Tetrahydroquinazoline-2-schiff base compounds as well as synthesis method and application thereof
A technology of tetrahydroquinazoline and synthetic method, applied in tetrahydroquinazoline-2-amine Schiff base fluorescent probe molecule and its preparation, tetrahydroquinazoline-2-amine Schiff base compound and its synthesis, detection of zinc ions in the field of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthetic method of tetrahydroquinazolin-2-amine Schiff base compound, synthetic process is:
[0035]
[0036] Specific steps are as follows:
[0037] 1) Preparation of 3-benzylidene nopinone:
[0038]In a 100mL three-necked flask equipped with a thermometer, a stirrer and a reflux condenser, add 1.38g (0.01mol) nopinone, 30mL distilled water, 6.00g (0.15mol) NaOH and 1.27g (0.012mol) benzaldehyde, and heat After about 8 hours of reflux reaction, the conversion rate of nopinone reaches over 95% (GC tracking detection). After cooling, saturated brine was added to the reaction solution and extracted three times with ethyl acetate (20mL×3), the combined organic phase was washed with saturated brine until neutral, washed with anhydrous Na 2 SO 4 After drying, filtering, and concentrating, the yellow solid crude product was obtained, which was then dissolved evenly with acetone and a small amount of ethanol, and left to crystallize to obtain 1.77 g of colorless tra...
Embodiment 2
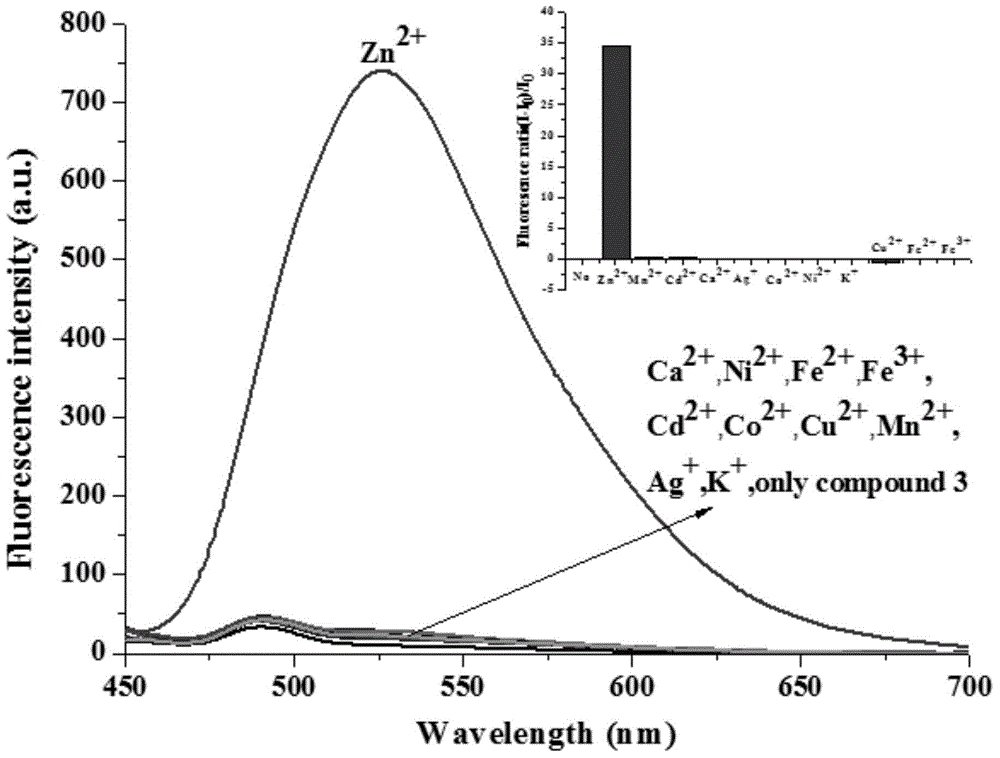
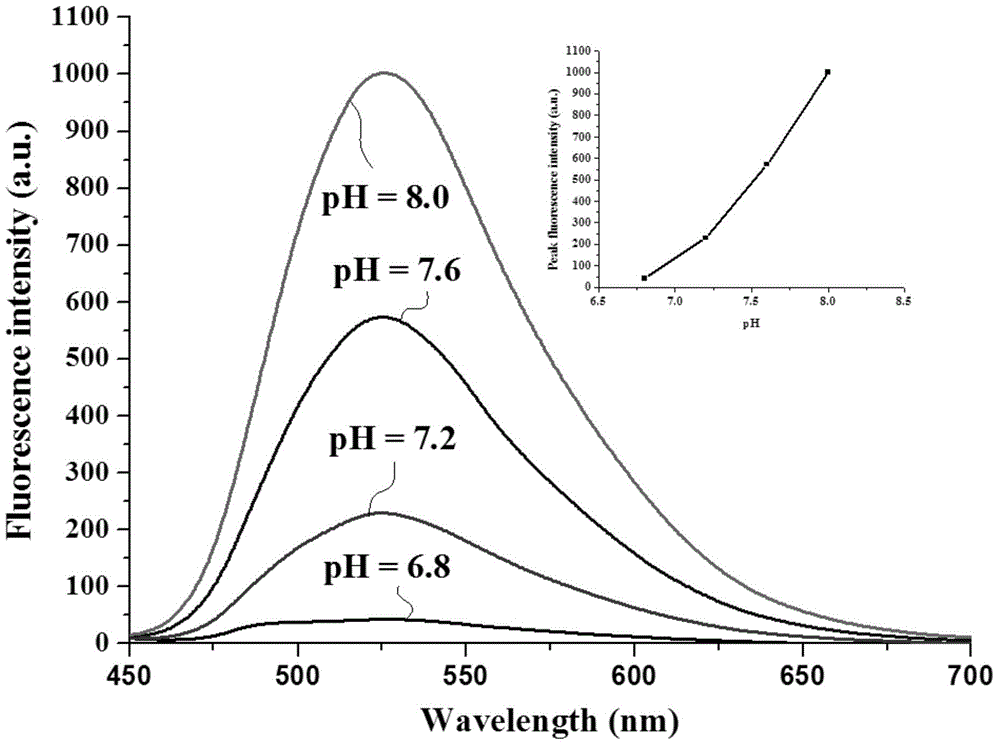
[0044] The solid 2-[[5,6,7,8-tetrahydro-4-phenyl-7,7-dimethyl-6,8-methanoquinazolin-2-imino]methyl ] Phenol compounds and different metal ions were dissolved in 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer solution (20mM, pH = 7.2, 50% (v / v) C 2 h 5 OH), formulated as a probe compound and Zn 2+ The concentration is 1×10 -5 Eleven solutions of M. Under the condition of excitation wavelength of 425nm, the fluorescence intensity changes in the presence of different metal ions were measured, such as figure 1 shown. The results showed that the compounds on Zn 2+ has a strong fluorescence enhancement effect, while Cu 2+ , Fe 2+ and Fe 3+ It has a strong fluorescence quenching, indicating that the compound has a strong effect on Zn 2+ Has better selectivity.
