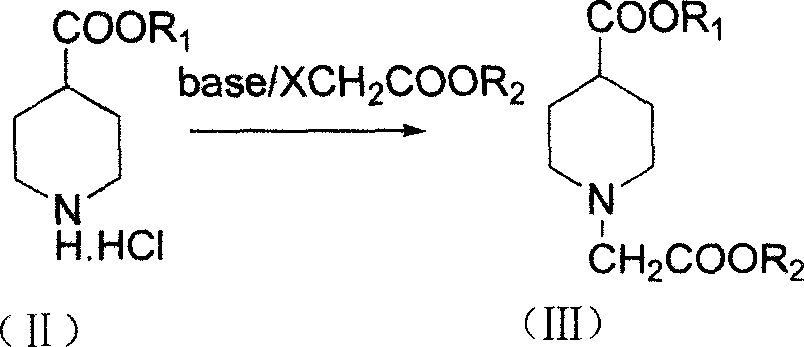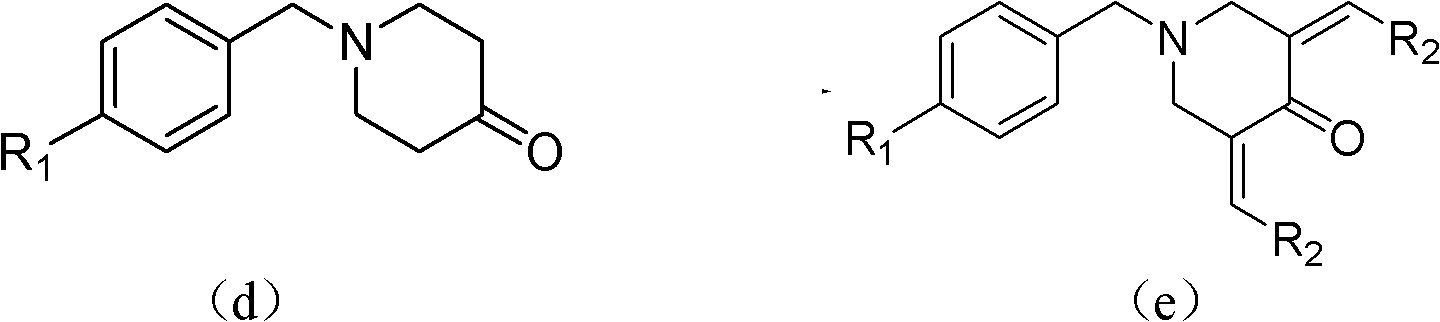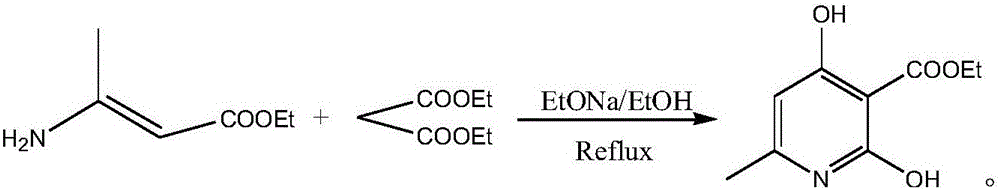Patents
Literature
37 results about "Dieckmann condensation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation.
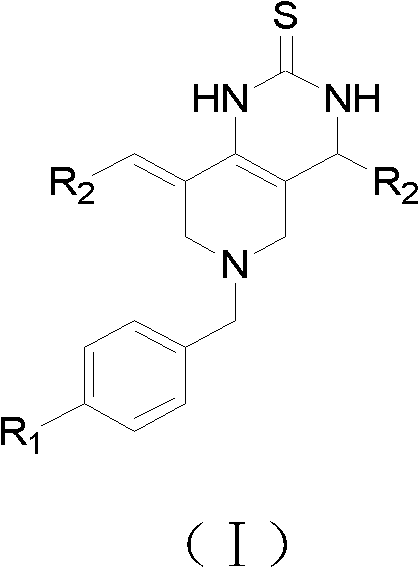
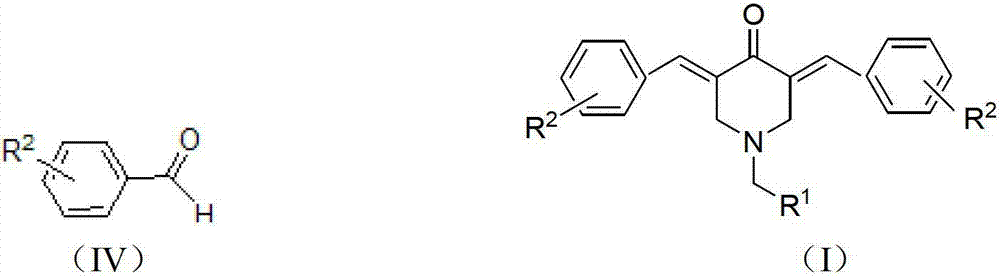
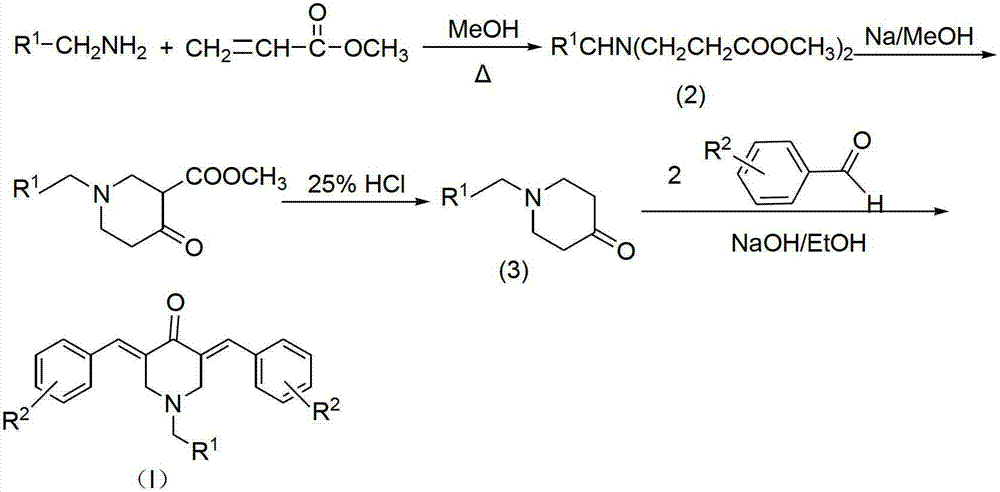
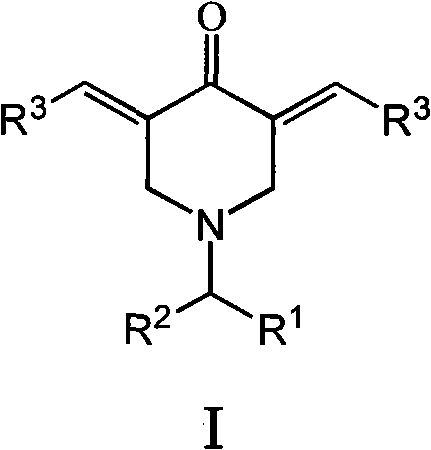
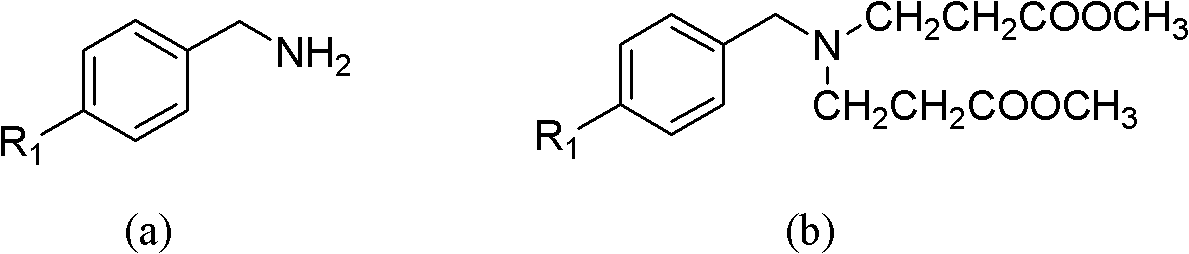
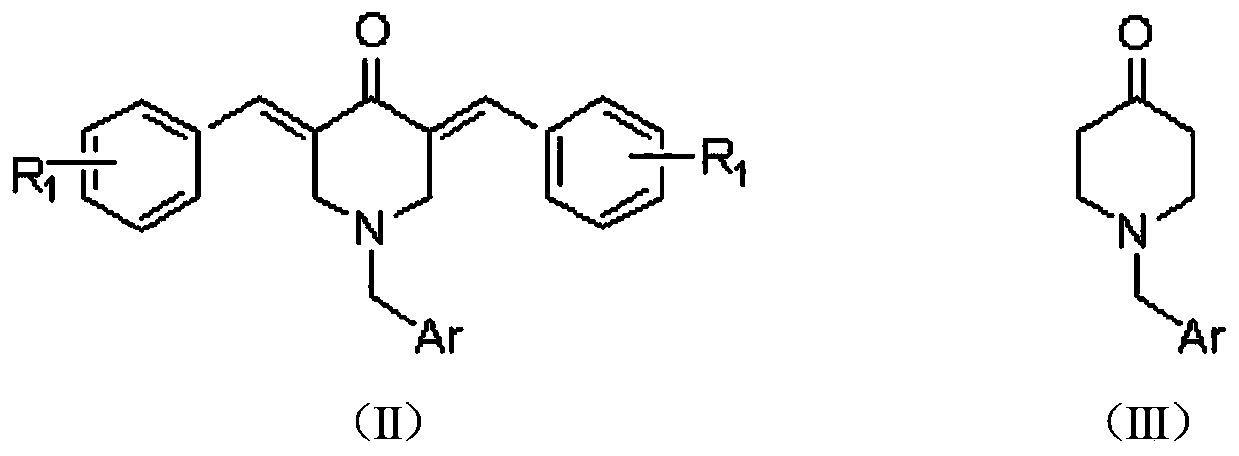
N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and preparation method and application thereof
InactiveCN102863376AInhibit biological activitySmall side effectsOrganic chemistryAntineoplastic agentsCarcinoma cell lineCancer cell
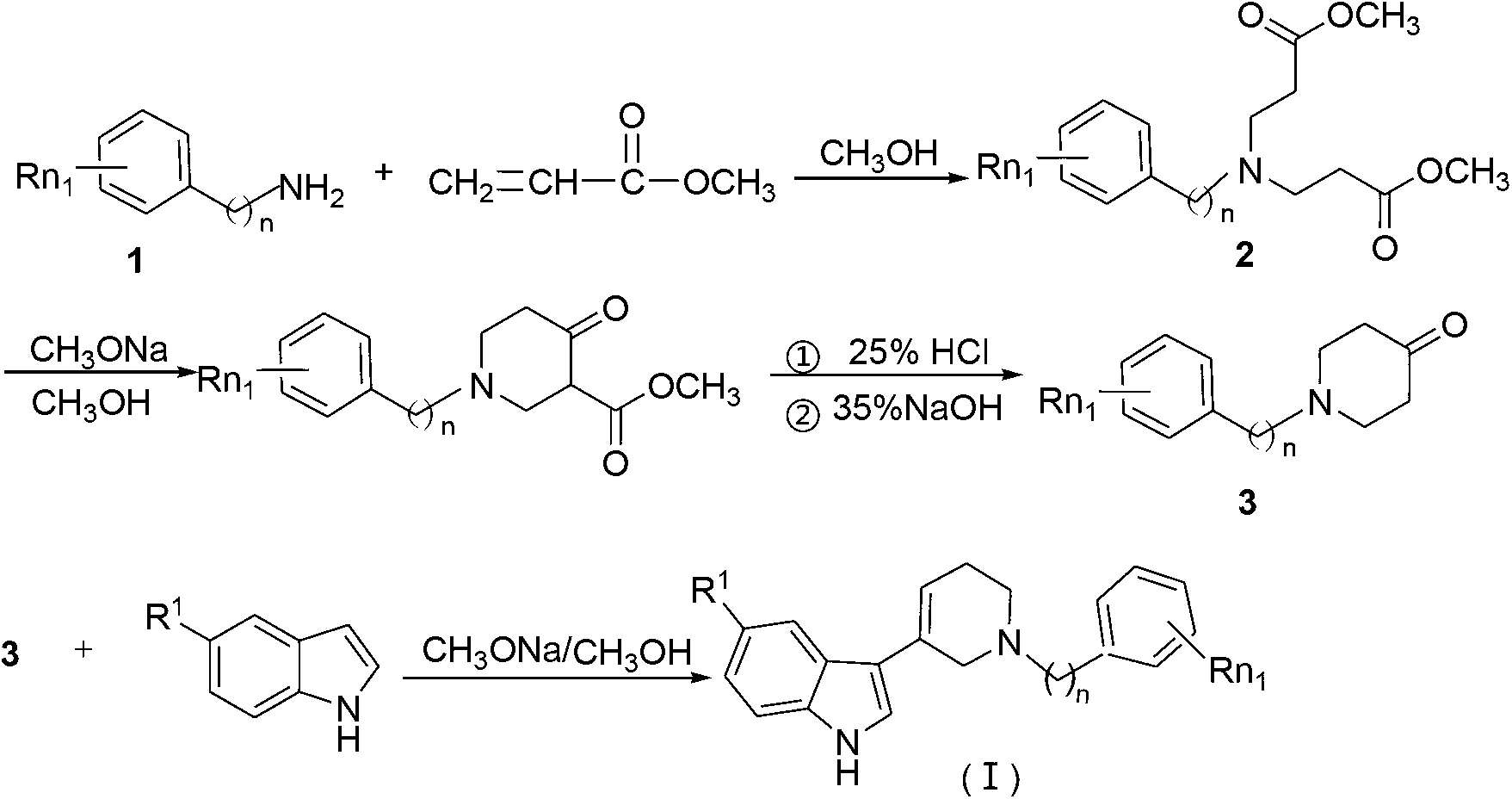
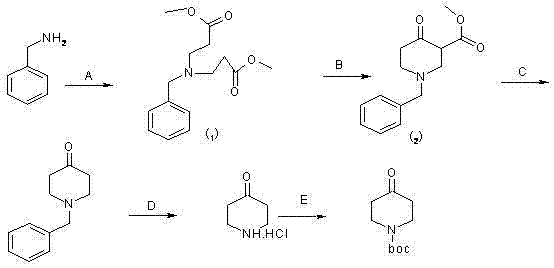
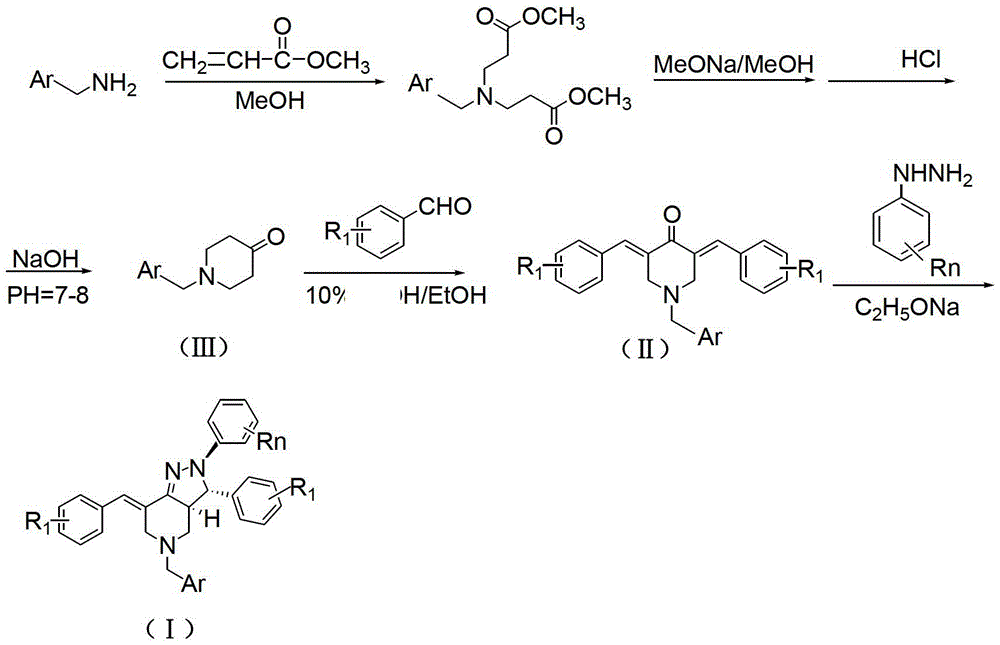
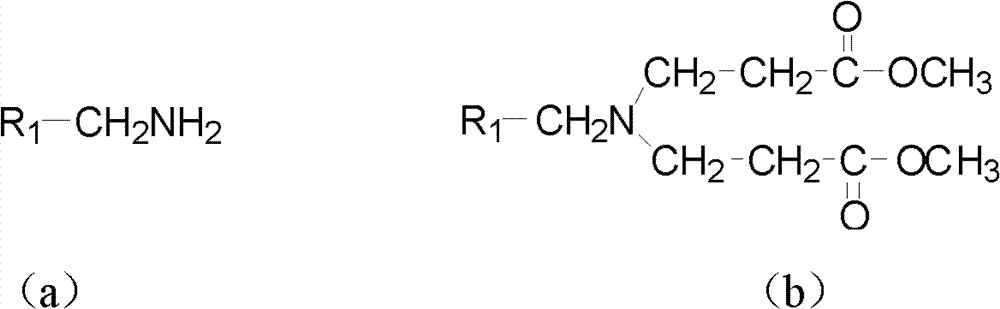
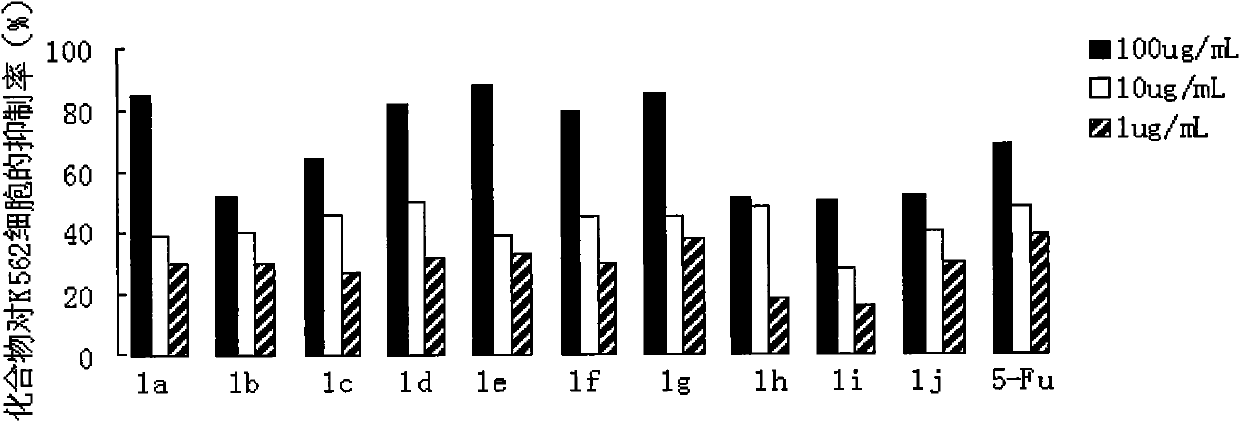
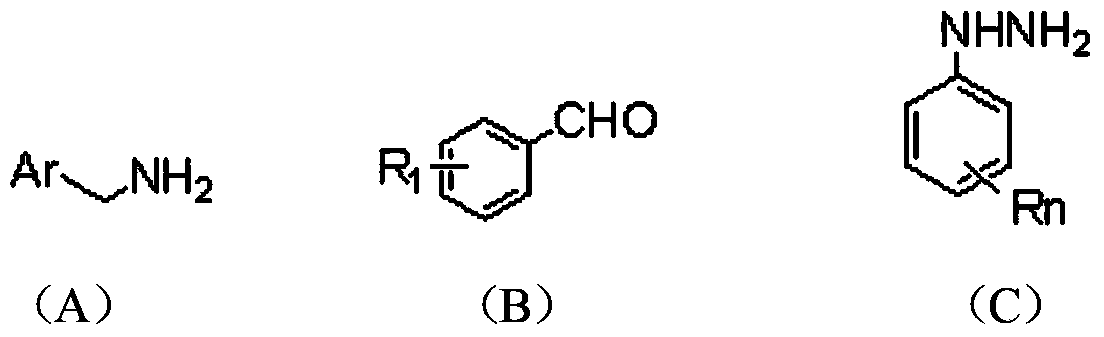
The invention relates to the field of organic synthesis and medicine, and discloses a preparation method for N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and biological activity for efficiently inhibiting cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer. The method includes: starting from various substituted methylamine and methyl acrylate, sequentially going through Michael addition, Dieckmann condensation, acidolysis and decarboxylation to obtain N-substituted methyl-4-piperidone, and subjecting the N-substituted methyl-4-piperidone to aldol reaction with substituted benzaldehyde to obtain a target compound N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone. The target compound can selectively and efficiently inhibit cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer, and activity of inhibiting carcinoma cell line proliferation is obviously higher than conventional chemotherapeutic 5-fluorouracil.
Owner:SHANGHAI NORMAL UNIVERSITY
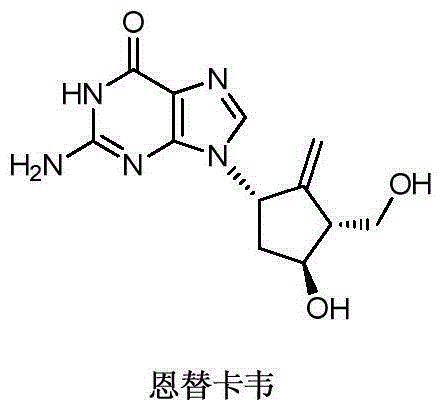
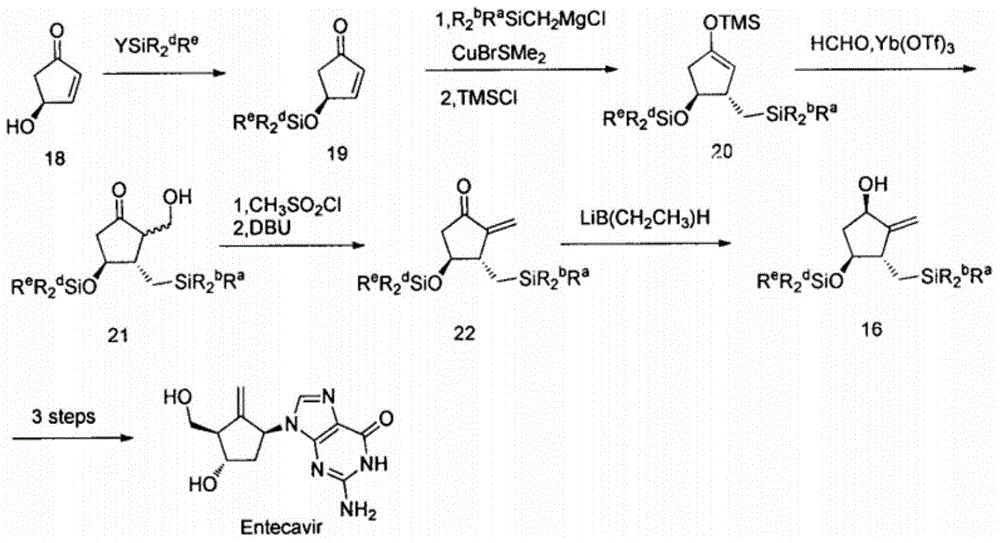
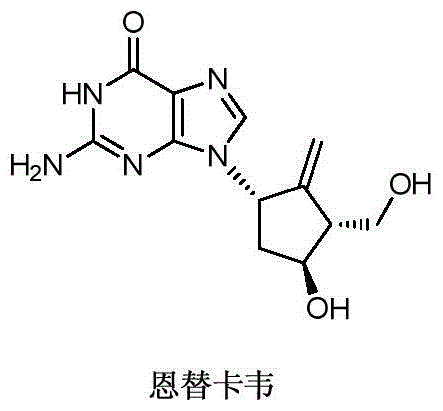
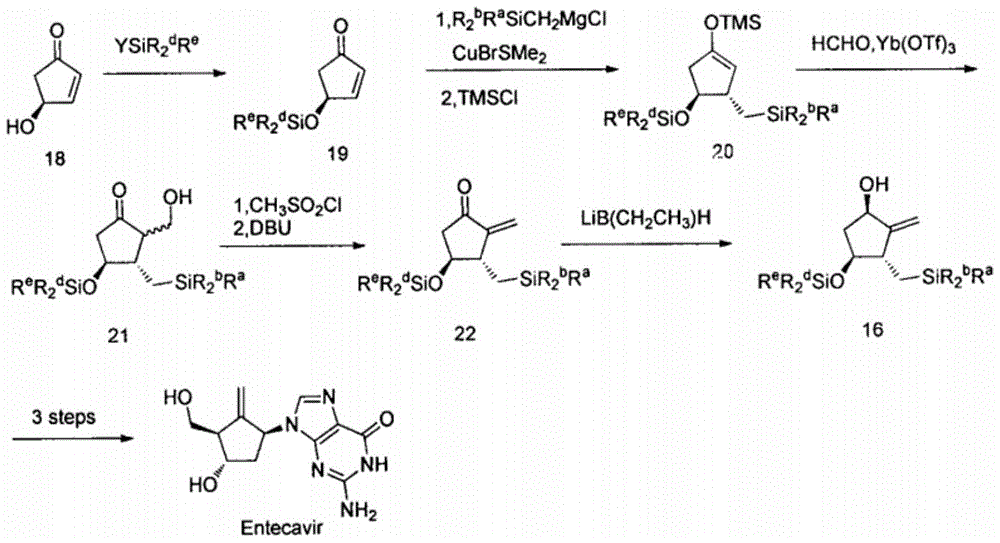
Novel synthetic method for entecavir compound
ActiveCN105037363ASolve rare problemsDosage controllableGroup 4/14 element organic compoundsKetoneAdipate
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
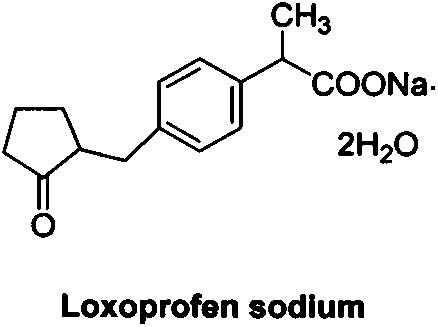
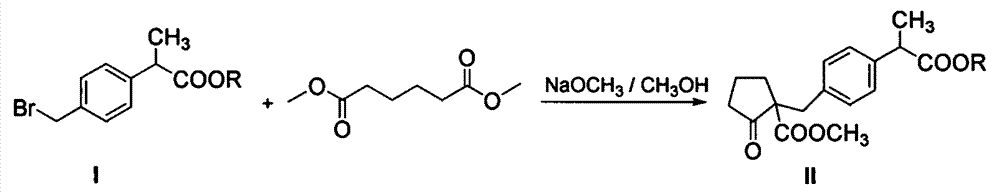
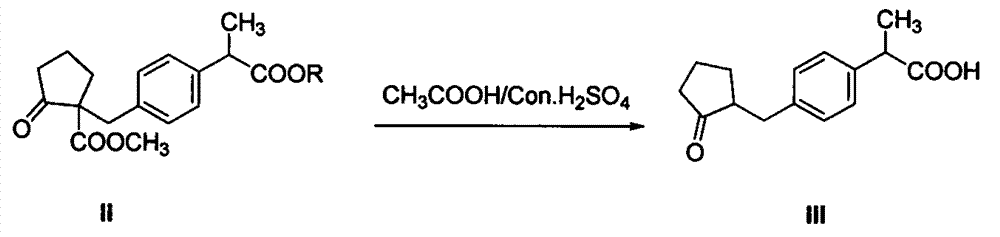
Industrial production method of high purity loxoprofen sodium dehydrate
