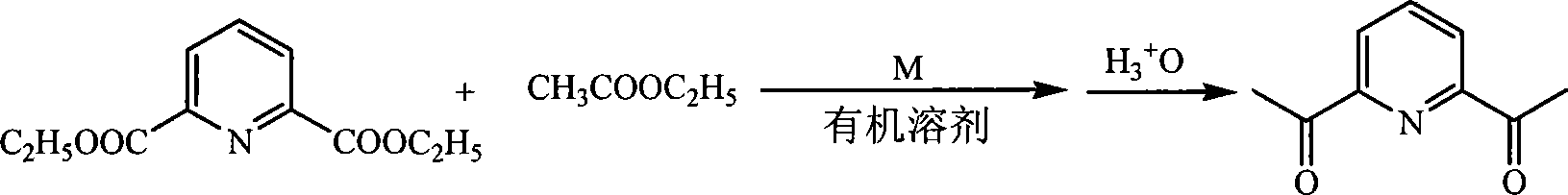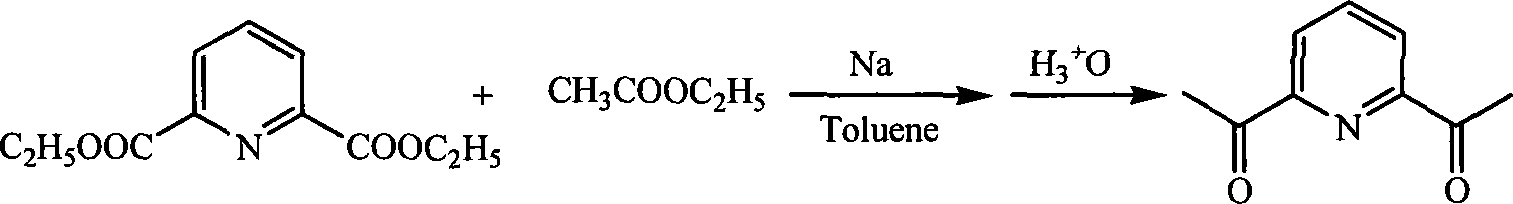Method for preparing 2,6-diacetyl pyridine
A technology of diacetylpyridine and diethyl pyridinedicarboxylate, which is applied in the direction of organic chemistry, can solve the problems of product failure, cumbersome operation, and yield decline, and achieve product yield improvement, simplification of experimental operations, and The effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] In a 250ml three-necked flask, add 1.115g (0.005mol) of pyridine diester, 20ml of ethyl acetate and 20ml of toluene in sequence, and add 0.575g (0.025mol) of sodium metal while stirring. After the sodium metal is completely dissolved, heat up to 120°C and reflux for 8 -9h, then add 20ml of water and 20ml of concentrated hydrochloric acid, react at 120°C for 4h, stop the reaction, adjust the pH of the mixture to neutral with sodium carbonate or sodium bicarbonate, then extract with ether 3-4 times, combine the solutions with anhydrous Na 2 SO 4 After drying, the solvent was evaporated and recrystallized with anhydrous methanol to obtain 0.717 g of white crystals with a yield of 88%. 1 HNMR (300MHz, CDCl 3 ,) δ8.20(d, 2H, Py-Hm), 8.04(t, 1H, Py-Hp), 2.81(s, 6H, O=C(CH 3 )).Anal.Calcd for C 9 h 9 NO 2 : C, 66.25; H, 5.56; N, 8.58. Found: C, 66.25; H, 5.52; N, 8.73.mp 79°C.
Embodiment 2
[0021] Add 1.115g (0.005mol) of pyridine diester, 20ml of ethyl acetate and 30ml of toluene to a 250ml three-necked flask, and add 0.69g (0.03mol) of sodium metal while stirring. After the sodium metal is completely dissolved, heat up to 120°C and reflux for 8- 9h, then add 20ml of water and 20ml of concentrated hydrochloric acid, react at 120°C for 4h, stop the reaction, adjust the pH of the mixture to neutral with sodium carbonate or sodium bicarbonate, then extract with ether 3-4 times, combine the solutions with anhydrous Na 2 SO 4 After drying, the solvent was evaporated and recrystallized with anhydrous methanol to obtain 0.701 g of white crystals with a yield of 86%. 1 HNMR (300MHz, CDCl 3 ,) δ8.20(d, 2H, Py-Hm), 8.04(t, 1H, Py-Hp), 2.81(s, 6H, O=C(CH 3 )).Anal.Calcd for C 9 h 9 NO 2 : C, 66.25; H, 5.56; N, 8.58. Found: C, 66.23; H, 5.55; N, 8.62.mp 79°C.
Embodiment 3
[0023] Add 1.115g (0.005mol) of pyridine diester and 30ml of ethyl acetate into a 250ml three-necked flask, and add 0.80g (0.02mol) of calcium metal while stirring. After the calcium metal is completely dissolved, heat up to 100°C and reflux for 8 hours, then slowly add 20ml Water and 20ml of concentrated hydrochloric acid were reacted at 120°C for 4 hours, and the reaction was stopped. The excess acid was neutralized with sodium carbonate, and then extracted with ether for 3-4 times. After combining the solutions, use anhydrous Na 2 SO 4 After drying and evaporating the solvent, it was purified with a silica gel column. The developing solvent was petroleum ether: ethyl acetate (5:1) to obtain 0.75 g of white crystals with a yield of 92%. 1 HNMR (400MHz, CDCl 3 ,) δ8.20(d, 2H, Py-Hm), 8.04(t, 1H, Py-Hp), 2.81(s, 6H, O=C(CH 3 )).Anal.Calcd for C 9 h 9 N0 2 : C, 66.25; H, 5.56; N, 8.58. Found: C, 66.20; H, 5.58; N, 8.63.mp 79.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com