Preparation method of 4-ethylpyridine
A technology of ethylpyridine and acetylpyridine, applied in organic chemistry and other fields, can solve problems such as difficult industrialization and dangerous processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of preparation method of 4-ethylpyridine, comprises the following steps:
[0030] (1) After mixing ethyl 4-pyridinecarboxylate and sodium ethoxide, heat it to 90-110°C, then add ethyl acetate dropwise to carry out Claisen ester condensation reaction to obtain 3-oxo 3-(4-pyridyl ) ethyl propionate;
[0031] (2) After mixing the ethyl 3-oxo 3-(4-pyridyl)propionate obtained in the step (1), dimethyl sulfoxide and water, heat treatment is carried out to obtain 4-acetylpyridine;
[0032] (3) Mix ethylene glycol and potassium hydroxide and cool, add hydrazine hydrate, heat up to 60-80°C, then mix with the 4-acetylpyridine obtained in the step (2), and perform a reduction reaction to obtain 4-acetylpyridine base pyridine.
[0033] In the present invention, after mixing ethyl 4-pyridinecarboxylate and sodium ethoxide, heat it to 90-110°C, then add ethyl acetate dropwise to carry out Claisen ester condensation reaction to obtain 3-oxo 3-(4-pyridyl...
Embodiment 1
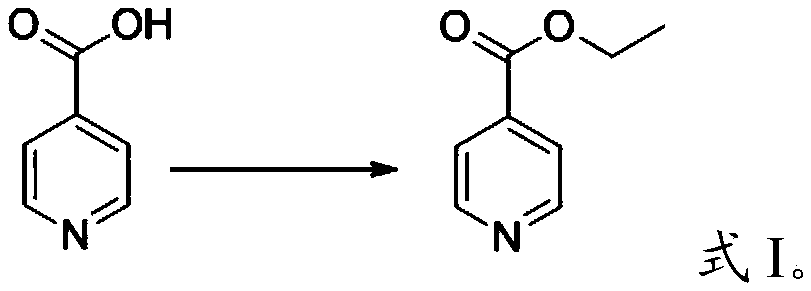
[0061] (1) Preparation of ethyl 4-pyridinecarboxylate
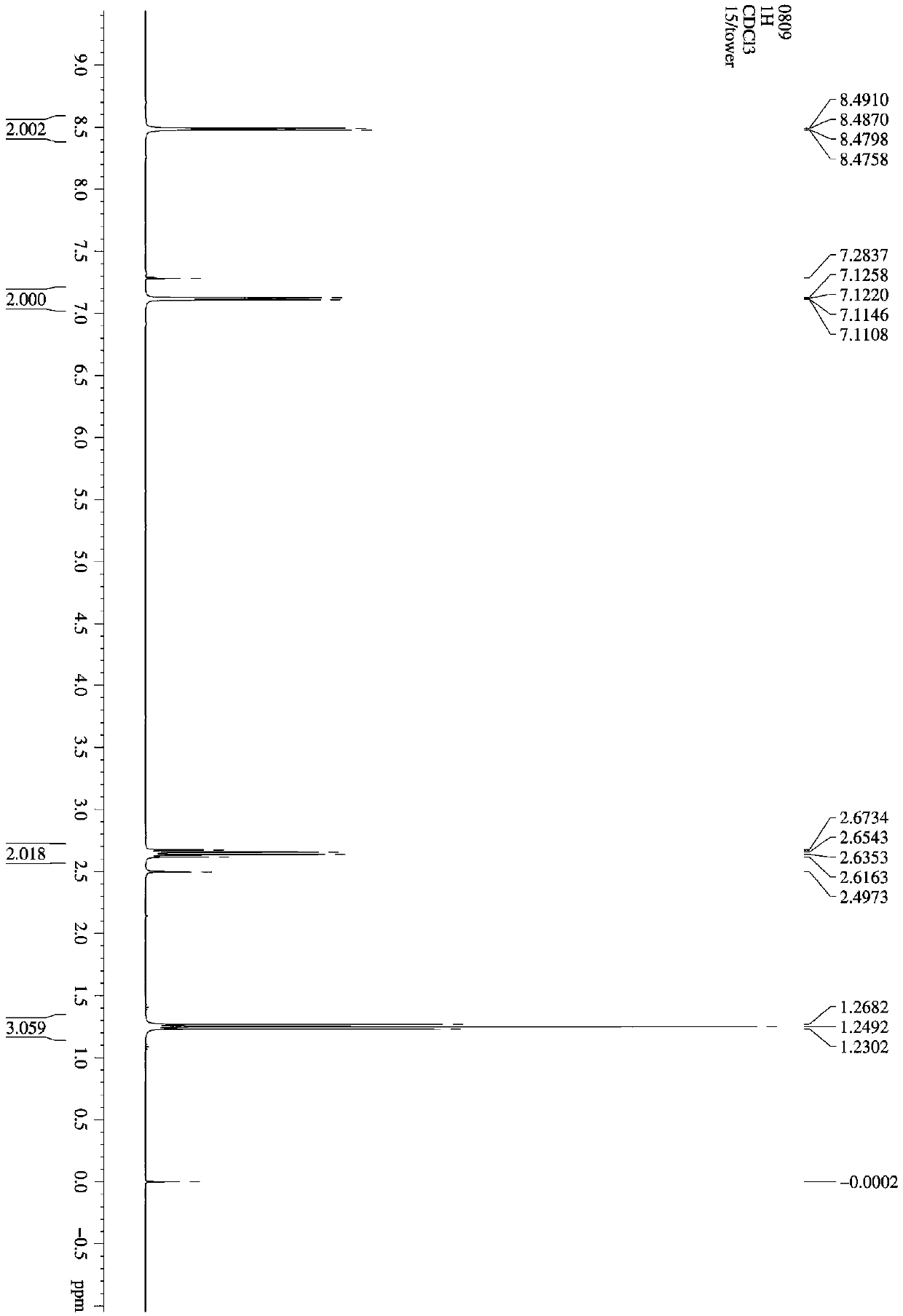
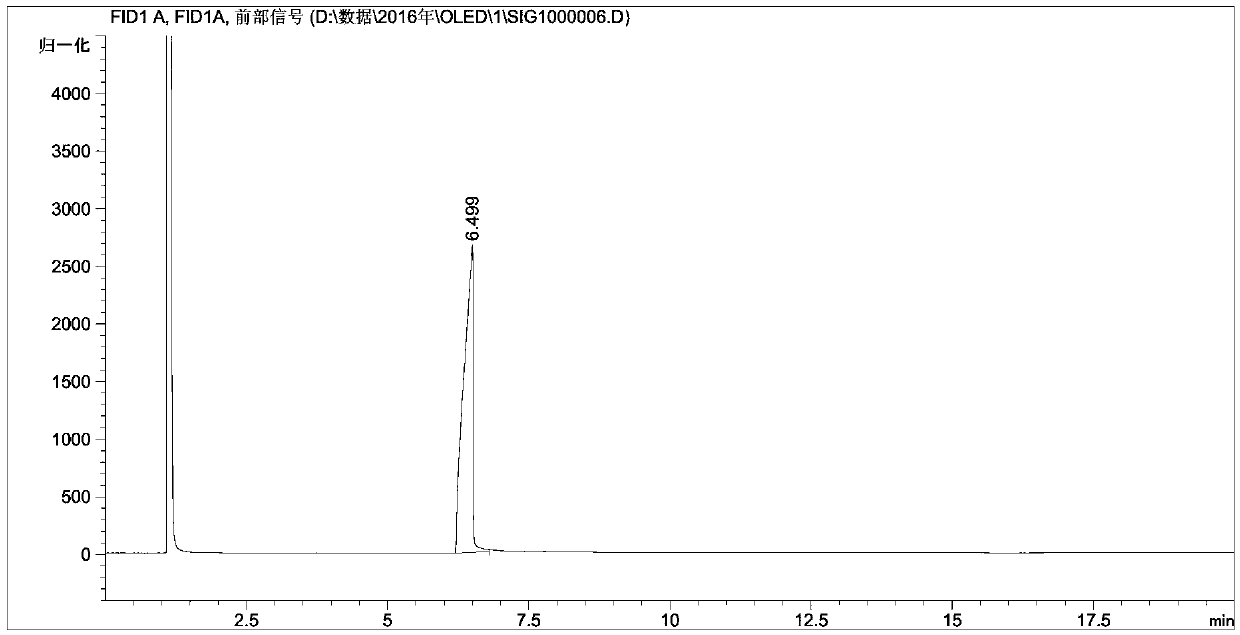
[0062] 0.8 mol of isonicotinic acid, 0.64 mol of absolute ethanol, 2.0 g of activated powdered activated carbon were successively added into a 100 mL three-necked flask as a catalyst, and toluene as a solvent. Microwave reaction time 10min (power 200W; temperature 130°C). with saturated Na 