InactiveCN104326903AHigh yieldImprove qualityOrganic compound preparationCarboxylic acid esters preparationDistillationAdipic acid
The invention discloses an industrial production method of high purity loxoprofen sodium dehydrate; according to the method, adipic acid diester is taken as a starting material for Dieckmann condensation reaction with 2-(4-bromomethyl phenyl) propionate under strong alkaline conditions, the yield and quality of compound II are effectively improved, because the adipic acid diester on the market is lower in cost than 2-ethoxy carbonyl cyclopentanone, the production cost can be greatly reduced; the compound obtained by reaction is processed sequentially by hydrolysis and decarboxylation under acidic conditions, in the hydrolysis and decarboxylation process, generated low boiling point alcohol solvents and carbon dioxide gas can be removed by atmospheric distillation, the reaction can be effectively promoted, the reaction time is shortened, the conversion rate is improved; and finally the final product loxoprofen sodium dehydrate is generated under the condition of aqueous sodium hydroxide solution. The preparation method can greatly reduce the manufacturing cost of loxoprofen sodium, and is more suitable for the industrialized production than the existing technology.
Owner:合肥远志医药科技开发有限公司
Preparation method and application of N-substituted-3,5-dibenzal piperidine-4-one
InactiveCN101973935APracticalStrong inhibitory activityOrganic chemistryAntineoplastic agentsKetoneHydrolysis
The invention belongs to a lead compound of a novel drug for preventing leukaemia K562 cell proliferation and relates to a preparation method and application of N-substituted-3,5-dibenzal piperidine-4-one which can effectively inhibit leukaemia K562 cell proliferation. The preparation method comprises the following steps of: performing Michael addition reaction for substituted amine and methyl acrylate to prepare a yellow oily object N,N-di(beta-methyl propionate) substituted amine; performing Dieckmann condensation under effect of sodium alkoxide and performing hydrolysis and decarboxylationunder effect of acid to obtain a yellow oily object N-substituted piperidine-4-one; and dehydrating the product obtained to obtain the N-substituted-3,5-dibenzal piperidine-4-one with the general formula (I). The product of the invention has higher inhibition activity to eukaemia K562 cell proliferation and the method has the advantages of simple process and easy production.
