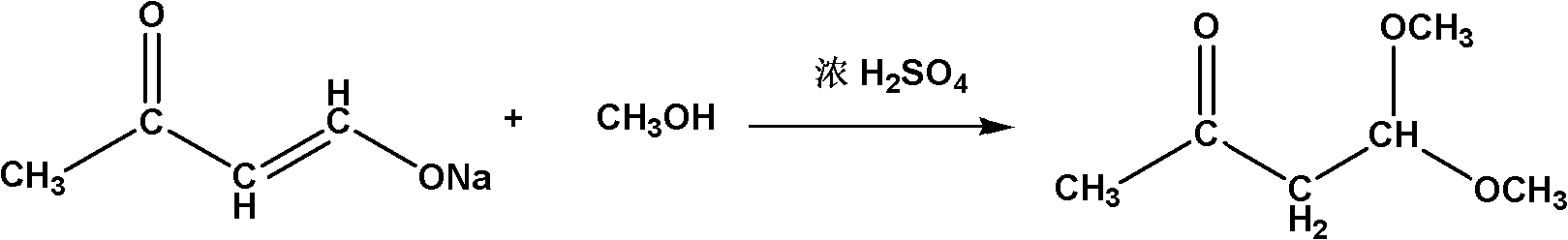
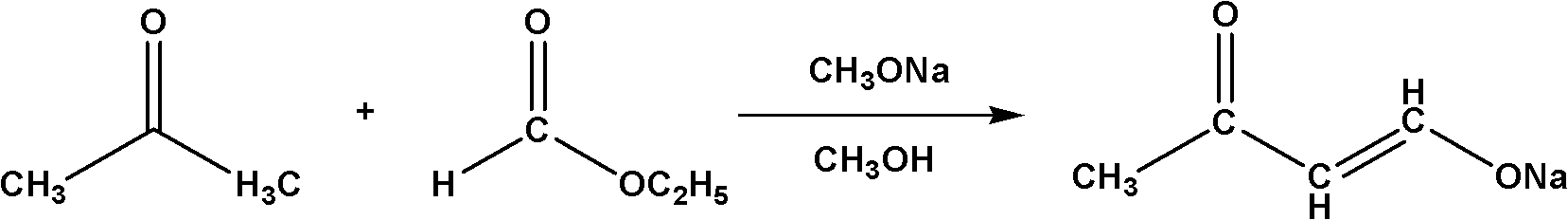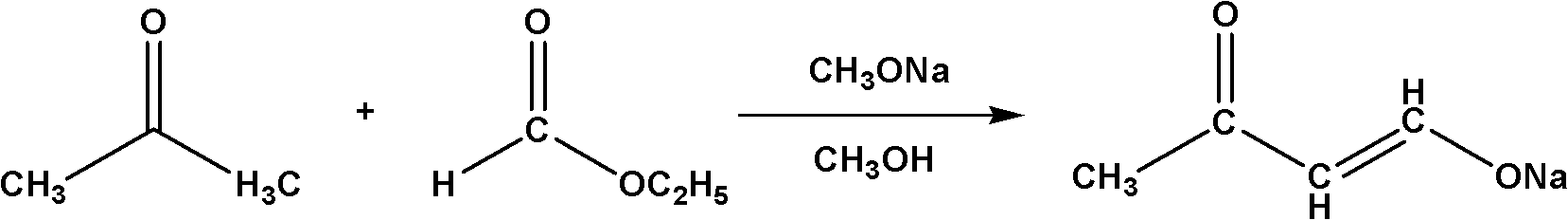Method for synthesizing 4,4-dimethoxy-2-butanone
The technology of a dimethoxy group and a synthetic method is applied in the field of fine chemical raw materials, and can solve the problems of expensive methyl formate, inhalation by operators, and troublesome operation, and achieve the effects of low price, easy operation and convenient storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) In a 1L four-neck flask equipped with a thermometer, a spherical condenser, a stirrer and a nitrogen protection device, add 135g of liquid sodium methoxide, start stirring, heat up to 30°C, and drop 43.5g of acetone and 55.5g of formic acid into it The ethyl ester mixture was added dropwise after 4 hours, and then kept at 30°C for 5 hours to obtain a suspension containing sodium butanone enolate.
[0018] (2) Rotary evaporation was performed on the sodium butanone enolate suspension to remove the solvent and liquid impurities to obtain 53.2 g of a white solid of sodium butanone enolate, and 100 g of methanol was added to form a solution. In another 1L four-necked flask equipped with a thermometer, a spherical condenser, a stirrer and a nitrogen protection device, a mixed solution of 48.3g of concentrated sulfuric acid (98%) and 160g of methanol was added, and the temperature was raised to 25°C. Add the prepared sodium butanone enolate solution dropwise into a four-n...
Embodiment 2
[0021] (1) In a 1L four-neck flask equipped with a thermometer, a spherical condenser, a stirrer and a nitrogen protection device, add 135g of liquid sodium methoxide, start stirring, raise the temperature to 40°C, and drop 43.5g of acetone and 111g of ethyl formate into it The ester mixture was added dropwise after 6 hours, and kept at 40°C for 3 hours to obtain a suspension containing sodium butanone enolate.
[0022] (2) Rotary evaporation was performed on the sodium butanone enolate suspension to remove solvent and liquid impurities to obtain 57.0 g of a white solid of sodium butanone enolate, and 90 g of methanol was added to form a solution. In another 1L four-neck flask equipped with a thermometer, a spherical condenser, a stirrer and a nitrogen protection device, a mixed solution of 77.5g of concentrated sulfuric acid (98%) and 200g of methanol was added, and the temperature was raised to 30°C. Add the prepared sodium butanone enolate solution dropwise into a four-neck...
Embodiment 3
[0025] (1) In a 1L four-neck flask equipped with a thermometer, a spherical condenser, a stirrer and a nitrogen protection device, add 122.7g of liquid sodium methoxide, start stirring, raise the temperature to 50°C, and drop 43.5g of acetone and 166.5g of The mixture of ethyl formate was added dropwise within 7 hours, and then kept at 50°C for 2 hours to obtain a suspension containing sodium butanone enolate.
[0026] (2) Rotary evaporation was performed on the sodium butanone enolate suspension to remove solvent and liquid impurities to obtain 63.3 g of a white solid of sodium butanone enolate, and 80 g of methanol was added to form a solution. In another 1L four-necked flask equipped with a thermometer, a spherical condenser, a stirrer and a nitrogen protection device, a mixed solution of 95.3 g of concentrated sulfuric acid (98%) and 300 g of methanol was added, and the temperature was raised to 35° C. Add the prepared sodium butanone enolate solution dropwise into a four-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com