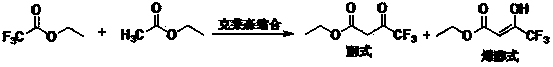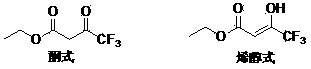Preparation method of ethyl 4,4,4-trifluoroacetoacetate
A technology of ethyl trifluoroacetoacetate and ethyl trifluoroacetate is applied in the field of preparation of ethyl 4,4,4-trifluoroacetoacetate, and can solve the problem that the end point of the dealcoholization process is difficult to control, the feeding process is difficult to control, and the solvent is difficult to control. Recycling energy consumption increases and other problems, to achieve the effect of novel preparation process, avoid severe heat release, and convenient and easy-to-obtain raw materials
Inactive Publication Date: 2014-04-02
SINOCHEM LANTIAN +1
View PDF2 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
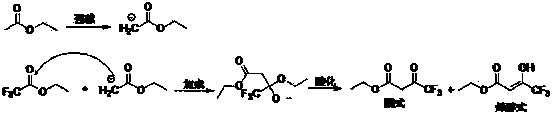
In this production process: solid sodium alkali releases heat violently during the feeding process, the feeding process is difficult to control, and it is easy to cause dust pollution; using metal sodium and ethanol to prepare sodium ethoxide will generate a large amount of hydrogen, which has potential safety hazards in industrial production; Boiling dealcoholization, high energy consumption, difficult to control the end point of the dealcoholization process, resulting in unstable process; dealcoholization uses a large amount of cyclohexane, resulting in increased energy consumption and reaction time for solvent recovery
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a preparation method of ethyl 4,4,4-trifluoroacetoacetate. With an ethanol solution of sodium ethoxide as a catalyst and in the presence of an organic solvent, ethyl trifluoroacetate and ethyl acetate are subjected to a Claisen condensation reaction, and ethyl 4,4,4-trifluoroacetoacetate is synthesized. The method provided by the invention has the advantages of mild conditions, simple operation, relatively high conversion rate of raw materials, relatively high product selectivity, easy separation of the product, low energy consumption and the like, and is suitable for industrialized production. The prepared ethyl 4,4,4-trifluoroacetoacetate is an important pesticide and medicine intermediate, can be used for preparation of thifluzamide, fluacrypyrim, thiazopyr and other pesticides, and also can be used for preparation of befloxatone and other medicines.
Description
technical field [0001] The invention relates to a preparation method of ethyl 4,4,4-trifluoroacetoacetate. Background technique [0002] 4,4,4-Ethyl trifluoroacetoacetate (hereinafter referred to as ethyl trifluoroacetoacetate), the English name is ethyl 4,4,4-trifluoroacetoacetate, and the CAS number is 372-31-6. Molecular formula is C 6 h 7 f 3 o 3 , the molecular weight is 184.11, the density is 1.259, the melting point is -39 ℃, the boiling point is 129.5 ℃ (760 mmHg), the refractive index is 1.375-1.378, the flash point is 28 ℃, and the solubility in water is 10 g / L (20 ℃). Ethyl trifluoroacetoacetate has two configurations, enol and ketone, which can be converted freely under normal circumstances. The specific structure is as follows: Ethyl trifluoroacetoacetate is an important pesticide and pharmaceutical intermediate, which can be used to prepare pesticides such as flufenam, pyrimidin, and thiazopyr, and can also be used to prepare medicines such as beflux...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C69/716C07C67/343
CPCC07C67/343
Inventor 蒋强徐卫国李华戴佳亮杨汪松
Owner SINOCHEM LANTIAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com