Owner:SHANGHAI NORMAL UNIVERSITY
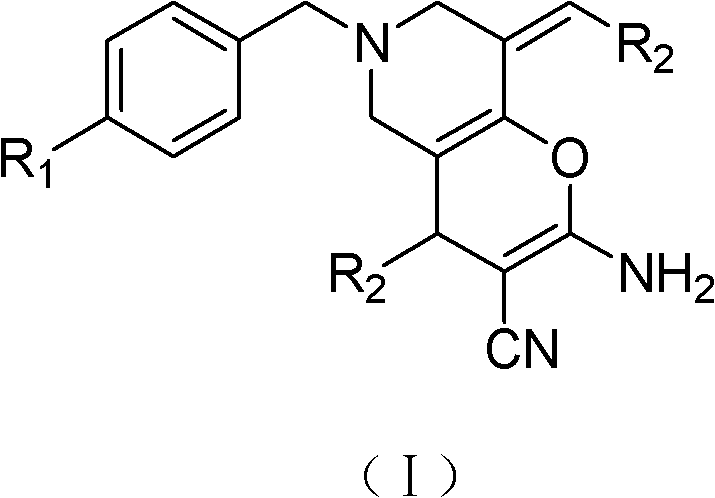
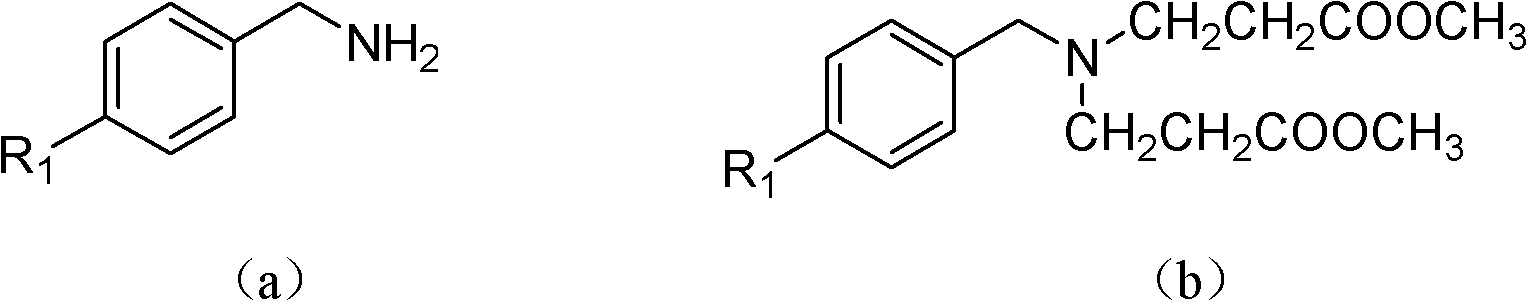
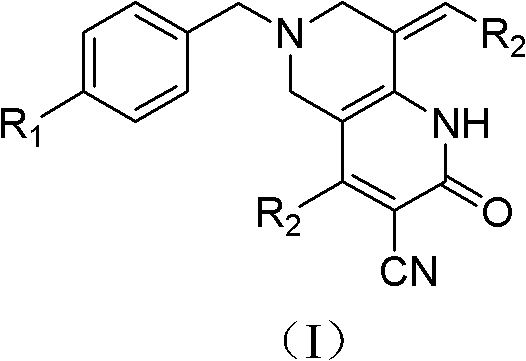
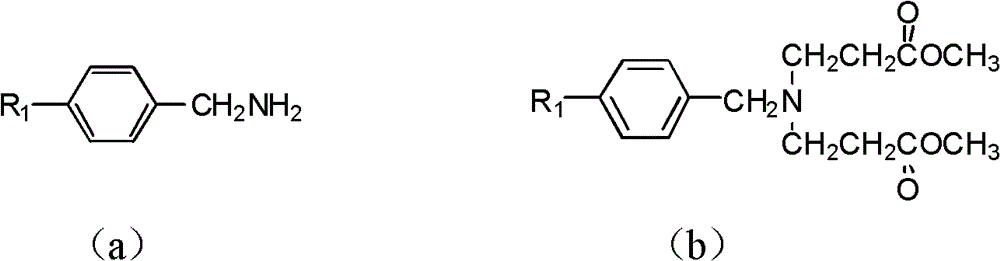
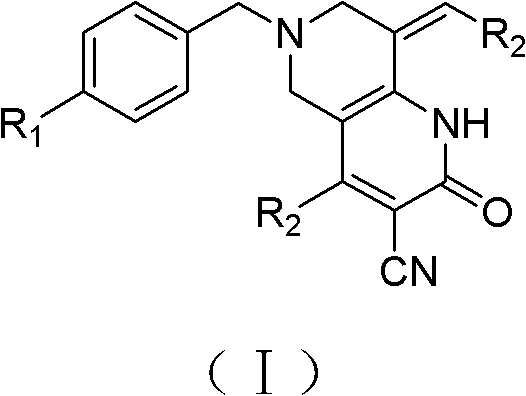
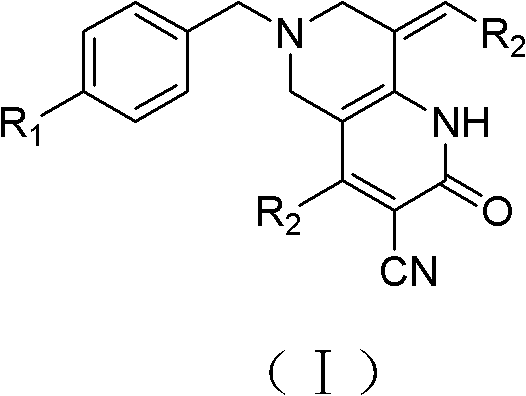
Tetrahydropyridopyridone derivative as well as preparation method and application thereof
ActiveCN103288824APrevent proliferationHigh activityOrganic active ingredientsOrganic chemistryKetoneHydrolysis
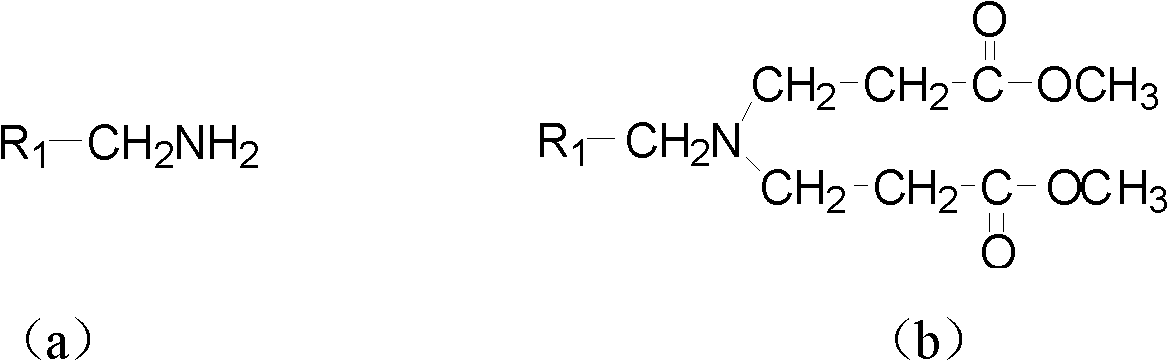
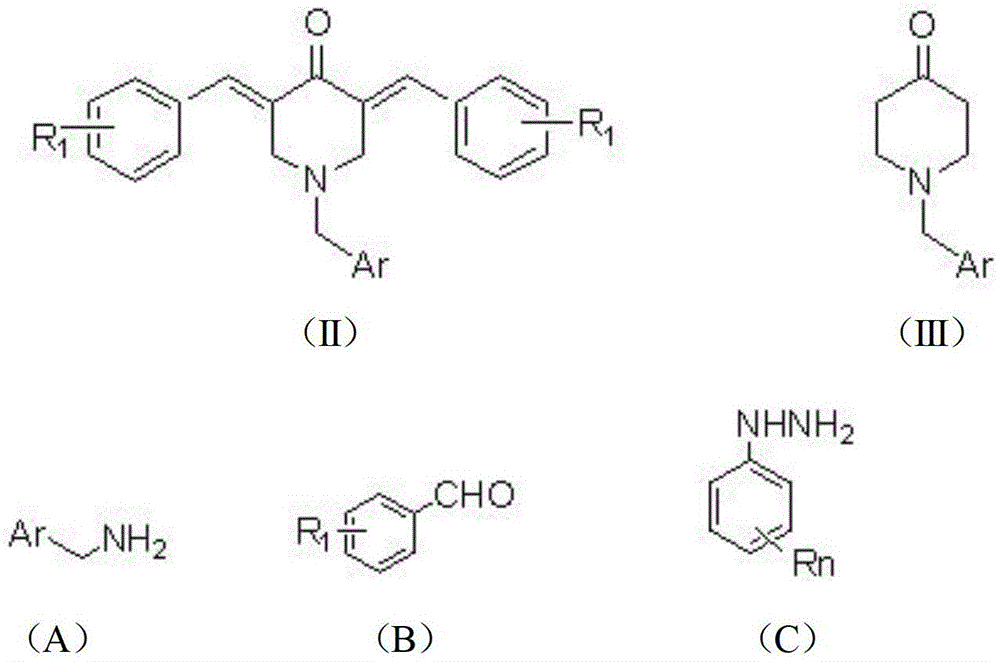
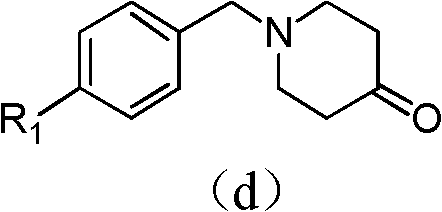
The invention provides a tetrahydropyridopyridone derivative as shown in a general formula (I), and a preparation method thereof. The method comprises the following steps of: preparing N,N-bi(methoxycarbonyl group) substituted benzylamine (b) by carrying out Michael addition on substituted benzylamine (a) and methyl acrylate, carrying out Dieckmann condensation on (b) under the action of sodium alcoholate, subsequently carrying out hydrolysis and decarboxylation under the action of acid so as to obtain N-substituted benzyl-4-piperidone (d), carrying out an aldol condensation reaction on (d) and bimolecular aromatic aldehyde so as to obtain N-substituted-3,5-bi(substituted benzylidene piperidine-4-ketone (e), and refluxing and heating (e) malononitrile and ammonium acetate in ethanol so as to obtain a final product as shown in the general formula (I). The tetrahydropyridopyridone derivative is simple in process and convenient to produce in scale, and the compound (I) has a good inhibition effect on multiplication of leukemia K562 cells, ovarian cancer HO-8910 cells and liver cancer SMMC-7721 cells. Therefore, the invention further provides an application of the compound as shown in the general formula (I) in preparing medicaments for preventing multiplication of the leukemia K562 cells, the ovarian cancer HO-8910 cells and the liver cancer SMMC-7721 cells.
Owner:SHANGHAI NORMAL UNIVERSITY +1
A kind of new synthetic method of entecavir compound
ActiveCN105037363BSolve rare problemsDosage controllableGroup 4/14 element organic compoundsWittig reactionDrugs synthesis
The invention belongs to the field of drug synthesis and relates to an entecavir compound and a synthetic method of an intermediate of the entecavir compound. The novel synthetic method comprises: by taking (S)-3-hydroxyl dimethyl adipate as an initial raw material, preparing an intermediate 9 through hydroxyl TBS protection, Dieckmann condensation reaction, ketone protection to ketal, ester group reduction to hydroxyl, hydroxyl protection, deprotection, ketone to silyl enol ether and Rubottom oxidizing reaction; and preparing entecavir from the intermediate 9 through wittig reaction, Mitsunobu reaction, silicon preventing radical group removal and basic hydrolysis. The novel synthetic method provided by the invention is mild and easily controllable in reaction condition, simple to operate, high in product yield, high in purity and suitable for industrialized mass production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +1
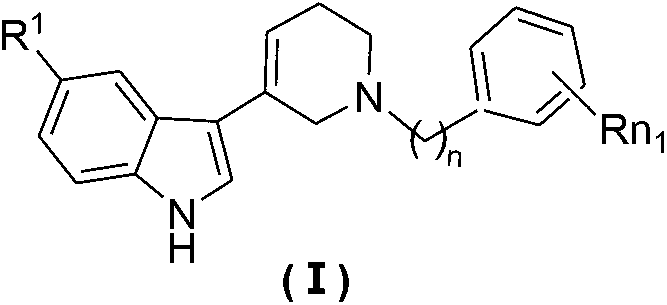
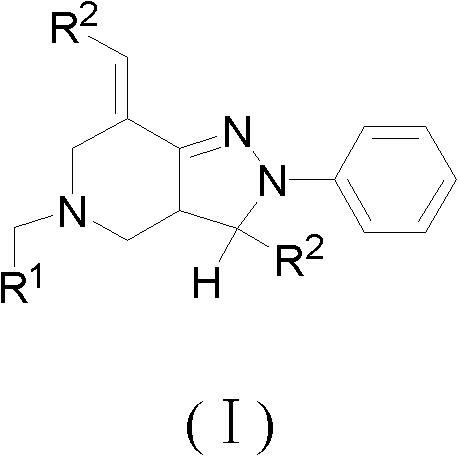
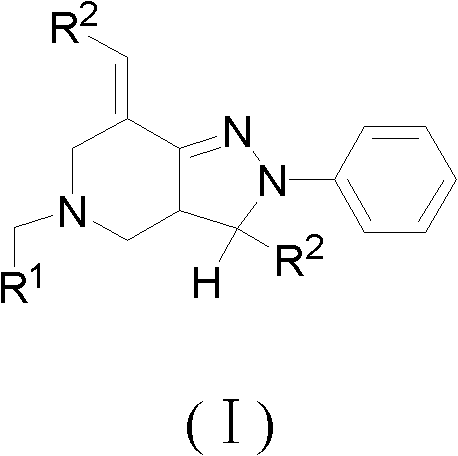
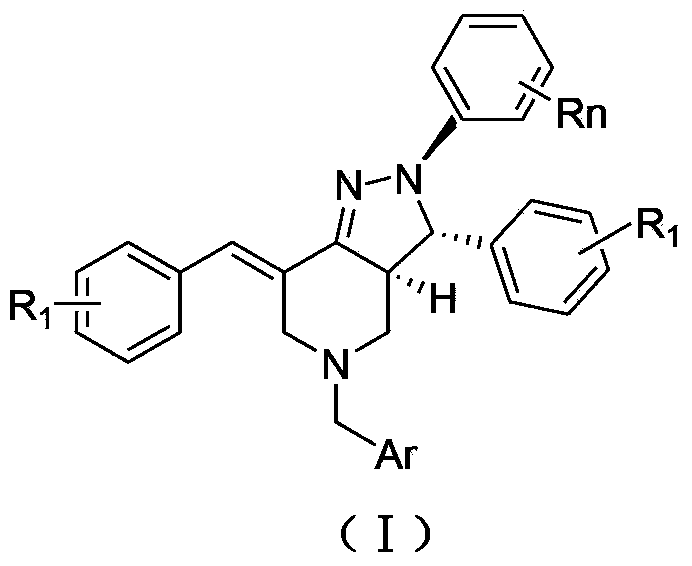
2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and preparation method and application thereof
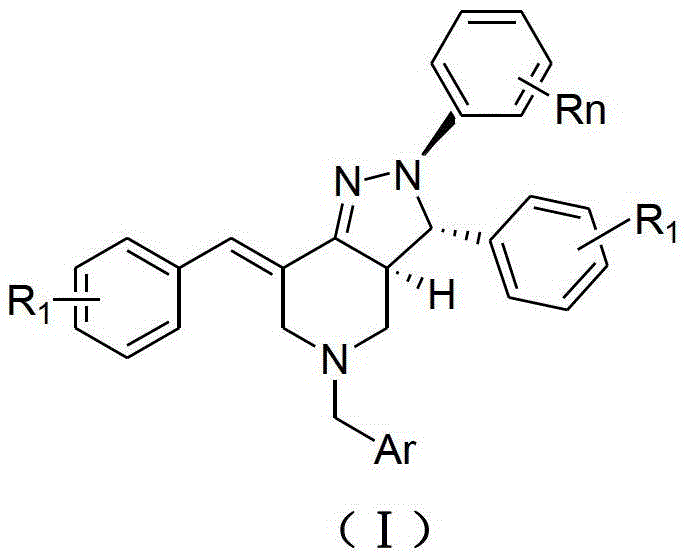
The invention provides 2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and a preparation method and application thereof. The derivative is 2, 3-bis(substituted phenyl)-5-subsituted arylmethyl-7-substituted benzylidene dihydro-pyrazolo piperidine derivative, having the following formula (I). The preparation method includes using substituted arylmethyl amine and methyl acrylate as raw materials; subjecting the materials to Michael addition, Dieckmann condensation and hydrolysis-decarboxylation sequentially; allowing for Aldol reaction with substituted benzaldehyde to obtain intermediate N-substituted arylmethyl-3, 5-bis(substituted benzylidene)-4-piperidone; allowing for condensation with substituted phenylhydrazine to obtain a compound according to the formula (I). The derivative is efficient in inhibiting multiplication of various carcinoma cell lines such as leukemia, esophagus cancer, ovarian cancer and breast cancer in human, is well stably metabolic in liver microsomes of human and rat, is free of direct and competitive inhibition on five enzymes of liver microsomes, such as CYP3A4, CYP2D6, CYP2C9, CYP1A2 and CYP2C19, is highly bioavailable, is low in toxicity to normal cells, and is available for the preparation of drugs for the cancers.