2 CO 3 The aqueous solution was used to neutralize the reaction solution until the pH was 7, and the layers were allowed to stand to separate, and the upper organic layer was taken; the aqueous phase was extracted with 20 mL of chloroform, and the extract and the organic layer were combined. Chloroform was first recovered by atmospheric distillation and then vacuum distillation to obtain colorless transparent liquid ethyl 4-pyridinecarboxylate with a purity of 96.3% (GC) and a yield of 97.2%.
[0063] Activated powdered activated carbon is obtained by immersing the powdered activated carbon in 60 mL of 25% p-toluenesulfonic acid aque...
Embodiment 2
[0071] (1) Preparation of ethyl 4-pyridinecarboxylate
[0072] 0.8 mol of isonicotinic acid, 0.64 mol of absolute ethanol, 2.0 g of activated powdered activated carbon were successively added into a 100 mL three-necked flask as a catalyst, and toluene as a solvent. Microwave reaction time 10min (power 200W; temperature 130°C). with saturated Na 2 CO 3 The aqueous solution was used to neutralize the reaction solution until the pH was 7, and the layers were allowed to stand to separate, and the upper organic layer was taken; the aqueous phase was extracted with 20 mL of chloroform, and the extract and the organic layer were combined. Chloroform was first recovered by atmospheric distillation and then vacuum distillation to obtain colorless transparent liquid ethyl 4-pyridinecarboxylate with a purity of 96.3% (GC) and a yield of 97.2%.
[0073] Activated powdered activated carbon is obtained by immersing the powdered activated carbon in 60 mL of 25% p-toluenesulfonic acid aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com