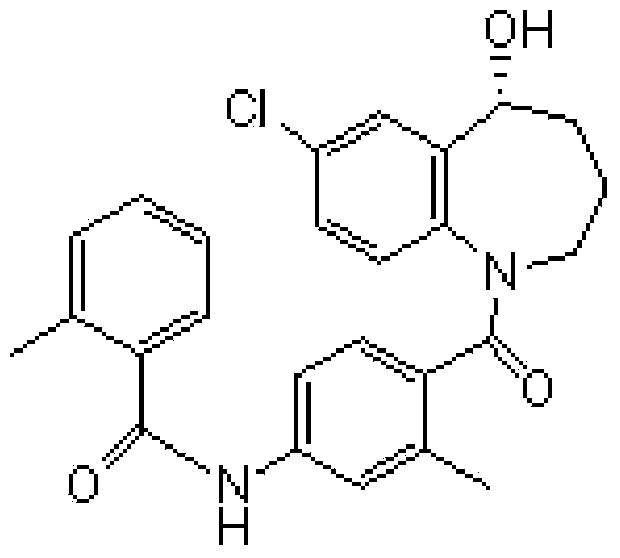
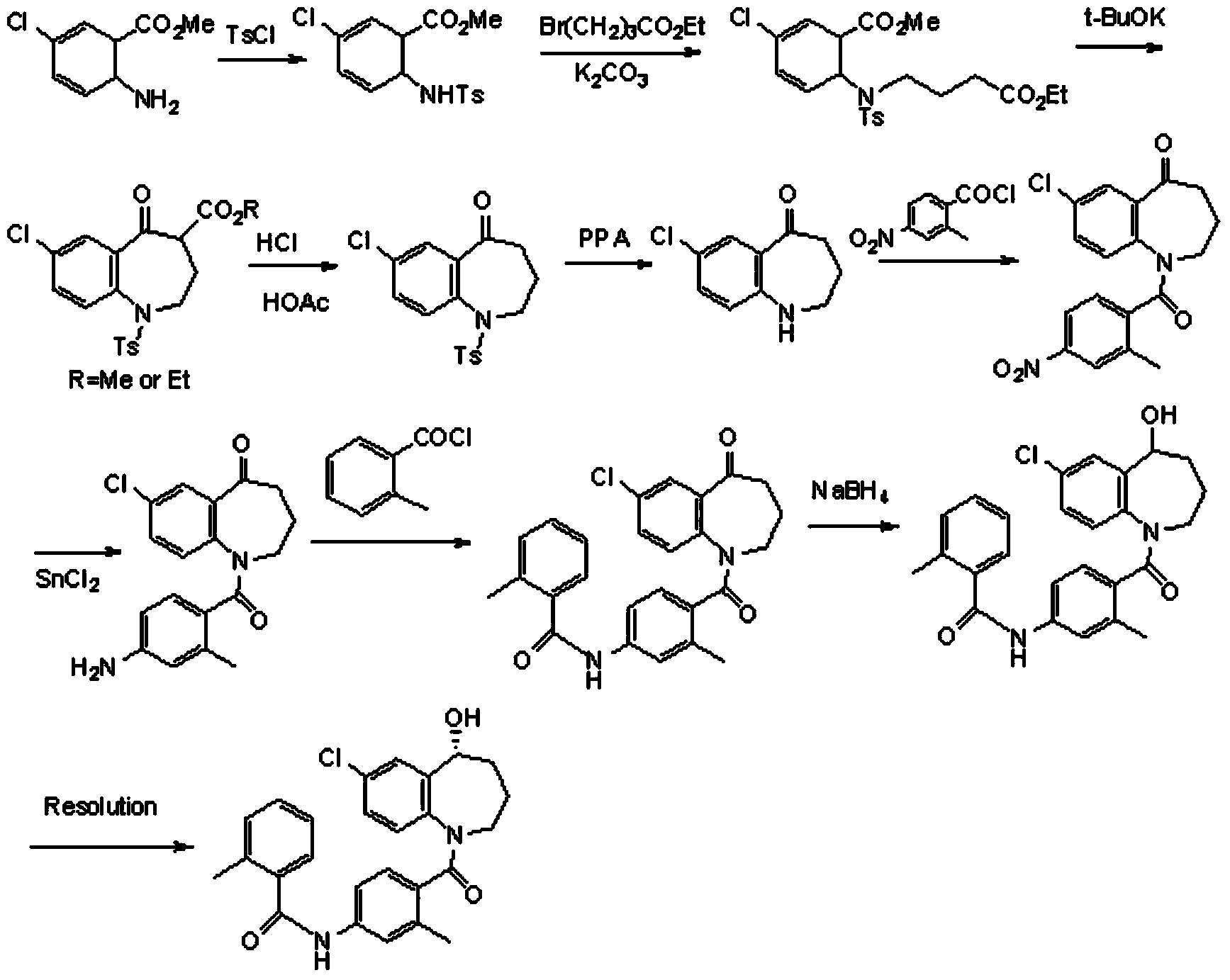
Preparation method of tolvaptan intermediate
A technology of tolvaptan and intermediates, applied in the field of drug synthesis, to achieve the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
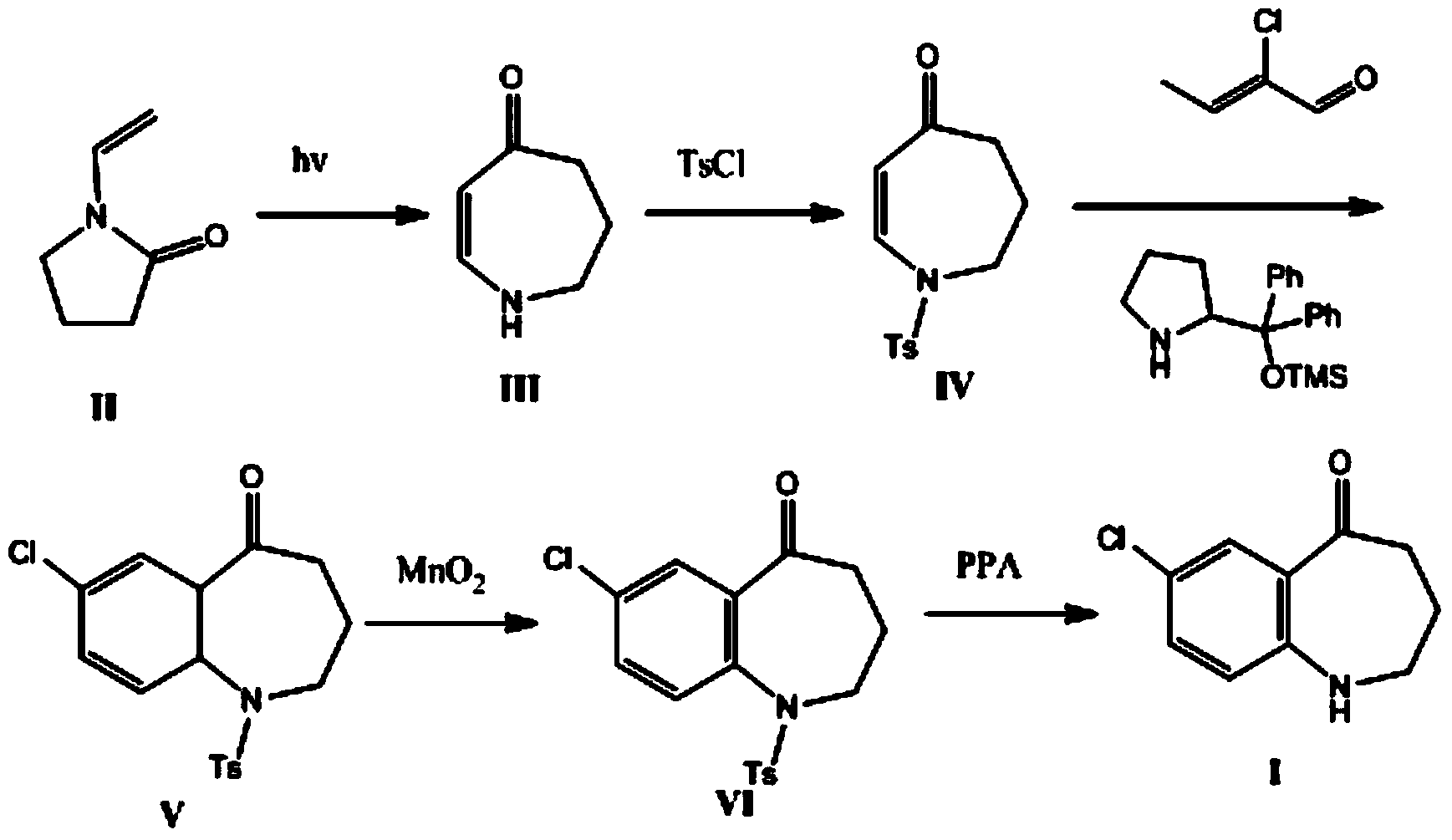
[0024] The synthesis of embodiment 1 compound 4-azepanenone
[0025] N-vinylpyrrolidone (30 g, 0.27 mol) was irradiated in 1600 mL of methanol at 40 °C with a 50-watt low-pressure mercury UV lamp for 45 hours, concentrated under reduced pressure and recrystallized in acetonitrile to obtain 24 g of 4-azepanenone. The yield is 80%. Melting point: 74-76°C. 1 H NMR (400MHz, CDCl 3 ):δ6.90(d,J=10.9Hz,1H),5.14(d,J=10.9Hz,1H),2.99-2.90(m,4H),2.00(s,1H),1.96-1.86(m, 2H).