Owner:SHANGHAI NORMAL UNIVERSITY
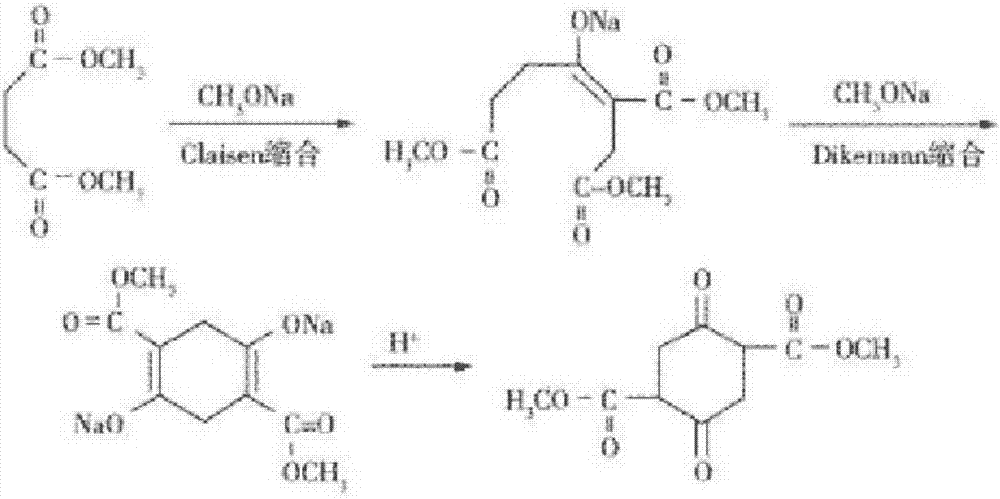
Method for continuous production of dimethyl succinylo succinate
InactiveCN106986771AOvercome the problem of large quality differencesShort reaction timeOrganic compound preparationCarboxylic acid esters preparationSodium methoxideClaisen condensation
The invention provides a method for continuous production of dimethyl succinylo succinate. The technical scheme employs continuous drip adding to carry out Claisen condensation reaction on dimethyl succinate and sodium methoxide and Dieckmann condensation reaction, and can realize continuous discharge, after a certain product is collected, and then dilute sulfuric acid acidification is carried out to obtain dimethyl succinylo succinate. The method provided by the invention is designed based on classical experimental principles, and the operation mode is improved to form a brand-new technological process. The technical scheme shortens the reaction time, also reduces energy consumption, significantly improves the production efficiency, and at the same time overcomes the problem of large quality difference between different batches in batch production. The method provided by the invention takes innovative technological improvement and achieves good technical effect, simultaneously has the characteristics of low cost and easy realization, thus having good popularization prospect.
Owner:淄博鸿润新材料有限公司
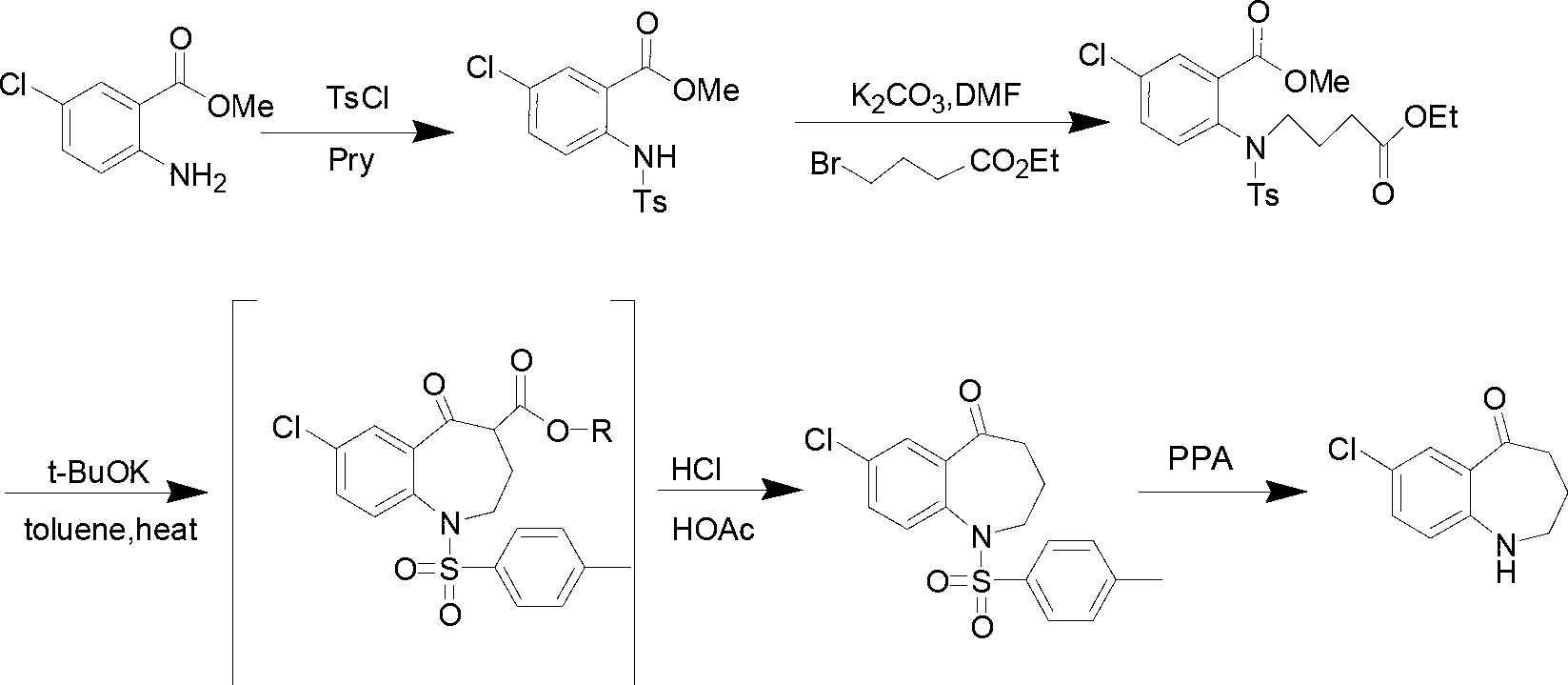
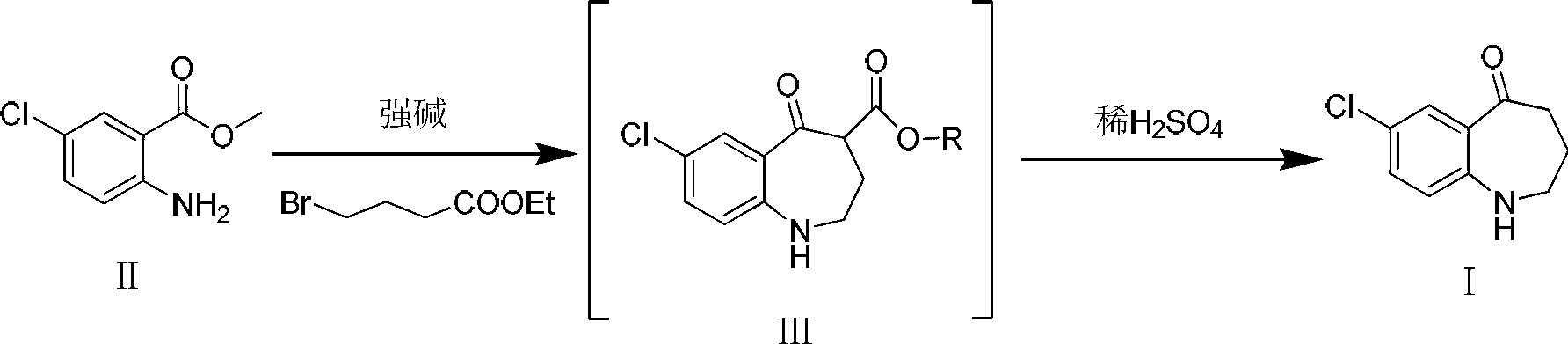
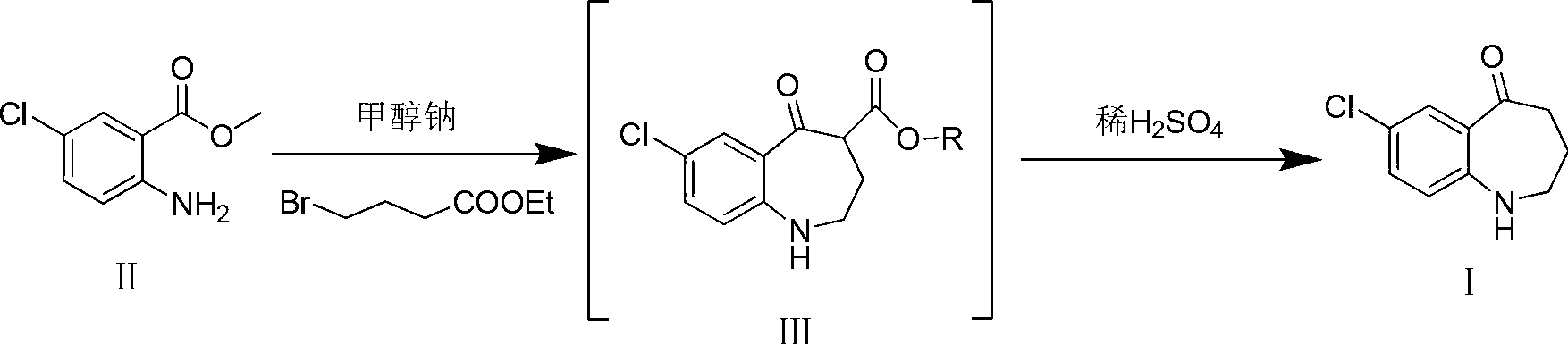
Preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine
The invention relates to a preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine, which is an important intermediate for preparing arginine vasopressin V2 receptor antagonist Tolvaptan. The preparation method comprises the following steps: with methyl 2-amido-5-chlorobenzoate and ethyl 4-bromobutyrate as starting raw materials, reacting at a low temperature under the effect of an acid binding agent to generate secondary amine first, then carrying out Dieckman condensation reaction at a raised temperature to generate an intermediate mixture III, and finally carrying out hydrolysis to obtain the target compound 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine. Compared with the existing method, the method provided by the invention has the advantages that the reaction steps are reduced, the two-step reaction is simplified to one-step reaction through temperature control, so the operation is simple, the processing is convenient, the product purity is high, and the yield is also greatly improved, thus the production cost can be reduced, and the benefits are increased.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
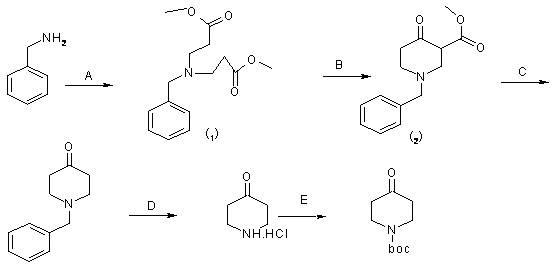
Synthesis method of 1-teriary butoxy carbonyl-4-piperidone
InactiveCN102070513AReduce consumptionReduce manufacturing costOrganic chemistryDicarbonatePtru catalyst
The invention relates to a synthesis method of 1-teriary butoxy carbonyl-4-piperidone. The synthesis method comprises the following steps: dissolving benzylammine and methyl acrylate with methyl alcohol to carry out Michael addition reaction at room temperature, removing the methyl alcohol, and then obtaining piperidine intermediate 1; dissolving the piperidine intermediate1 and sodium in toluene to carry out Diekman condensation reaction at high temperature, and then obtaining piperidone intermediate 2; carrying out decarboxyl reaction on the piperidone intermediate 2 and concentrated hydrochloric acid, and then obtaining crude 1-benzyl-4-piperidone hydrochloride; dissolving with ethanol solution, crystallizing, and then obtaining fine 1-benzyl-4-piperidone hydrochloride; adding a catalyst and the fine 1-benzyl-4-piperidone hydrochloride in the concentrated hydrochloric acid, and then preparing 4-piperidone hydrochloride; and reacting triethylamine, di-tert-butyl dicarbonate with the 4-piperidone hydrochloride at room temperature to obtain a reaction product; and recrystallizing the reaction product and then preparing the 1-teriary butoxy carbonyl-4-piperidone. The synthesis method in the invention is simple and convenient in process, and the prepared product has the advantages of high purity and low energy consumption.
