Nopinyl indazole silver ion fluorescent probe and preparation method thereof
A technology of nopinyl indazoles and silver ions, applied in the field of fine organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation process of the Novol-carbazole compound is:
[0028]
[0029] Specific steps are as follows:
[0030] 1) Preparation of 3- (2'-pyridyl) nobil:
[0031] 0.06 mol sodium hydride was added to three flasks equipped with a stirrer, a thermometer and reflow condenser, and 8 ml of glycol dimethyl ether was slowly injected under nitrogen protection, and 0.02 mol nobil is dissolved in 9 ml of glycol dimethyl ether. In the case of slow injection into the flask, the temperature rise control reaction temperature at 82 ° C, heat reflow 0.5 h, 0.024 mol pyridyl hydrochloride is dissolved in 9 ml of glycol dimethyl ether after nitrogen protection under nitrogen protection under nitrogen protection, thin layer chromatography tracking Process, reaction 7-8h. The reaction solution was extracted 3 times with 100 mL of ethyl acetate, combined with a organic phase, washed with saturated brine to neutral, and the organism was dried over anhydrous sodium sulfate, filtered, conce...
Embodiment 2
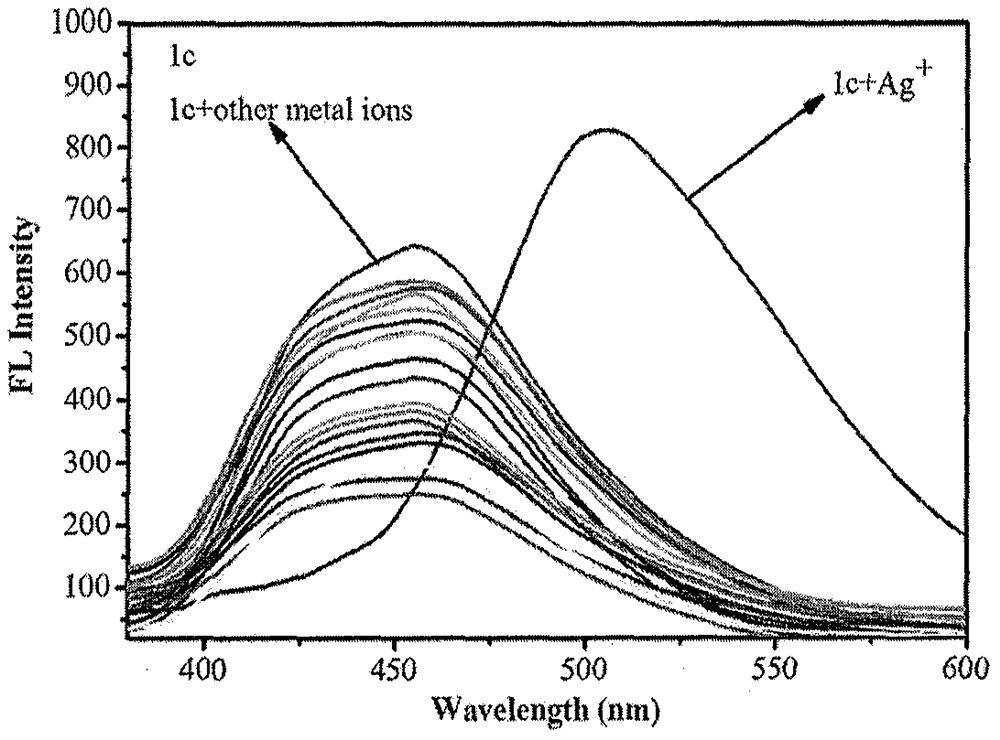
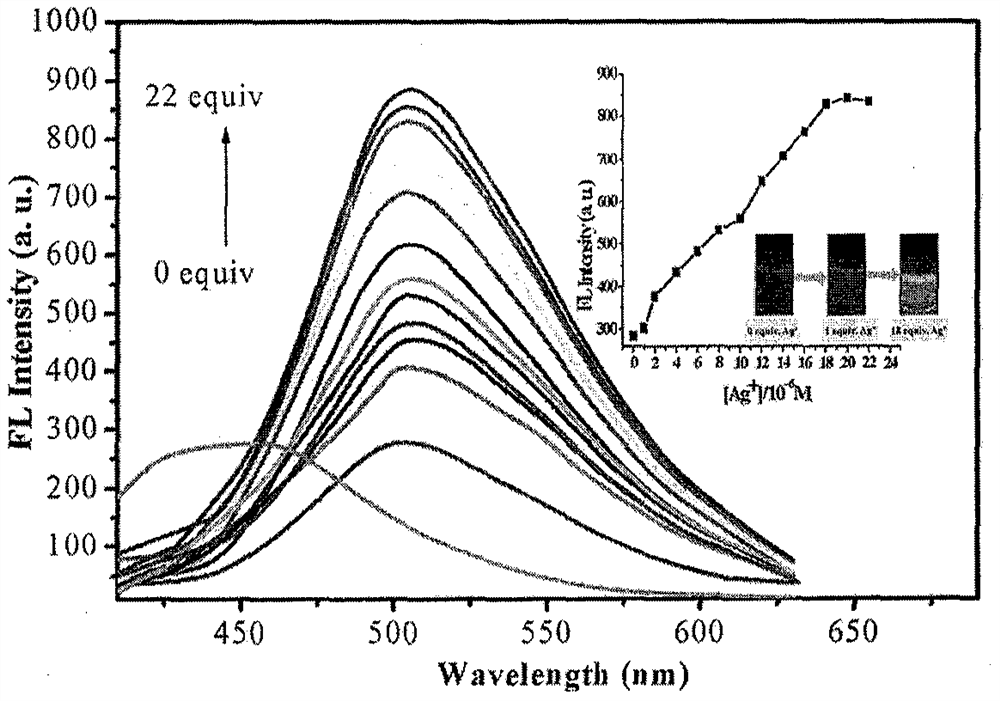