Embodiment 3
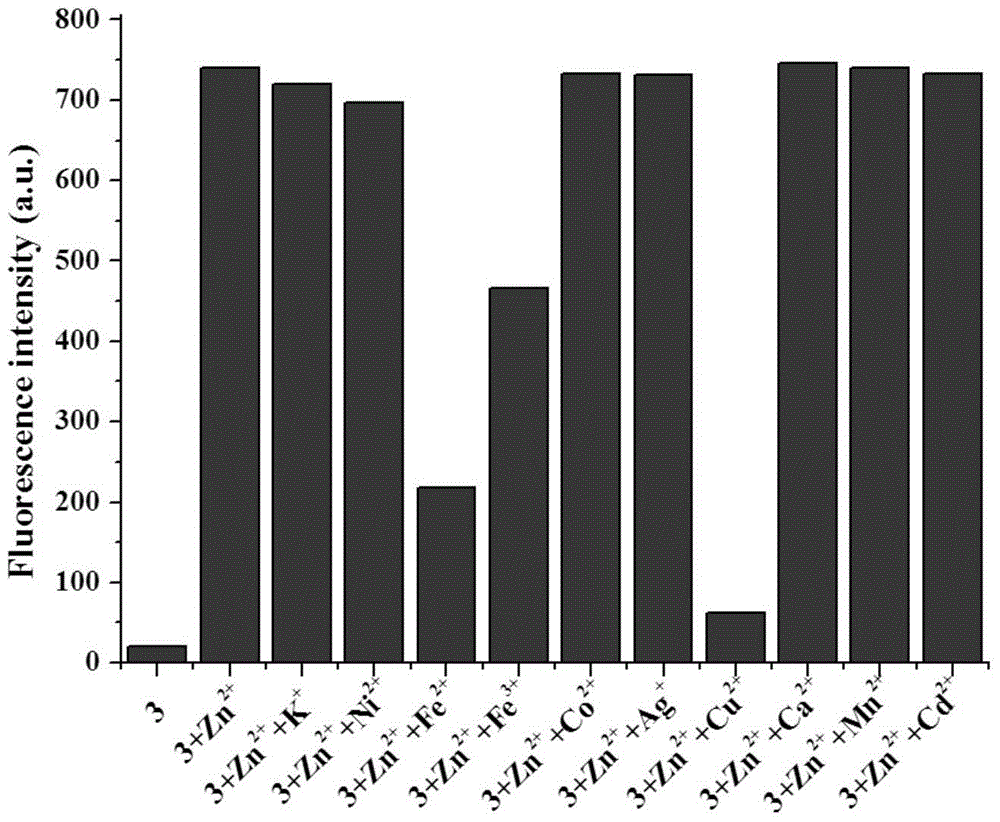
[0046] The configured 2-[[5,6,7,8-tetrahydro-4-phenyl-7,7-dimethyl-6,8-methanoquinazoline-2-imino] Methyl]phenol (1×10 -5 M)+Zn 2+ (1×10 -5 M) solution (HEPES buffer, 20mM, pH=7.2, 50% (v / v) C 2 h 5 OH) were added with Zn 2+ Equimolar amount of K + , Ca 2+ , Ni 2+ ,Fe 2+ ,Fe 3+ ,Cd 2+ ,Co 2+ ,Mn 2+ , Ag + and Cu 2+ , the fluorescence spectrum was measured under the condition of excitation wavelength of 425nm, the results are as follows figure 2 shown. When adding Cu 2+ ,Fe 2+ and Fe 3+ Ionic, 2-[[5,6,7,8-tetrahydro-4-phenyl-7,7-dimethyl-6,8-methanoquinazolin-2-imino]methanol base]phenol+Zn2+ The fluorescence intensities of 2+ >Fe 2+ >Fe 3+ , while K + , Ca 2+ , Ni 2+ ,Cd 2+ ,Co 2+ ,Mn 2+ , Ag + Plasma has little effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com