Owner:兰州博实生化科技有限责任公司
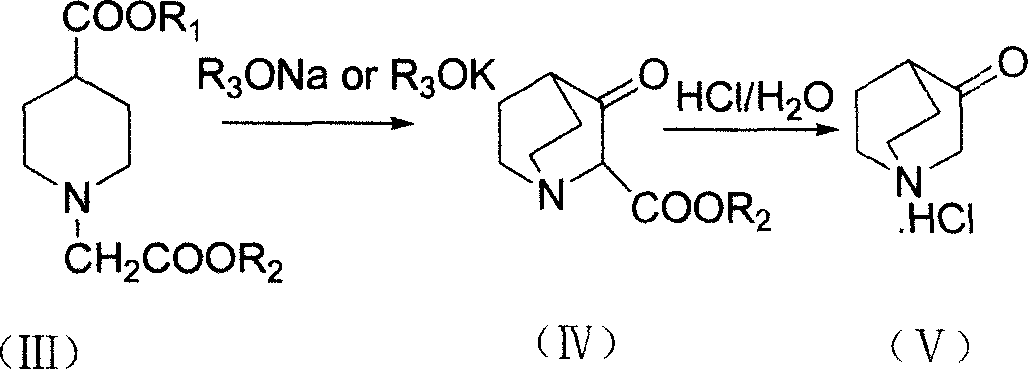
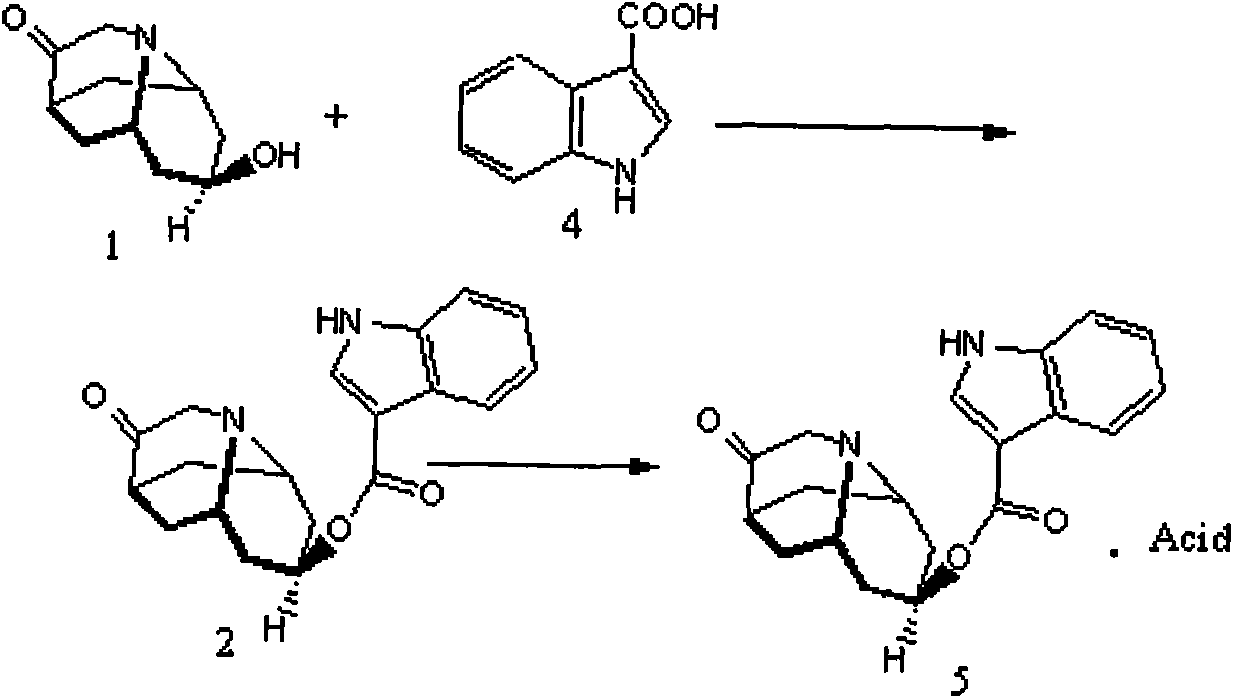
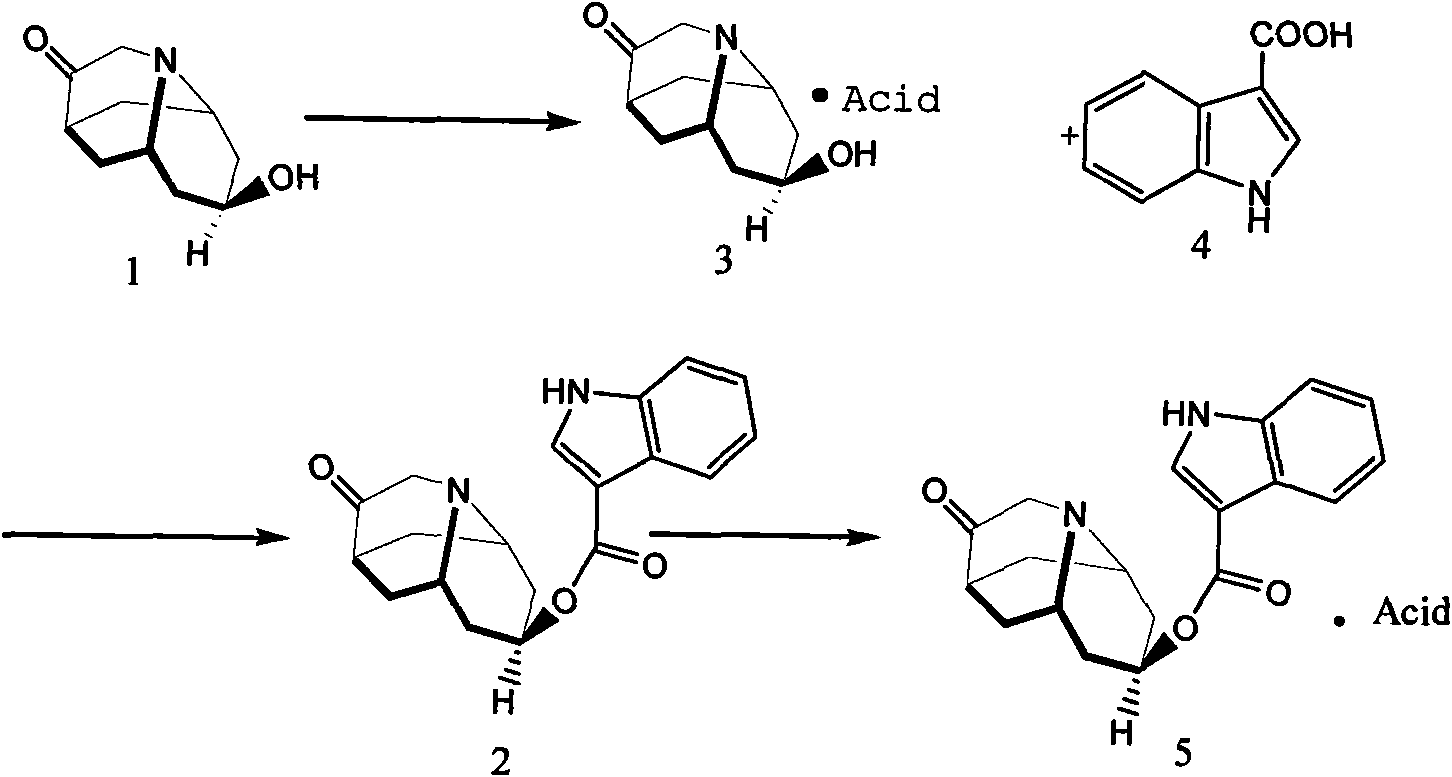
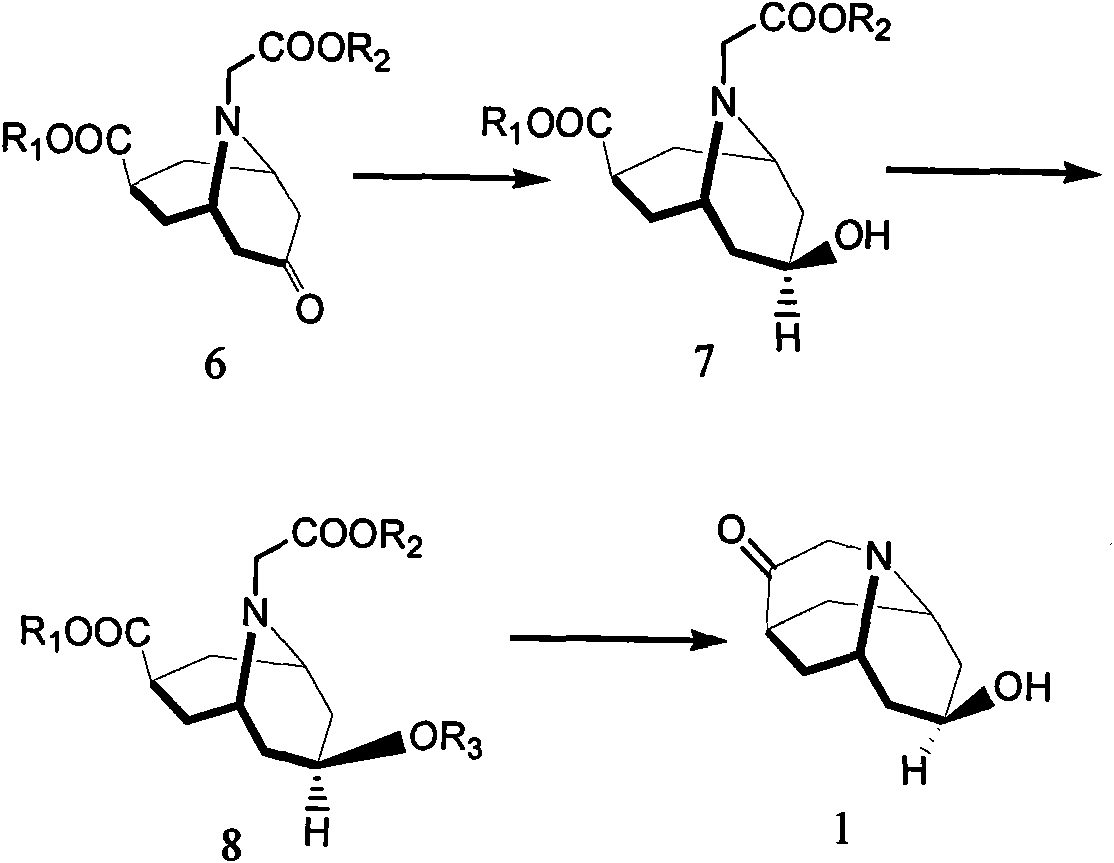
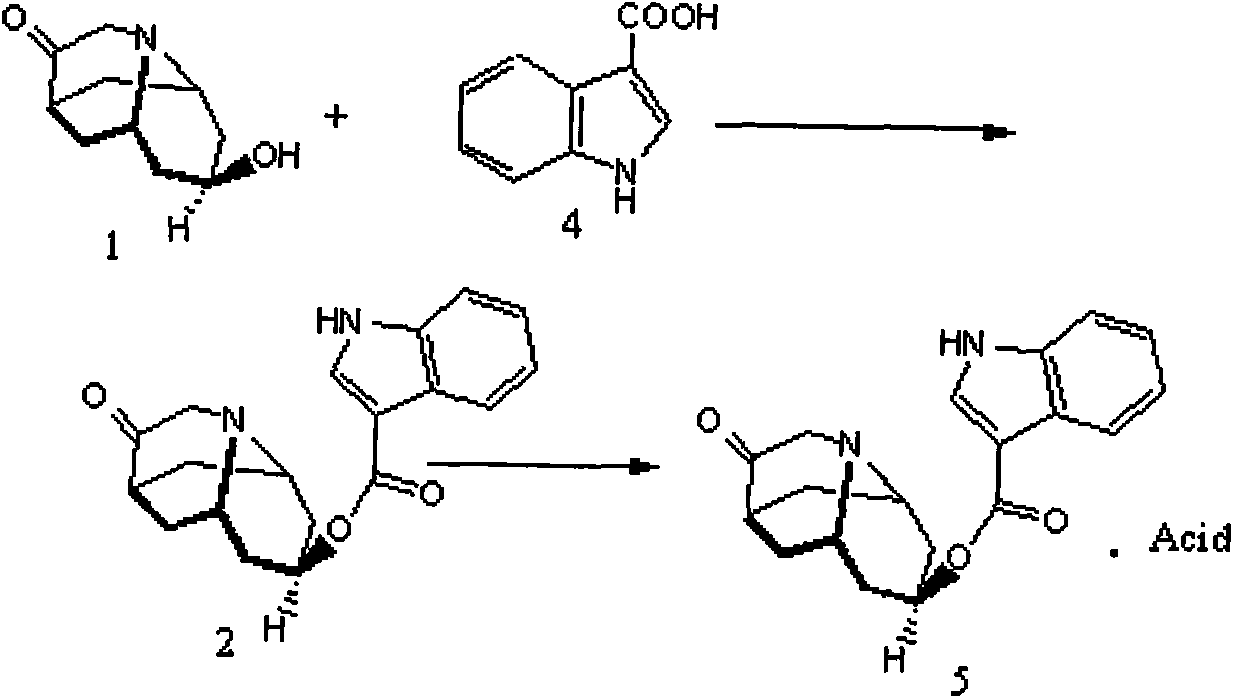
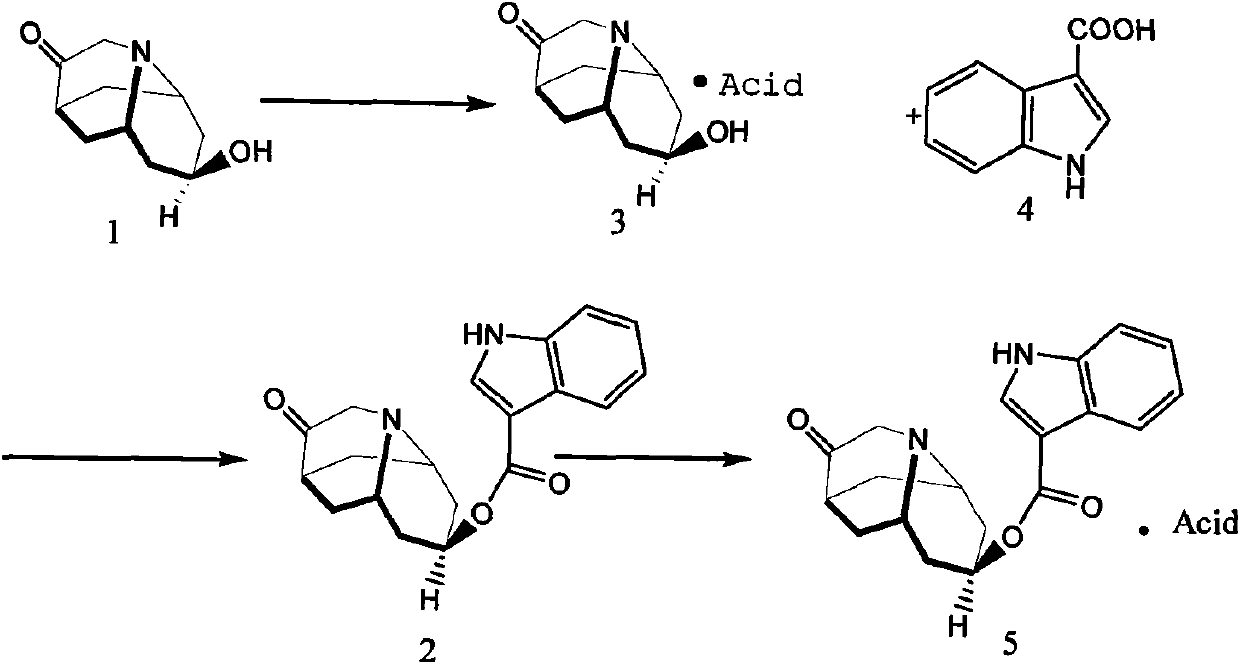
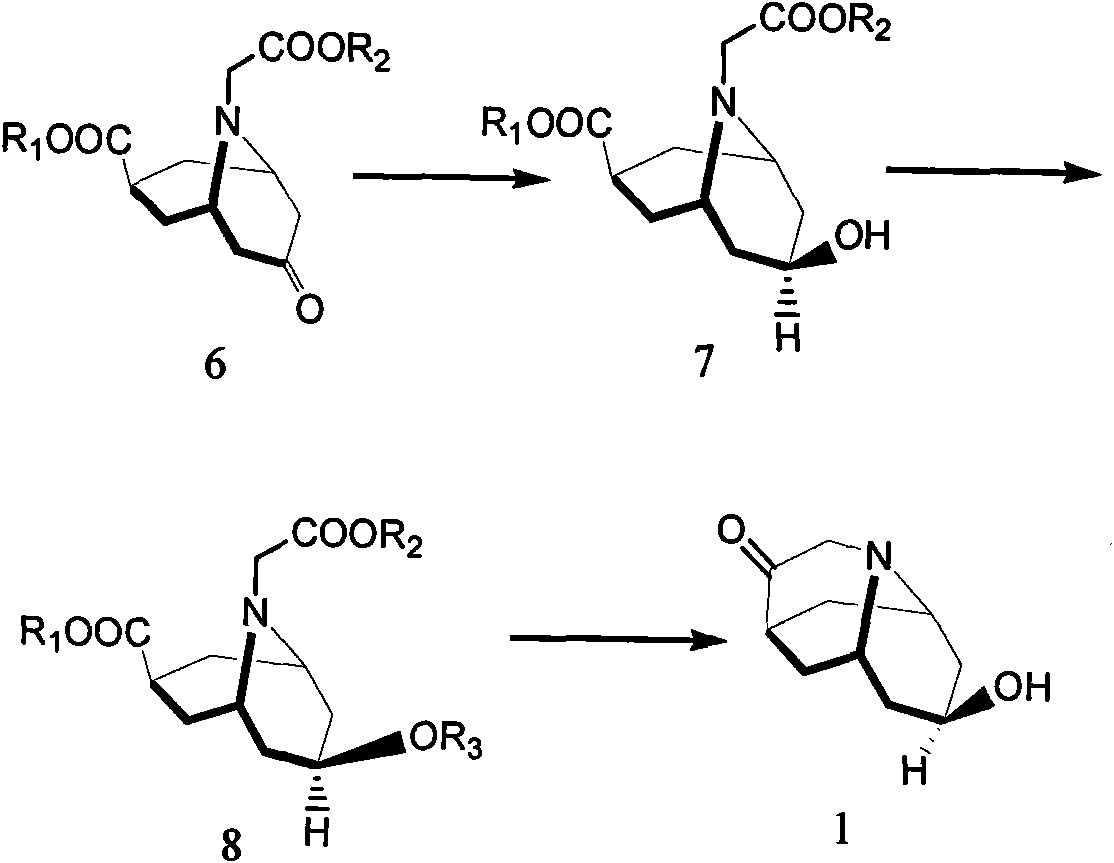
Dolasetron isomer or salt thereof, preparation method for the Dolasetron isomer or salt thereof and application of the Dolasetron isomer or salt thereof
The invention relates to a Dolasetron isomer or salt thereof, a preparation method for the Dolasetron isomer or salt thereof and application of the Dolasetron isomer or salt thereof. In the preparation method, the Dolasetron isomer or salt thereof is prepared from outside-hexahydro-8-hydroxy-2,6-methylene-2H-quinolizidine-3(4H)-ketone or salt thereof serving as a raw material by esterifying and salifying. The invention also provides two methods for synthesizing outside-hexahydro-8-hydroxy-2,6-methylene-2H-quinolizidine-3(4H)-ketone. The outside-hexahydro-8-hydroxy-2,6-methylene-2H-quinolizidine-3(4H)-ketone is prepared from the compound (6) shown in the specification and serving as a raw material by the steps of reducing, protecting hydroxyl, performing Dieckmann condensation and performing hydrolysis and decarboxylation, or by the steps of reducing, performing Mitsunbu esterification, performing hydrolysis, protecting hydroxyl, performing Dieckmann condensation and performing hydrolysis and decarboxylation. The invention also relates to application of the Dolasetron isomer or the salt thereof to preparation of Dolasetron, or application of the salt of the Dolasetron isomer to preparation of control products for detection.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method
InactiveCN110563726AHigh yieldThe reaction is easy to scale upOrganic chemistrySodium methoxideFormate
