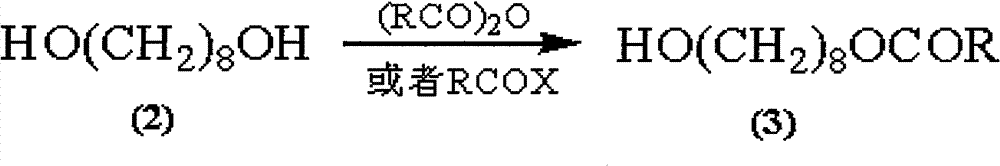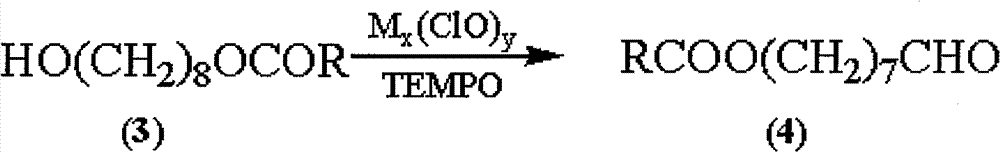Patents
Literature
34 results about "Monoethyl malonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
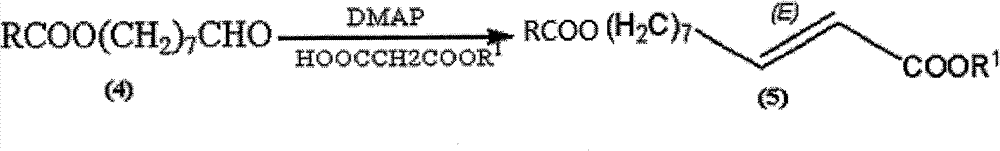
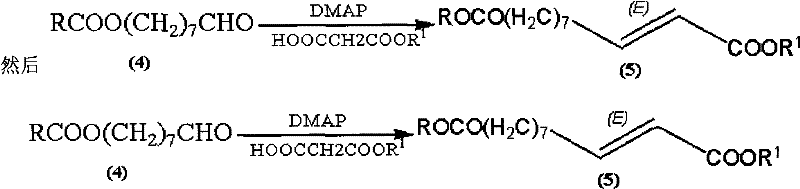
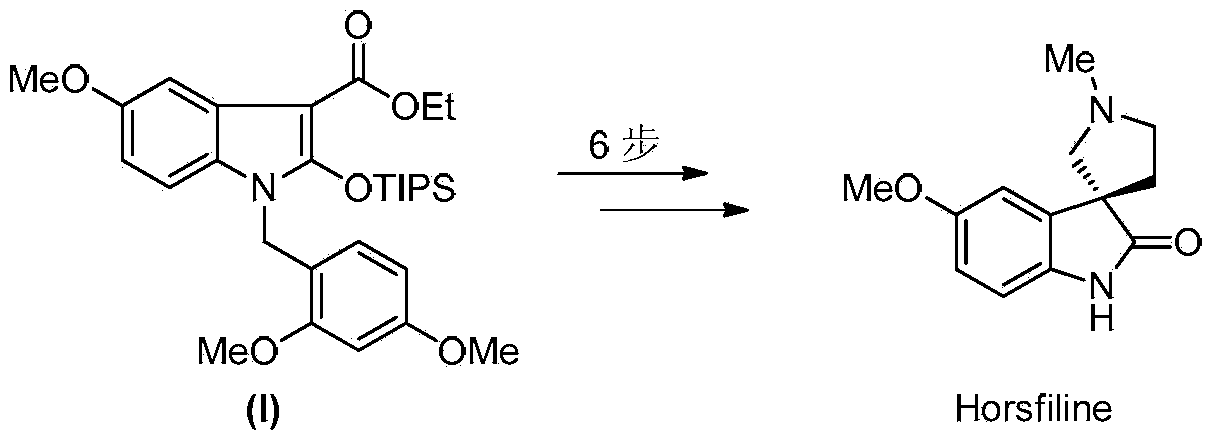
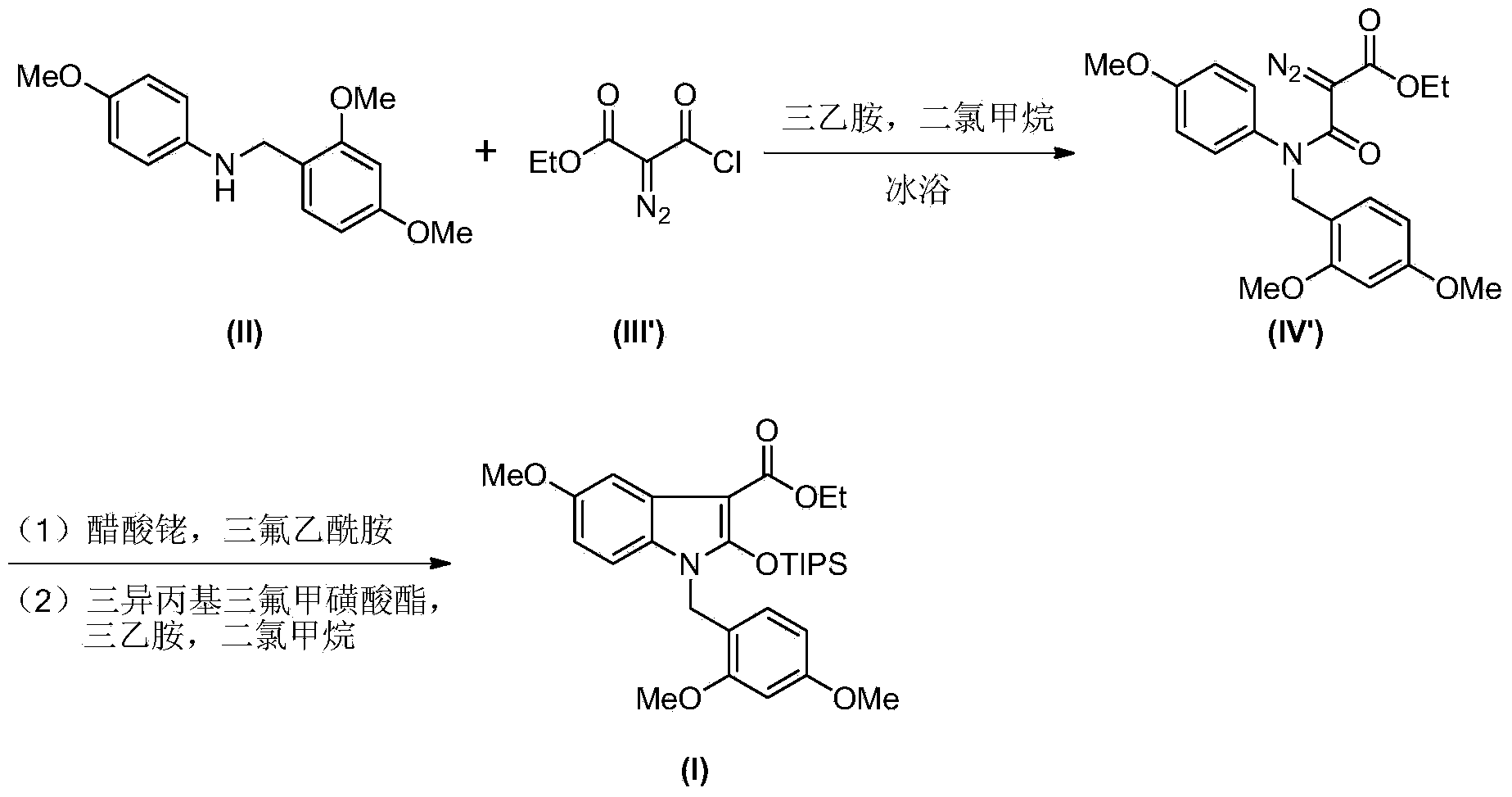
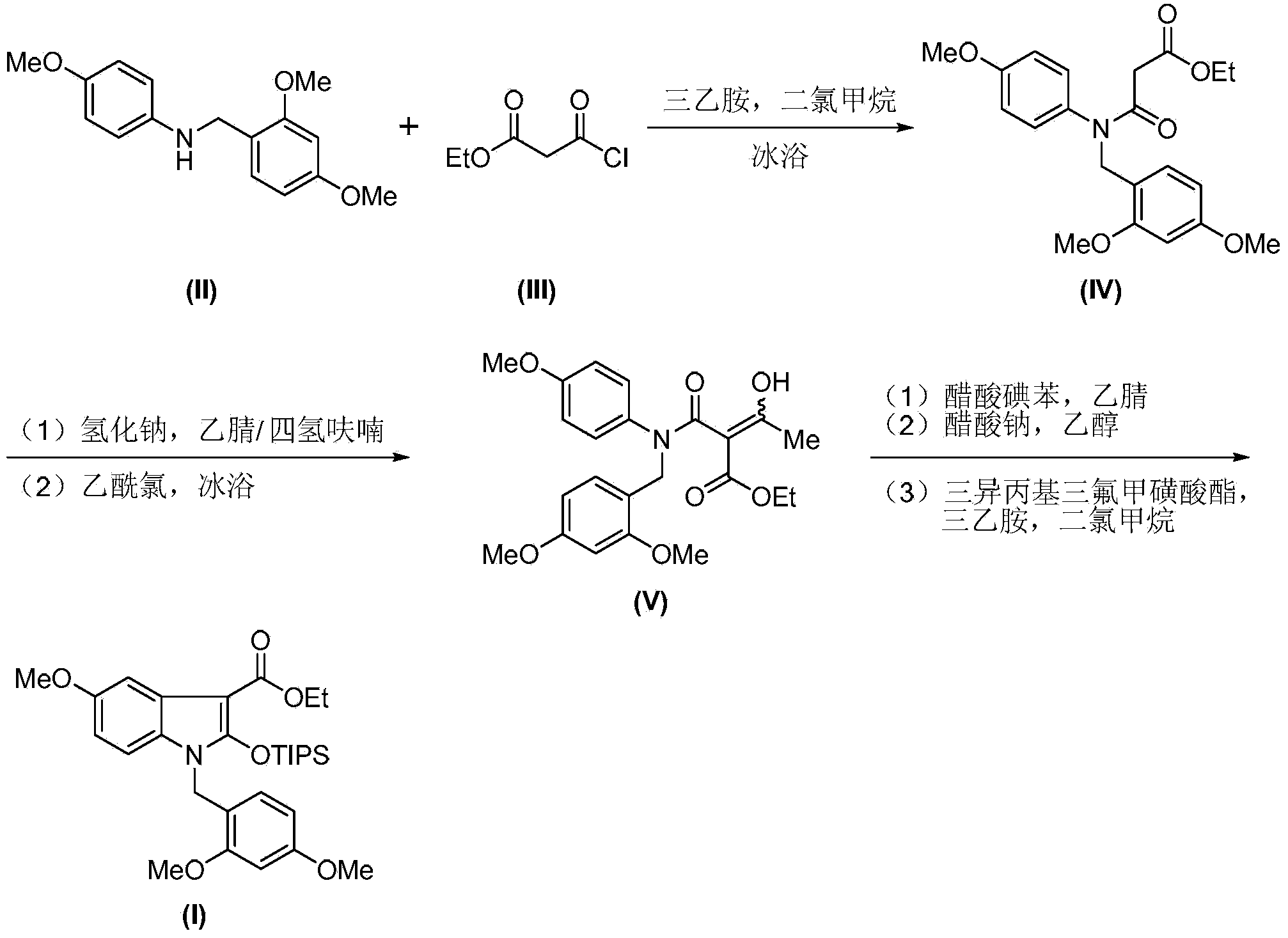
Synthetic method of royaljelly acid
ActiveCN102206151AOrganic compound preparationCarboxylic compound preparationSolventAqueous solution
The invention discloses a synthetic method of royaljelly acid, comprising steps of: firstly, performing a reaction between 8-alkanoyloxy octanal (4) and monoethyl malonate in the presence of DMAP in a solvent, and collecting (E)-10-alkanoyloxy-2-decenoic acid alkyl ester (5) from the reaction product; secondly, performing a saponification reaction of (E)-10-alkanoyloxy-2-decenoic acid alkyl ester(5) in an aqueous solution of alkaline materials, and collecting the target product royaljelly acid from the saponification product. According to the invention, the synthesis of royaljelly acid is designed again, avoiding problems of difficult product purification and low yield in traditional synthesis technology of the compound and greatly reducing production cost. Any other method can not accomplish the result in the invention. According to the invention, reagents used in the whole reaction are all easily available. The process route provided by the invention is of great innovation and convenient for industrial enforcement.
