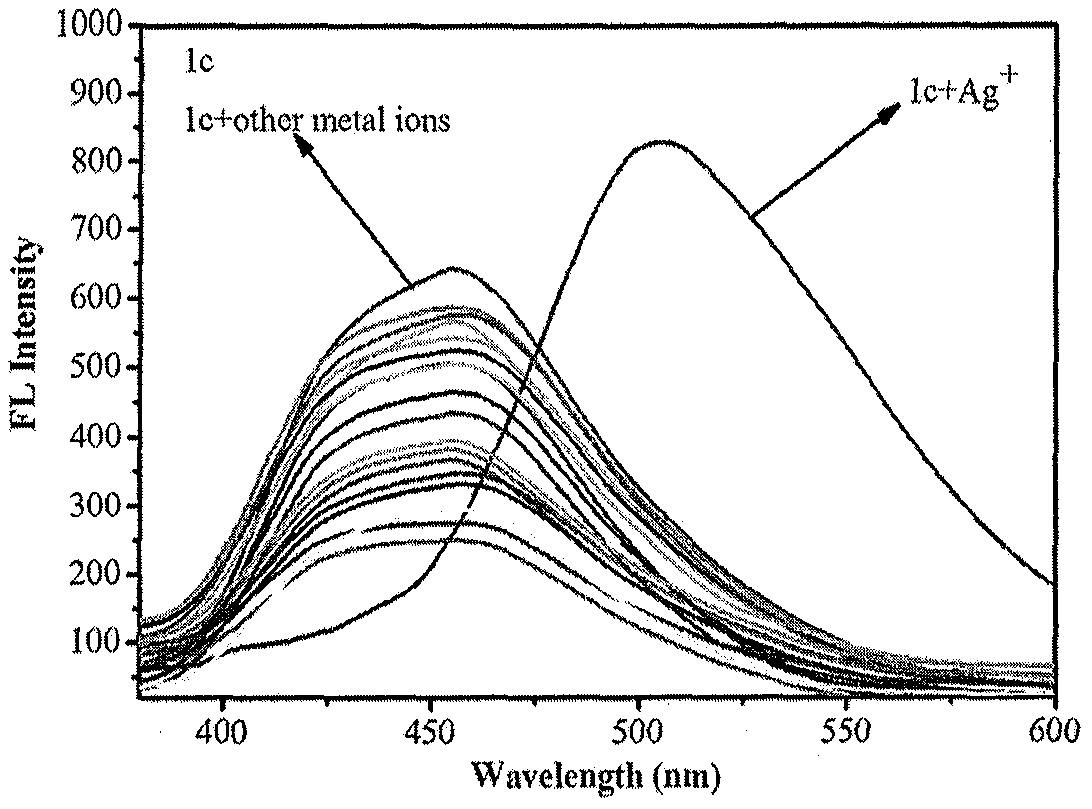
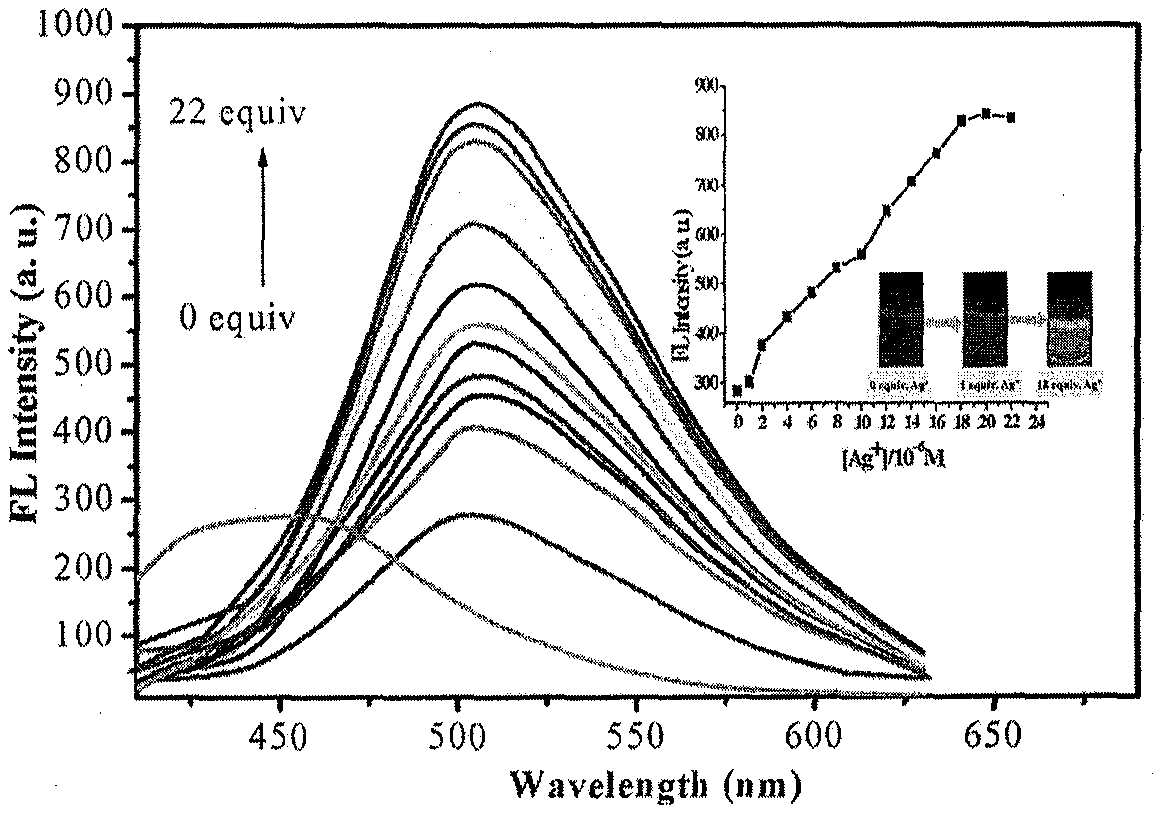
[0035] The solid 6,6-dimethyl-2-phenyl-3-(2′-pyridyl)-4,5,6,7-tetrahydro-2H-5,7-methanoindazole and different The metal ions were dissolved in 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer solution (20mM, pH=7.2, 7 / 3 (v / v) ethanol / water), and the concentration of the probe compound was prepared as 1× 10 -6 M and Ag + The concentration is 1×10 -5 19 solutions of M. Under the condition of excitation wavelength of 320nm, the fluorescence intensity changes in the presence of different metal ions were measured, such as figure 1 shown. The results showed that the compounds on Ag + It has a strong fluorescence enhancement effect, the maximum absorption wavelength is red-shifted from 455nm to 505nm, and the fluorescent color changes from blue to green under ultraviolet light, indicating that the compound has a strong effect on Ag + Has better selectivity.