The invention relates to a tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method. A purpose of the present invention is to mainly solve the technical problem that no method is suitable for industrial synthesis in the prior art. The method comprises four steps, and comprises: dissolving a compound 1 in ethanol to generate a compound 2 under the action of ammonium acetate and monoethyl malonate; in the presence of potassium carbonate, making the compound 2 act with ethyl malonyl chloride in a mixed solution of tetrahydrofuran and water to obtain a compound 3; and carrying out a Dieckmann condensation reaction on the compound 3, toluene and sodium methoxide, treating to obtain a compound 4, and carrying out heating decarboxylating on the compound 4 in water and acetonitrile to obtain a compound 5, wherein the reaction formula is defined in the specification. According to the present invention, the obtained compound can be used as the useful intermediates or productsfor synthesis of a plurality of medicaments.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
2, 4-pyrrolidine-diketone compounds and synthesis method thereof
InactiveCN103483341AMild reaction conditionsShort routeOrganic chemistrySynthesis methodsDrug biological activity
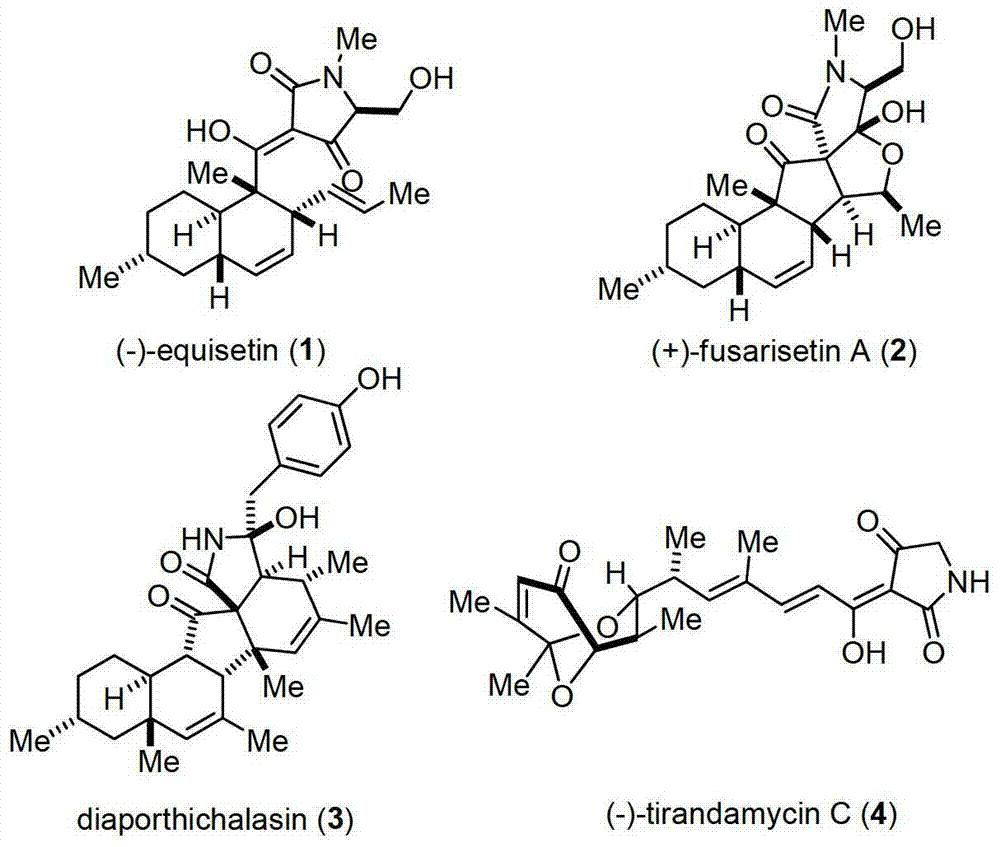
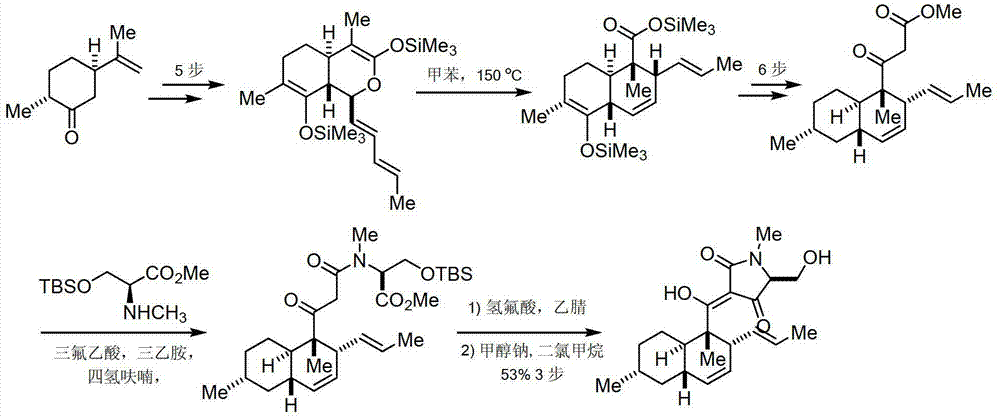
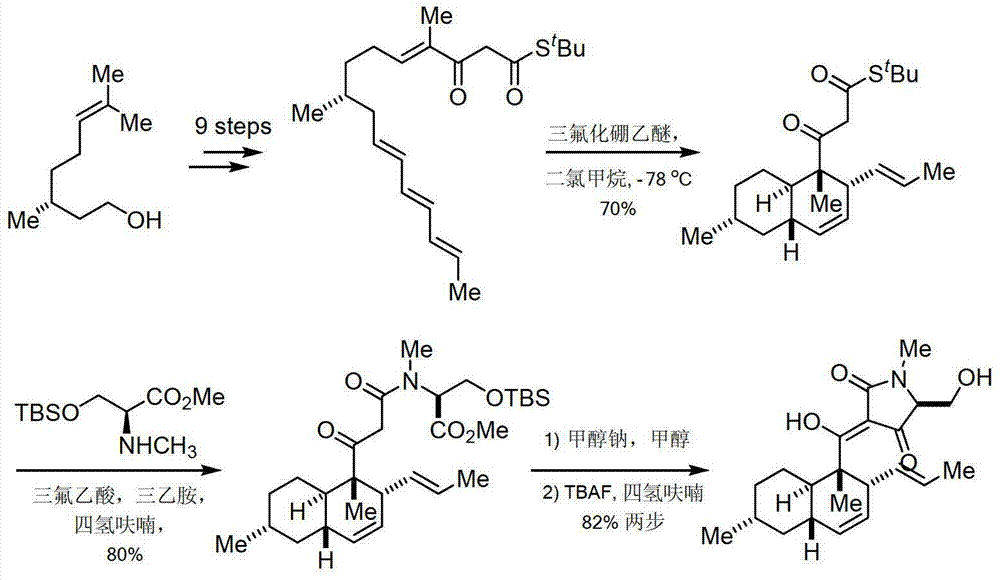
The invention discloses equisetin-derived 2, 4-pyrrolidine-diketone compounds. The diketone compounds have the structure shown in the specification, wherein R1 is hydrogen, alkyl, aryl or a heteroatom group containing O and N; R2 is hydrogen or alkyl; R3 is hydrogen or alkyl. A synthesis method of the diketone compounds comprises eight steps, wherein aldehyde II is prepared in the four steps; a polyene compound III is prepared through a Wittig reaction or Horner-Wadswort-Emmons reaction; a compound IV' is prepared through a Diels-Alder reaction; finally a compound V is prepared by ammonolysis and Dieckmann condensation. The compounds have relatively high biological activity and bring the foundation to the research and development of anti-cancer medicaments; the raw materials used in synthesis are simple and easy to obtain; the synthesis process is simple to carry out; the yield is relatively high.