Embodiment 2
[0026] The preparation of embodiment 2N-p-toluenesulfonyl-4-azepanenone
[0027] Add 24g (0.216mol) of 4-azepanenone, 125ml of dichloromethane, 125ml of pyridine, and 0.3g of 4-dimethylaminopyridine into a 500mL single-necked round bottom flask, and then add 41.18g of p-toluenesulfonyl chloride ( 0.216mol), the mixture was stirred at room temperature for 8 hours. Then add 100mL1N hydrochloric acid, extract with dichloromethane (50mL×3), combine the organic phases, wash with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure to obtain 55.0 g (0.21 mol) of a white solid, with a yield of 96%.
[0028] 1 H NMR (400MHz, CDCl 3 ):δ7.74(dd,J=7.5,1.5Hz,2H),7.40(dd,J=7.5,1.5Hz,2H),6.92(d,J=10.9Hz,1H),5.14(d,J= 10.9Hz, 1H), 3.16(m, 2H), 2.94(m, 2H), 2.34(s, 3H), 1.96-1.86(m, 2H).
Embodiment 3
[0029] Example 3 Preparation of N-p-toluenesulfonyl-7-chloro-2,3,4,5,10,11-hexahydro-1H-1-benzazepin-5-one
[0030] Add N-p-toluenesulfonyl-4-azepanenone (265mg, 1.0mmol), 2-chlorobutenal (104mg, 1.0mmol), (S)-diphenyl Trimethylsiloxymethylpyrrolidine (5mg), chloroform (15ml), and p-nitrobenzoic acid (5mg) were reacted at room temperature for 48 hours and separated by column chromatography to obtain 238mg (0.68mmol) of the product, with a yield of 68%.
[0031] 1 H NMR (400MHz, CDCl 3 ):δ7.73(dd,J=7.5,1.5Hz,2H),7.39(dd,J=7.5,1.5Hz,2H),5.98(dd,J=6.2,1.0Hz,1H),5.90(d, J=10.9Hz,1H),5.80(dd,J=10.9,6.2Hz,1H),3.50(dd,J=7.0,7.0Hz,1H),3.30-3.10(m,3H),2.60-2.33(m ,2H), 2.33(s,3H), 1.97-1.86(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com