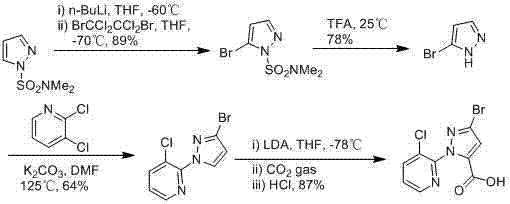
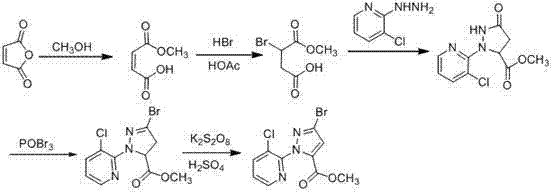
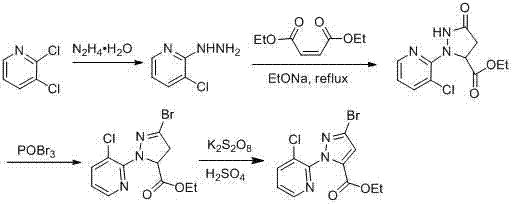
Synthesis method of 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-formate
A synthesis method and formate technology are applied in the field of broad-spectrum insecticide chlorantraniliprole pesticides, which can solve the problems of expensive raw materials and high industrial production costs, reduce equipment corrosion and harm to the health of operators, The effect of cost reduction and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Take a 50 mL three-necked flask, add a reflux condenser, and install a drying tube with anhydrous calcium chloride on the top of the condenser. Add 5 mL (123 mmol) of anhydrous methanol to the three-necked flask, add 0.54 g (10 mmol) of sodium methoxide, and when the sodium methoxide is completely dissolved, put the three-necked flask into a low-temperature reactor and cool to 0 °C. At this temperature, a mixture of methyl formate (0.78 mL, 13 mmol) and methyl pyruvate (0.75 mL, 8.3 mmol) was slowly added dropwise. After the dropwise addition was completed, the mixture was stirred at this temperature for 10 hours, and then rotary evaporated to dryness. Add 5 mL of cold diethyl ether to the evaporated solid to dissolve, filter to obtain a khaki solid sodium salt of methyl 2,4-dioxobutyrate, weigh 0.85 g after drying, and the yield is 67% (based on Calculate the amount of methyl pyruvate).
[0046](2) Take 1.52 g (10 mmol, sodium salt of methyl 2,4-dioxobutyrate) ob...
Embodiment 2
[0048] (1) Take a 50 mL three-necked flask, add a reflux condenser, and install a drying tube with anhydrous calcium chloride on the top of the condenser. Add 10 mL (246 mmol) of anhydrous methanol to the three-necked flask, and add 0.54 g (10 mmol) of sodium methoxide. When the sodium methoxide is completely dissolved, put the three-necked flask into a low-temperature reactor and cool to 0°C. At this temperature, a mixture of methyl formate (1.08 mL, 18 mmol) and methyl pyruvate (0.75 mL, 8.3 mmol) was slowly added dropwise. After the dropwise addition was complete, it was stirred at this temperature for 10 hours. Rotary evaporate to dryness, add 5 mL of cold diethyl ether to the solid, filter to obtain khaki solid sodium salt of methyl 2,4-dioxobutyrate, weigh 0.86 g after drying, and the yield is 55% (based on Calculate the amount of methyl pyruvate).
[0049] (2) Take 1.52 g (10 mmol) of methyl 2,4-dioxobutyrate sodium salt obtained in (1) above, dissolve it in 10 mL of ...
Embodiment 3
[0051] (1) Take a 50 mL three-necked flask, add a reflux condenser, and install a drying tube with anhydrous calcium chloride on the top of the condenser. Add 5 mL (123 mmol) of anhydrous methanol to the three-necked flask, and add 0.54 g (10 mmol) of sodium methoxide. When the sodium methoxide is completely dissolved, put the three-necked flask into a low-temperature reactor and cool to 0°C. At this temperature, a mixture of methyl formate (0.78 mL, 13 mmol) and methyl pyruvate (0.75 mL, 8.3 mmol) was slowly added dropwise. After the dropwise addition was complete, it was stirred at this temperature for 10 hours. Rotary evaporate to dryness, add 5 mL of cold diethyl ether to the solid, filter to obtain khaki solid sodium salt of methyl 2,4-dioxobutyrate, weigh 1.02 g after drying, and the yield is 75% (based on Calculate the amount of methyl pyruvate).
[0052] (2) Take 1.52 g (10 mmol) of methyl 2,4-dioxobutanoate sodium salt in (1) above, dissolve it in 10 mL of methanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com