Owner:EAST CHINA NORMAL UNIV
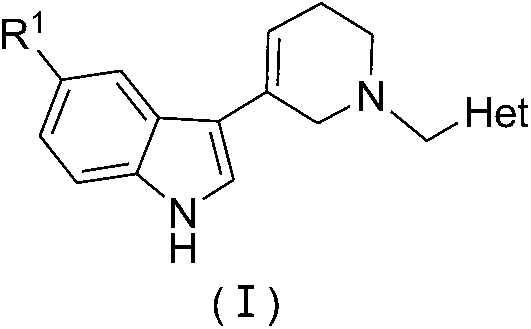
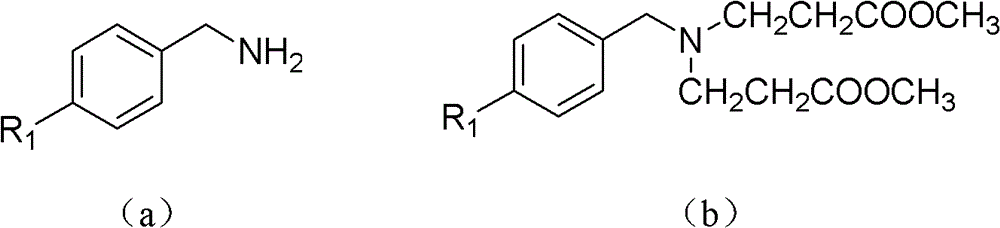
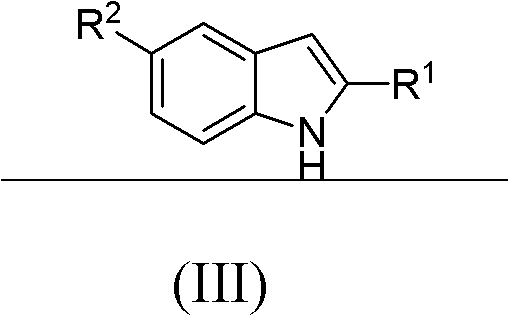
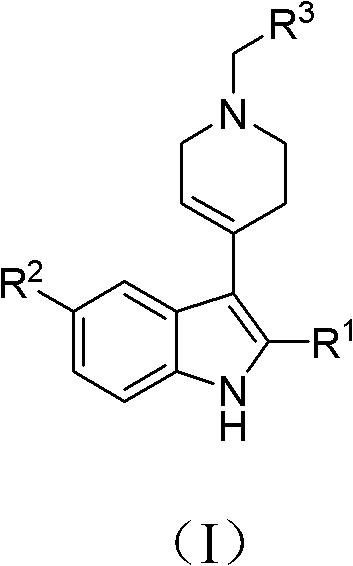
N-substituted benzyl tetrahydropyridine with indole and preparation method and application thereof
InactiveCN103232433APrevent proliferationPromote apoptosisOrganic chemistryAntineoplastic agentsHuman leukemiaEsophagus Cancers
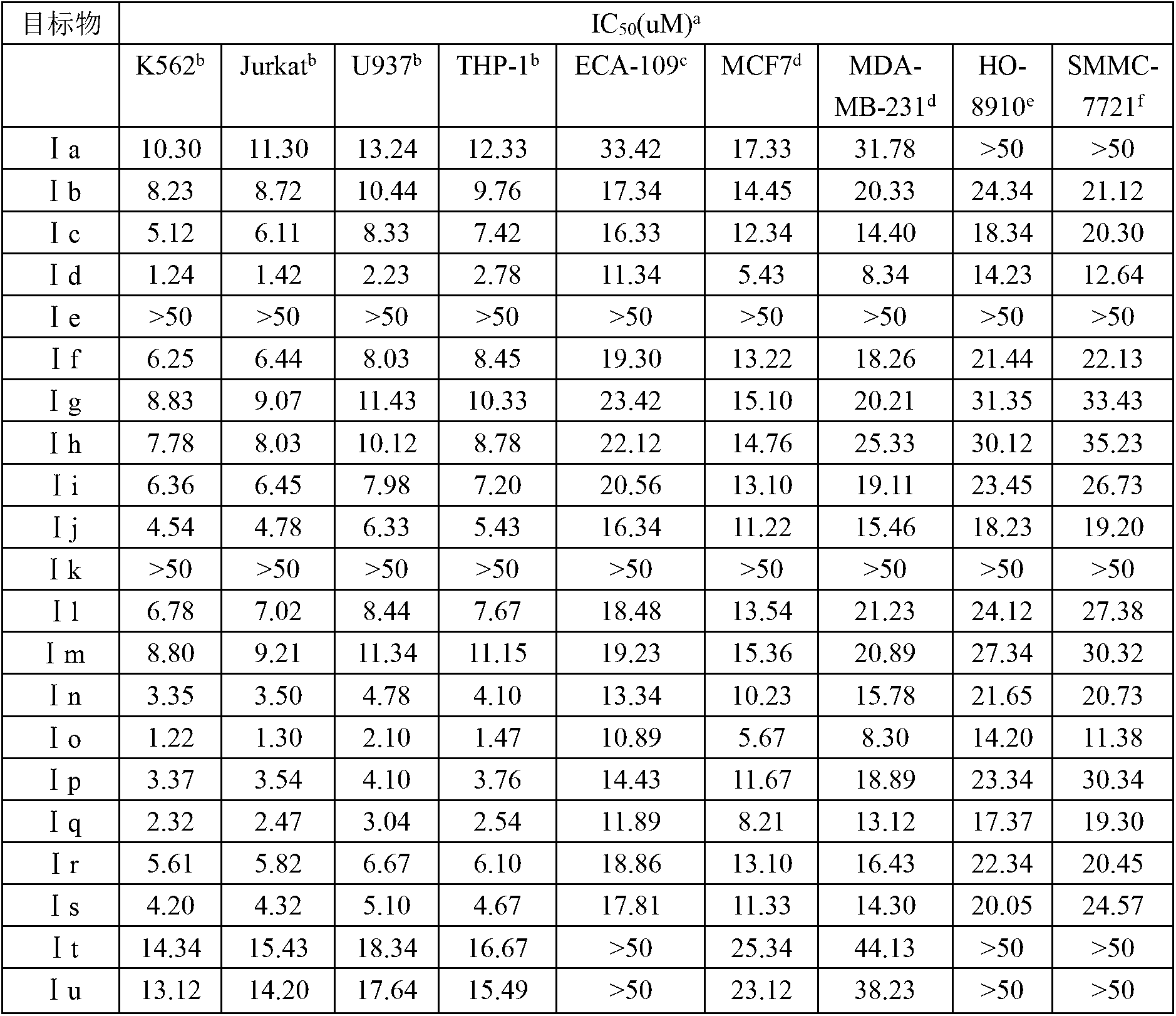
The invention discloses a N-substituted benzyl tetrahydropyridine with -5-substituted indole and preparation method and application thereof, and the structure is shown in the general formula (I): substituted benzene methylamine (ethylamine) and methyl acrylate are raw materials, and an intermediate N-substituted benzylpiperidine (phenylethylpiperidine)-4-ketone is obtained by three steps of reaction such as Michael addition, Dieckmann condensation and hydrolysis decarboxylation or the like in sequence, and the object (I) is obtained by condensation reaction of the intermediate and 5-substituted indole. The compound (I) can effectively inhibit proliferation of human leukemia K562, Jurkat, U937, THP-1 cell line, the human esophagus cancer ECA-109 cell line, human liver cancer SMMC-7721 cell line, human ovary cancer HO-8910 cell line, human breast cancer MCF-7 cell line, breast cancer MDA-MB-231 cell line; the compound has a good metabolism stability in the human and rat liver microsomes; The compound does not have mechanical inhibition effects for five enzymes of human liver microsomes such as CYP3A4, CYP 2D6, CYP2C9, CYP1A2 and CYP2C19 or the like; the compound can induce the cell cycle G2 / M retardance and promote cancer cell apoptosis and inhibit cancer cell propagation.
Owner:SHANGHAI NORMAL UNIVERSITY
Method of synthesizing 3-quininone hydrochlorate
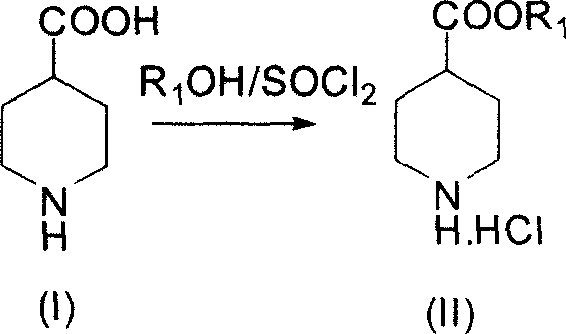
The invention discloses a synthesizing method of 3-quininone hydrochlorate, which comprises the following steps: adopting 4-piperidine aminic acid as raw material; esterifying; proceeding N-hydrocarbonization through halogenated acetic ester; obtaining diester catalyzed by alkaline to do Dieckmann condensation; stripping ester in the condensed acid condition to obtain the product.
Owner:WUHAN UNIV OF TECH
Tetrahydropyridopyran derivatives, preparation methods and applications thereof
InactiveCN102285993APrevent proliferationPracticalOrganic active ingredientsOrganic chemistryKetoneHydrolysis
The invention provides a tetrahydropyridine oxapicene derivative and a preparation method thereof. The method comprises the following steps: subjecting substituted amine (a) and methyl acrylate to Michael additive reaction to prepare N,N-bis(beta-methyl propionate) substituted amine (b), subjecting the N,N-bis(beta-methyl propionate) substituted amine (b) to Dieckmann condensation under the action of sodium alkoxide and hydrolysis and decarboxylation under the action of acid to obtain N-substituted piperidine-4-ketone (d), and subjecting two active methylenesand and bimolecular aromatic aldehydes of the N-substituted piperidine-4-ketone (d) to reaction to remove bimolecular water to obtain N-substituted-3,5-2-benzylpiperidine-4-ketone (e); and carrying out reflowing and heating on the N-substituted-3,5-2-benzylpiperidine-4-ketone (e) and malononitrile in normal butanol to obtain a final product shown as a general formula (I). The preparation method is simple in process and convenient for mass production; and the obtained product has a favorable inhibiting effect for the cell proliferation of leukemia K562, oophoroma HO-8910 and liver cancer SMMC-7721.
Owner:SHANGHAI NORMAL UNIVERSITY
Dihydropyrazolohexahydropyridine derivatives, preparation method and application thereof
InactiveCN102276605AStrong inhibitory activitySimple processOrganic active ingredientsOrganic chemistryModern medicineHexahydropyridine
The invention provides a dihydro-pyrazolo hexahydropyridine derivative and a preparation method thereof. The method comprises the following steps of: performing Michael addition reaction of substituted amine (a) and methyl acrylate to prepare N,N-bis(beta-methyl propionate) substituted amine (b); performing Dieckmann condensation of (b) under the action of sodium alcoholate and hydrolyzing and decarboxylating under the action of acid to obtain yellow oily matter N-substituted piperidine-4-ketone (d); reacting (d) and aromatic aldehyde to remove bimolecular water to obtain N-substitution-3,5-dibenzal piperidine-4-ketone (e); and performing reaction of (e) to form the dihydro-pyrazolo hexahydropyridine derivative. The preparation method is simple, and the dihydro-pyrazolo hexahydropyridine derivative is convenient to produce in large scale; and a lead compound of the prepared dihydro-pyrazolo hexahydropyridine new medicine has the obvious inhibitory activity on leukemia K562 cancer cellproliferation, and has the obvious practicality in the production of modern medicines.
Owner:SHANGHAI NORMAL UNIVERSITY
Synthesis method of 1-teriary butoxy carbonyl-4-piperidone
InactiveCN102070513BReduce consumptionReduce manufacturing costOrganic chemistryDicarbonatePtru catalyst
The invention relates to a synthesis method of 1-teriary butoxy carbonyl-4-piperidone. The synthesis method comprises the following steps: dissolving benzylammine and methyl acrylate with methyl alcohol to carry out Michael addition reaction at room temperature, removing the methyl alcohol, and then obtaining piperidine intermediate 1; dissolving the piperidine intermediate1 and sodium in tolueneto carry out Diekman condensation reaction at high temperature, and then obtaining piperidone intermediate 2; carrying out decarboxyl reaction on the piperidone intermediate 2 and concentrated hydrochloric acid, and then obtaining crude 1-benzyl-4-piperidone hydrochloride; dissolving with ethanol solution, crystallizing, and then obtaining fine 1-benzyl-4-piperidone hydrochloride; adding a catalyst and the fine 1-benzyl-4-piperidone hydrochloride in the concentrated hydrochloric acid, and then preparing 4-piperidone hydrochloride; and reacting triethylamine, di-tert-butyl dicarbonate with the4-piperidone hydrochloride at room temperature to obtain a reaction product; and recrystallizing the reaction product and then preparing the 1-teriary butoxy carbonyl-4-piperidone. The synthesis method in the invention is simple and convenient in process, and the prepared product has the advantages of high purity and low energy consumption.
Owner:兰州博实生化科技有限责任公司
2,3,5,7-Tetrasubstituted dihydropyrazolohexahydropyridine derivatives, preparation method and application thereof
Owner:SHANGHAI NORMAL UNIVERSITY
N-heterocyclic methyl tetrahydropyridine-5-substituted indole and preparation method and application thereof
InactiveCN103232439APrevent proliferationPromote apoptosisOrganic active ingredientsOrganic chemistryHuman leukemiaMda mb 231
The invention discloses a N-heterocyclic methyl tetrahydropyridine-5-substituted indole and preparation method and application thereof, and the structure is shown in the general formula (I): heterocyclic methylamine and methyl acrylate are raw materials, and an intermediate N-heterocyclic methyl-4-piperidone is obtained by three steps of reaction such as Michael addition, Dieckmann condensation and hydrolysis decarboxylation or the like in sequence, and the object (I) is obtained by condensation reaction of the intermediate and 5-substituted indole. The compound (I) can effectively inhibit propagation of cell lines such as human leukemia K562, Jurkat, U937, THP-1; human esophagus cancer ECA-109; human liver cancer SMMC-7721; human ovary cancer HO-8910; human breast cancer MCF-7 and breast cancer MDA-MB-231; the compound inhibits the propagation of cancer cells by promoting cancer cell apoptosis. The compound can be applied to preparation of anticancer medicament.
Owner:SHANGHAI NORMAL UNIVERSITY
Dihydro-pyrazolo hexahydropyridine derivative, preparation method and application thereof
InactiveCN102276605BStrong inhibitory activitySimple processOrganic active ingredientsOrganic chemistryModern medicineHexahydropyridine
The invention provides a dihydro-pyrazolo hexahydropyridine derivative and a preparation method thereof. The method comprises the following steps of: performing Michael addition reaction of substituted amine (a) and methyl acrylate to prepare N,N-bis(beta-methyl propionate) substituted amine (b); performing Dieckmann condensation of (b) under the action of sodium alcoholate and hydrolyzing and decarboxylating under the action of acid to obtain yellow oily matter N-substituted piperidine-4-ketone (d); reacting (d) and aromatic aldehyde to remove bimolecular water to obtain N-substitution-3,5-dibenzal piperidine-4-ketone (e); and performing reaction of (e) to form the dihydro-pyrazolo hexahydropyridine derivative. The preparation method is simple, and the dihydro-pyrazolo hexahydropyridine derivative is convenient to produce in large scale; and a lead compound of the prepared dihydro-pyrazolo hexahydropyridine new medicine has the obvious inhibitory activity on leukemia K562 cancer cellproliferation, and has the obvious practicality in the production of modern medicines.
