Nopinic alkyl beta-dione boron difluoride complexes and preparation method and application thereof
A technology of diketone boron difluoride and nopinyl group, which is applied in the field of nopinyl β-diketone boron difluoride complex and its preparation, achieving the effects of high yield, simple synthesis operation and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
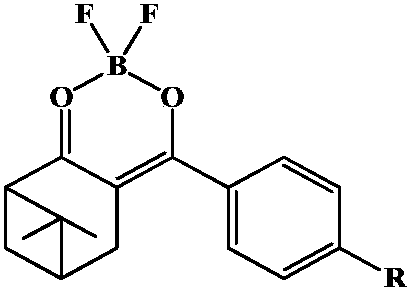
[0042] 1) Preparation of norpinyl β-diketone
[0043] Add 0.06mol sodium hydride into the reaction vessel, slowly inject 8mL of ethylene glycol dimethyl ether under the protection of nitrogen, dissolve 0.02mol of nopinone in 9mL of ethylene glycol dimethyl ether, slowly inject into the reaction vessel under the protection of nitrogen, and control the reaction by raising the temperature The temperature was 82°C, heated to reflux for 0.5h, 0.024mol of aromatic esters 1a and 1b were dissolved in 9mL of ethylene glycol dimethyl ether, and then slowly injected into the reaction vessel under the protection of nitrogen. The reaction progress was tracked by thin-layer chromatography, and the reaction was carried out for 7-8h. The reactant was extracted three times with 100 mL of ethyl acetate, and the organic phase was concentrated to recover the solvent to obtain the crude product of nopinone β-diketone compound. Recrystallize with methanol, get nopinyl β-diketone 2a and 2b, the stru...
Embodiment 2
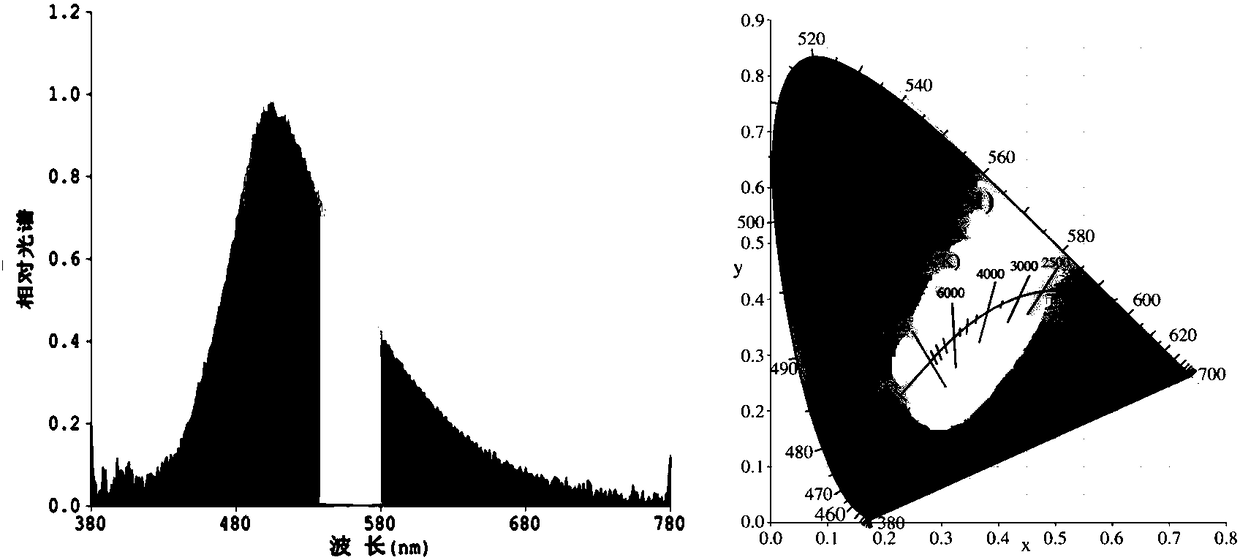
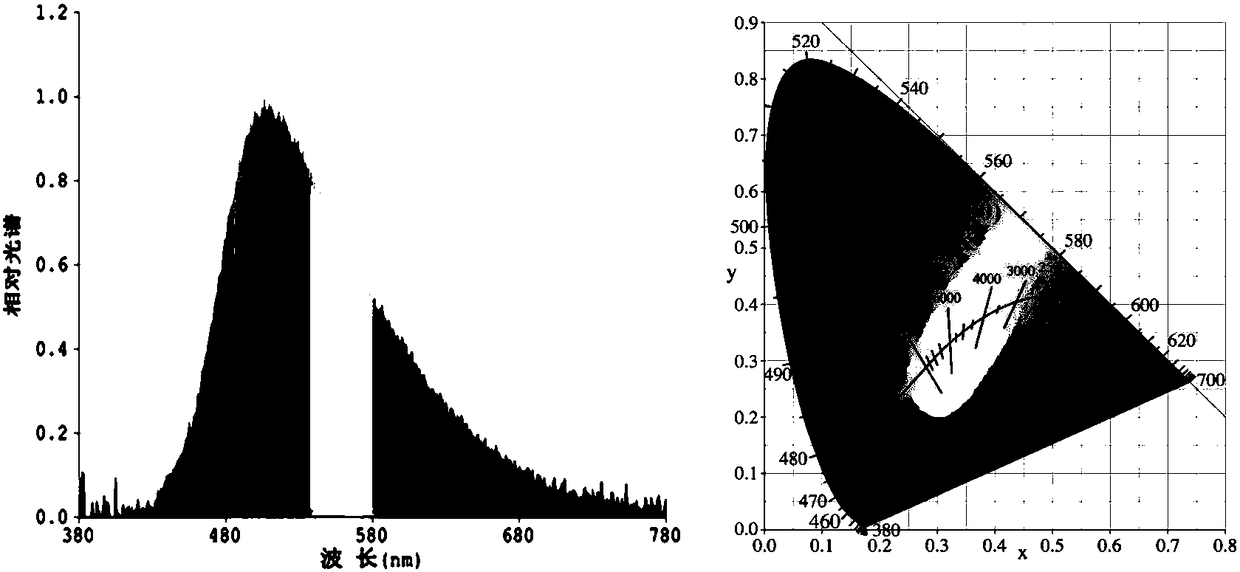
[0054] The norpinyl β-diketone boron difluoride complex is used as a light-emitting material in electroluminescent devices.
[0055] 1) Use a 0.001g electronic scale to weigh the conductive adhesive A glue and the curing agent B glue, and the weight ratio is 1:4.
[0056] 2) Conductive adhesive A needs to be stirred for more than 2 minutes before use. Then mix the conductive adhesive A glue with the fluorescent powder and stir well before adding the curing agent B glue. After adding the curing agent B glue, the stirring time generally takes 5 minutes. Make the fluorescent powder and the glue fully mix evenly, then defoam and set aside.
[0057] 3) The debubbled fluorescent glue must be properly stirred before dispensing. It is best to dispensing glue when the bracket is full and the cup is slightly convex, but not overflowing. After drying, the glue can be a flat cup.
[0058] 4) Baking conditions: 60°C / 40min+135°C / 90min. After dispensing, enter the oven for baking immedi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com