[0035] Solid 6,6-dimethyl-2-phenyl-3- (2'-pyridyl) -4,5,6,7-tetrahydro-2H-5, 7-bridge-methylhydrazole and different Metal ions are dissolved in 4-hydroxyethylpiperazine (HEPES) buffer solution (20 mM, pH = 7.2, 7 / 3 (v / v) ethanol / water), formulated into a probe compound concentration of 1 × 10 -6 M and AG + All concentration is 1 × 10 -5 The 19 solutions of M. The fluorescence intensity changes in different metal ions were measured under conditions of excitation wavelength of 320 nm, such as figure 1 Indicated. The results show that compounds are Ag + It has strong fluorescence enhanced, maximum absorption wavelength is transferred from 455 nm to 505 nm, and the fluorescent color under UV lamp is turned green, indicating that the compound is ag + Has good selectivity.
Embodiment 3
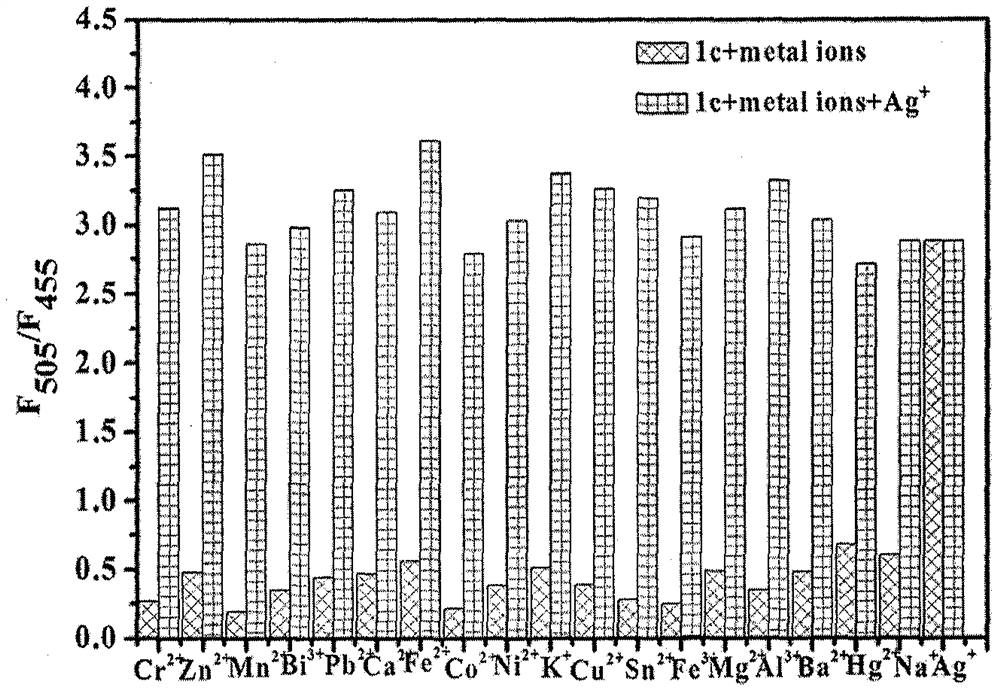
[0037] Penaged 6,6-dimethyl-2-phenyl-3- (2'-pyridyl) -4,5,6,7-tetrahydro-2H-5, 7-bridge-methylhydrazole (1 × 10 -6 M) + AG + (1 × 10 -5 M) solution (HEPES buffer, 10 mM, pH = 7.2, 50% (v / v) c 2 Hide 5 Add and AG in OH) + Wait molaron K + NA + Mg 2+ , Fe 2+ , Fe 3+ Mn 2+ CA 2+ Al 3+ , PB 2+ , Cr 2+ , CO 2+ , Zn 2+ , BI 3 + Ni 2+ , PB 2+ Cu 2+ SN 2+ , Ba 2+ AG + The fluorescence spectrum was measured under conditions of excitation wavelength of 320 nm. figure 2 Indicated. When other metal ions are added, 6,6-dimethyl-2-phenyl-3- (2'-pyridyl) -4, 5, 6, 7-tetrahydro-2H-5, 7-bridge Cantazole-AG + The fluorescence intensity of the complex has not been affected, indicating that other metal ions have not been detected by compounds. + Result in an impact.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com