Embodiment 3
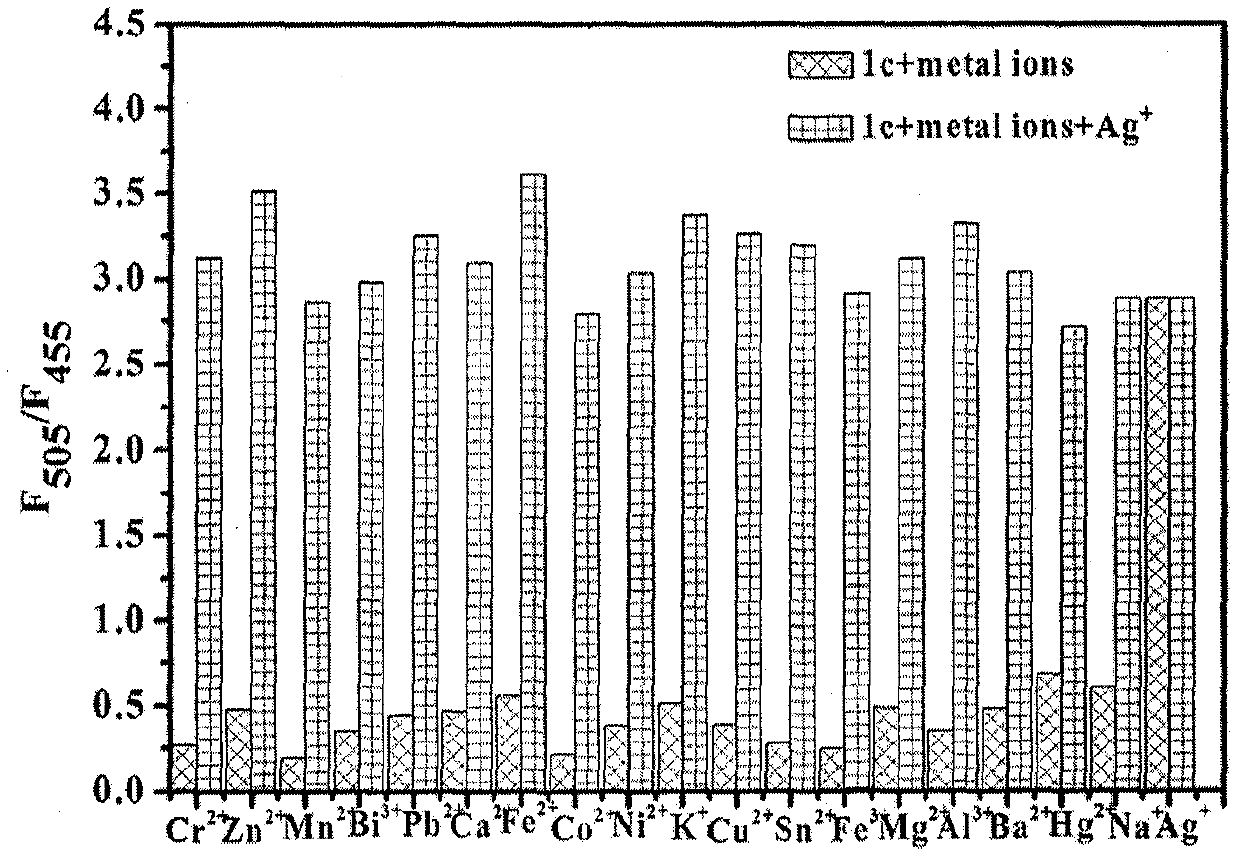
[0037] The configured 6,6-dimethyl-2-phenyl-3-(2′-pyridyl)-4,5,6,7-tetrahydro-2H-5,7-methanoindazole (1×10 -6 M)+Ag + (1×10 -5 M) solution (HEPES buffer, 10mM, pH=7.2, 50% (v / v) C 2 h 5 OH) were added with Ag + Equimolar amount of K + , Na + , Mg 2+ , Fe 2+ , Fe 3+ , Mn 2+ , Ca 2+ , Al 3+ , pb 2+ , Cr 2+ ,Co 2+ , Zn 2+ , Bi 3 + , Ni 2+ , Pb 2+ , Cu 2+ , Sn 2+ , Ba 2+ , Ag + , the fluorescence spectrum was measured under the condition that the excitation wavelength was 320nm, the results are as follows figure 2 shown. When other metal ions are added, 6,6-dimethyl-2-phenyl-3-(2′-pyridyl)-4,5,6,7-tetrahydro-2H-5,7-methanomethene Kindazole-Ag + The fluorescence intensity of the complex was not affected, indicating that other metal ions did not detect Ag for the compound + make an impact.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com