Owner:SHANGHAI NORMAL UNIVERSITY
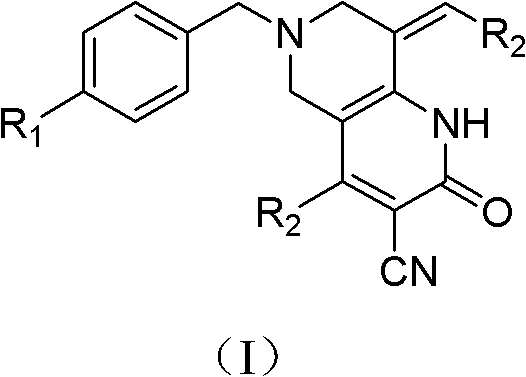
Tetrahydropyridopyridone derivatives, preparation methods and applications thereof
ActiveCN103288824BPrevent proliferationHigh activityOrganic active ingredientsOrganic chemistryKetoneHydrolysis
Owner:SHANGHAI NORMAL UNIVERSITY +1
Preparation method of substituted thiophene-3-one compound
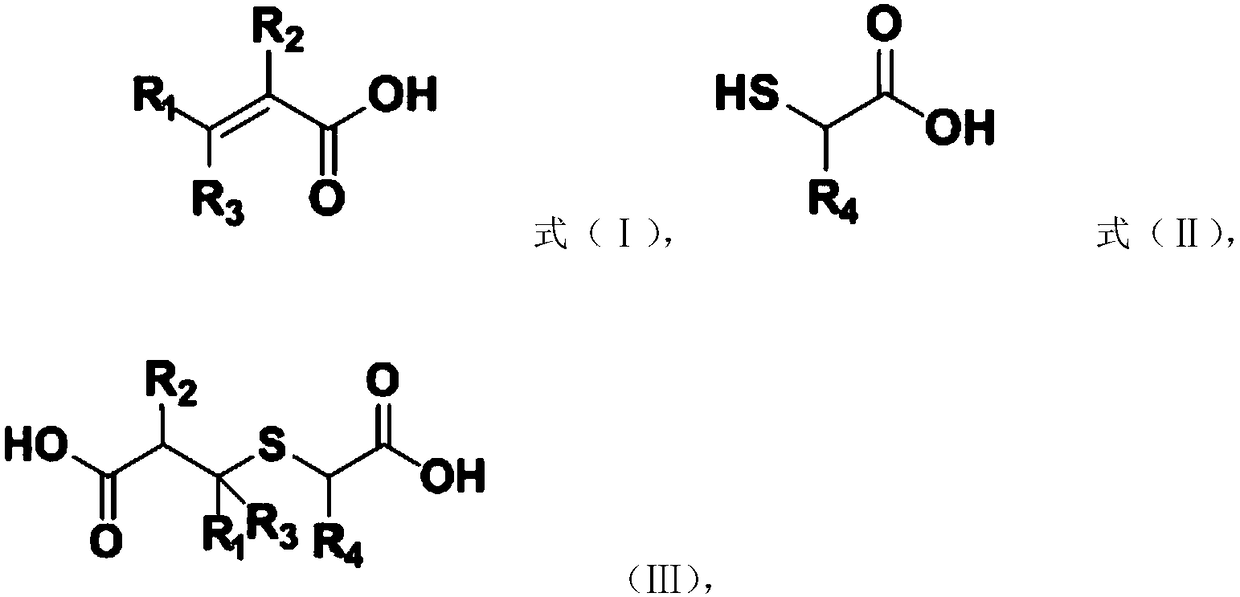
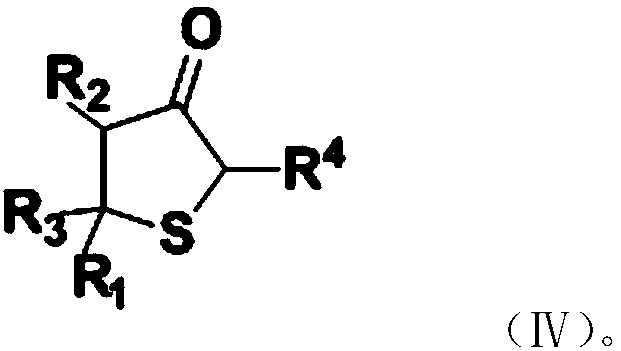
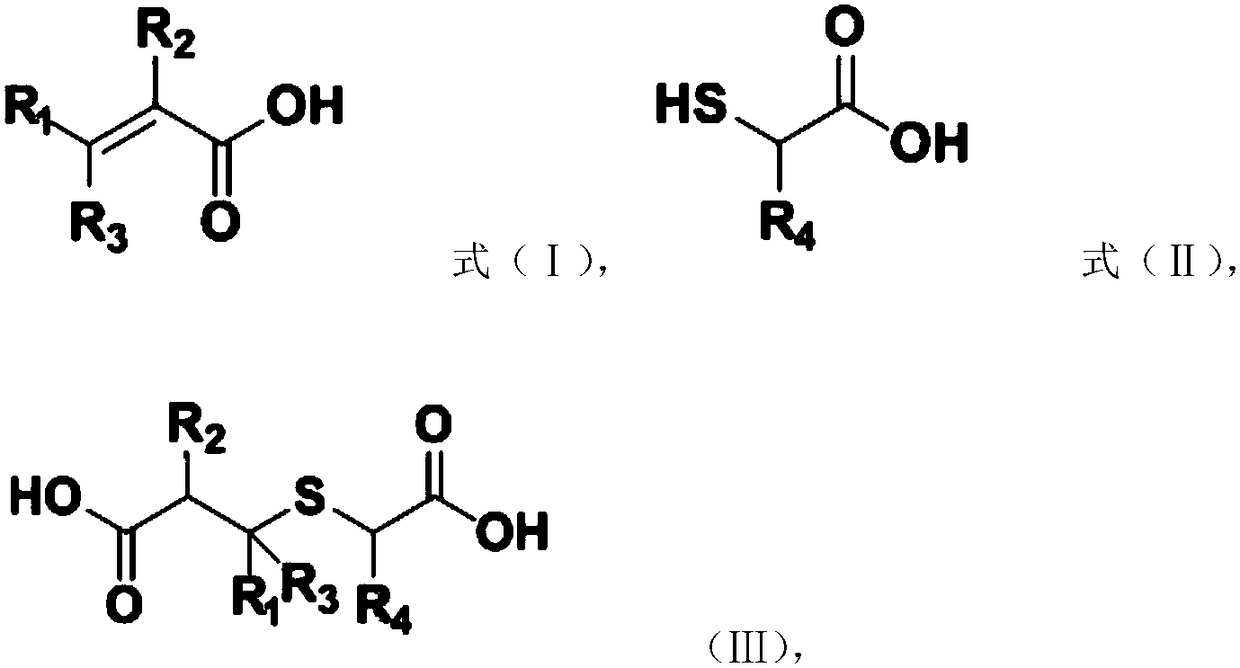
The invention provides a preparation method of a substituted thiophene-3-one compound, comprising: subjecting material A and material B to Michael addition reaction to obtain an addition product; subjecting the addition product to Dieckmann condensation reaction to obtain a substituted thiophene-3-one compound having a structure shown as formula (IV) that is shown in the description. The preparation method provided by the application has the advantages that using solvents and strong bases is not required, reaction materials are low in price and easy to obtain, no wastewater is produce, and therefore, the preparation method has good environmental friendliness; compared with existing preparation methods, the preparation method has a short synthetic route, can be performed under mild conditions and features good yield stability and good repeatability, which is helpful for greatly increasing total yield of the substituted thiophene-3-one compound and helpful for industrial popularization.
Owner:ASYMCHEM LAB TIANJIN
N-substituted tetrahydropyridine bound indole compound as well as preparation method and application thereof
InactiveCN102276581BSimple processEasy to produceOrganic active ingredientsOrganic chemistryKetoneHydrolysis
The invention relates to the field of chemical and medicinal chemistry, and discloses a N-substituted tetrahydropyridine bound indole compound with a structure shown as a formula (I), and the compound can be used for effectively inhibiting cell proliferation of leukemia K562, ovarian cancer HO-8910 and liver cancer SMMC-7721. The preparation method comprises the following steps: preparing N,N-bis(beta-methyl propionate) substituted amine from substituted amine and methyl acrylate by means of a Michael addition reaction; performing Dieckmann condensation in the action of sodium alcoholate, andperforming hydrolysis and decarboxylation in the action of acid to prepare N-substituted piperidine-4-ketone; and performing a condensation reaction to the product and indole or substituted indole. The preparation method has the advantages of simple process and easiness for production; and the compound can be used for preparing medicaments for treating leukemia, ovarian cancer or liver cancer.
Owner:SHANGHAI NORMAL UNIVERSITY
Sulfo-tetrahydro-pyridino-dihydro-pyrimidone derivative, preparation method for same and application thereof
InactiveCN102382111BStrong inhibitory activitySimple processOrganic active ingredientsOrganic chemistryKetoneHydrolysis
The invention provides a sulfo-tetrahydro-pyridino-dihydro-pyrimidone derivative, a preparation method for the same and application thereof. The sulfo-tetrahydro-pyridino-dihydro-pyrimidone derivative is capable of effectively inhibiting cell proliferation of leukaemia K562, ovarian cancer HO-8910 and liver cancer SMMC-7721. The method includes the steps that firstly, substituted amine (a) and acrylic acid methyl ester are manufactured into N by means of Michael addition reaction, secondly, N-bis (beta-acrylic acid methyl ester) substituted amine (b) further gives Dieckmann condensation under the action of sodium alcoholate and hydrolysis decarboxylation reaction under the action of acid to obtain N-substituted piperidine-4-ketone(d), thirdly, the N-substituted piperidine-4-ketone(d) and aromatic aldehyde are reacted by means of bimolecular dehydration so that N-substituted benzyl-3,5-bis benzylidene-piperidine-4-ketone(e) is obtained, and finally, the N-substituted benzyl-3,5-bis benzylidene-piperidine-4-ketone(e) is further reacted so as to obtain the sulfo-tetrahydro-pyridino-dihydro-pyrimidone derivative. The method is simple and high in efficiency, and the derivative prepared by the method can have remarkable inhibitory action on above disease cells.
Owner:SHANGHAI NORMAL UNIVERSITY
Dolasetron isomer or salt thereof, preparation method for the Dolasetron isomer or salt thereof and application of the Dolasetron isomer or salt thereof
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Processes for preparing 5,5-dimethyl-2-oxo-3-cyclopentene-1-carboxylate compounds and 3,5,5-trimethyl-2-oxo-3-cyclopentene-1-carboxylate compounds from 3,3-dimethyl-1-butene-1,4-dicarboxylate compounds and 1,3,3-trimethyl-1-butene-1,4-dicarboxylate compounds, and 1,3,3-trimethyl-1-butene-1,4-dicarboxylate compounds
ActiveUS20220024846A1High yieldOrganic compound preparationOrganic chemistry methodsCyclopenteneButene
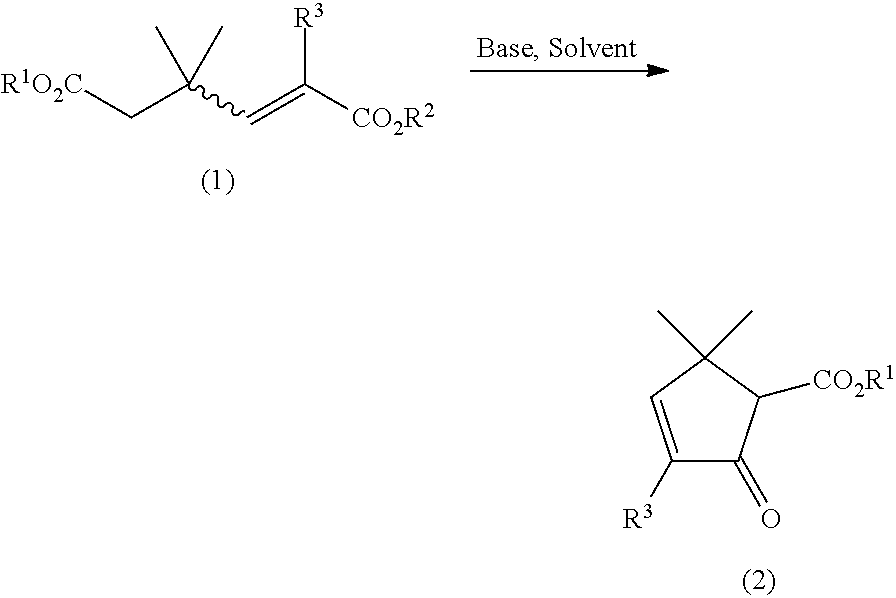
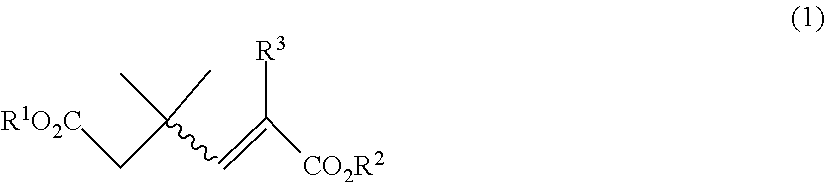
The present invention provides a process for preparing a compound of the following general formula (2): wherein R1 represents a monovalent hydrocarbon group having 1 to 10 carbon atoms, and R3 represents a hydrogen atom or a methyl group, the process comprising: subjecting a compound of the following general formula (1): wherein R1 and R2 represent, independently of each other, a monovalent hydrocarbon group having 1 to 10 carbon atoms, R3 represents a hydrogen atom or a methyl group, and the wavy bond represents an E-configuration, a Z-configuration, or a mixture thereof, to a Dieckmann condensation in the presence of base to form the compound (2).
Owner:SHIN ETSU CHEM IND CO LTD
N-substituted benzyl tetrahydropyridine with indole and preparation method and application thereof
InactiveCN103232433BPrevent proliferationPromote apoptosisOrganic chemistryAntineoplastic agentsHuman leukemiaEsophagus Cancers
The invention discloses a N-substituted benzyl tetrahydropyridine with -5-substituted indole and preparation method and application thereof, and the structure is shown in the general formula (I): substituted benzene methylamine (ethylamine) and methyl acrylate are raw materials, and an intermediate N-substituted benzylpiperidine (phenylethylpiperidine)-4-ketone is obtained by three steps of reaction such as Michael addition, Dieckmann condensation and hydrolysis decarboxylation or the like in sequence, and the object (I) is obtained by condensation reaction of the intermediate and 5-substituted indole. The compound (I) can effectively inhibit proliferation of human leukemia K562, Jurkat, U937, THP-1 cell line, the human esophagus cancer ECA-109 cell line, human liver cancer SMMC-7721 cell line, human ovary cancer HO-8910 cell line, human breast cancer MCF-7 cell line, breast cancer MDA-MB-231 cell line; the compound has a good metabolism stability in the human and rat liver microsomes; The compound does not have mechanical inhibition effects for five enzymes of human liver microsomes such as CYP3A4, CYP 2D6, CYP2C9, CYP1A2 and CYP2C19 or the like; the compound can induce the cell cycle G2 / M retardance and promote cancer cell apoptosis and inhibit cancer cell propagation.
Owner:SHANGHAI NORMAL UNIVERSITY
Preparation method of quininone derivative
The invention provides a preparation method of a quininone derivative, and belongs to the field of pharmaceutical chemical industry. According to the method disclosed by the invention, the 3-quininonederivative is obtained by taking a diethanolamine derivative as a starting material through chlorination or methanesulfonic acid and diethyl malonate cyclization and Dieckmann Condensation. The product produced by the method has the characteristics of high purity, high yield, low cost, simple operation and stable process.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for preparing 2,4-dyhydroxy-6-methylnicotinate
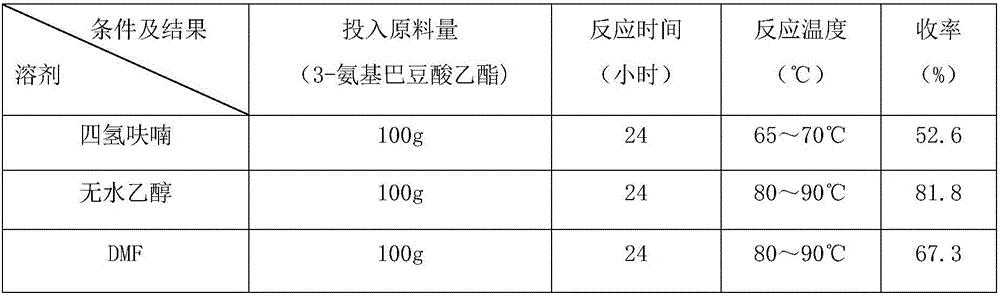
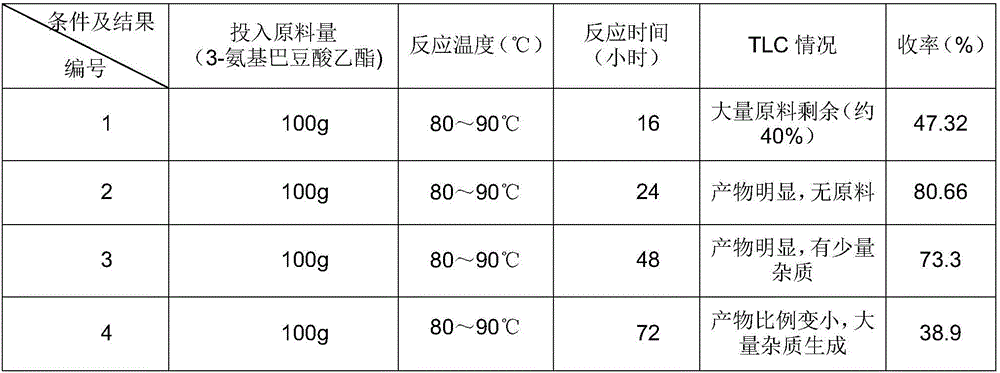
InactiveCN106279011AShort reaction timeReduce manufacturing costOrganic chemistryMethylnicotinateEthyl ester
The invention discloses a method for preparing 2,4-dyhydroxy-6-methylnicotinate. The method comprises the following steps: performing a Claisen ester condensation reaction and a Dieckmann condensation reaction on ethyl 3-aminocrotonate and diethyl malonate under the action of sodium ethoxide in sequence, thereby generating a product 2,4-dyhydroxy-6-methylnicotinate. The two steps of reactions, namely, the Claisen ester condensation reaction and the Dieckmann condensation reaction, of the method disclosed by the invention are both performed in one system, so that the reaction time is remarkably shortened, the production cost is greatly lowered, the method is simple, the reaction system is green and environmental-friendly, reaction conditions are not rigorous, the purity of a prepared product is 99% or greater, the yield of the product can be up to 80% or greater, and thus the method is applicable to industrial large-scale production.
Owner:CHENGDU BAISHIXING SCI & TECH IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method Tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method](https://images-eureka.patsnap.com/patent_img/2b60eb42-ee6d-4535-bfa9-adbcd291e04d/33093DEST_PATH_S.png)