Owner:上海灏翔生物科技有限公司
Method for preparing 5-aminolevulinic acid hydrochloride
InactiveCN101891639ARaw materials are easy to obtainSimple and fast operationOrganic compound preparationAmino-carboxyl compound preparationHydroxylamineHydroxylamine Hydrochloride
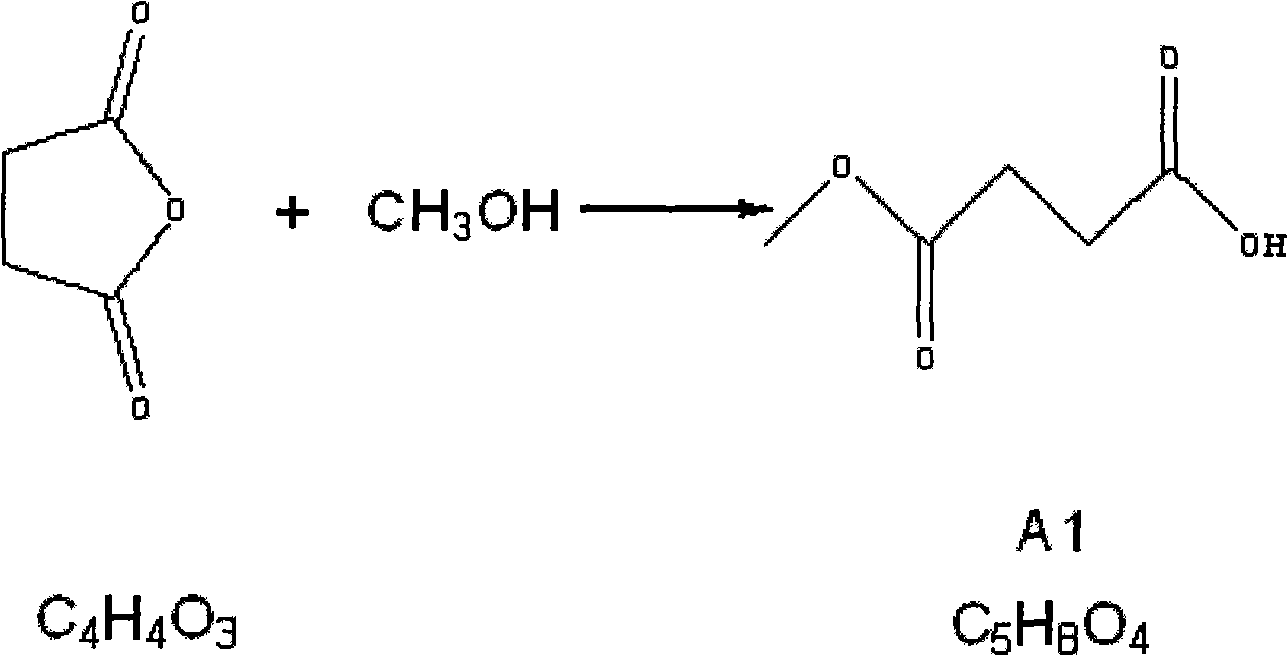
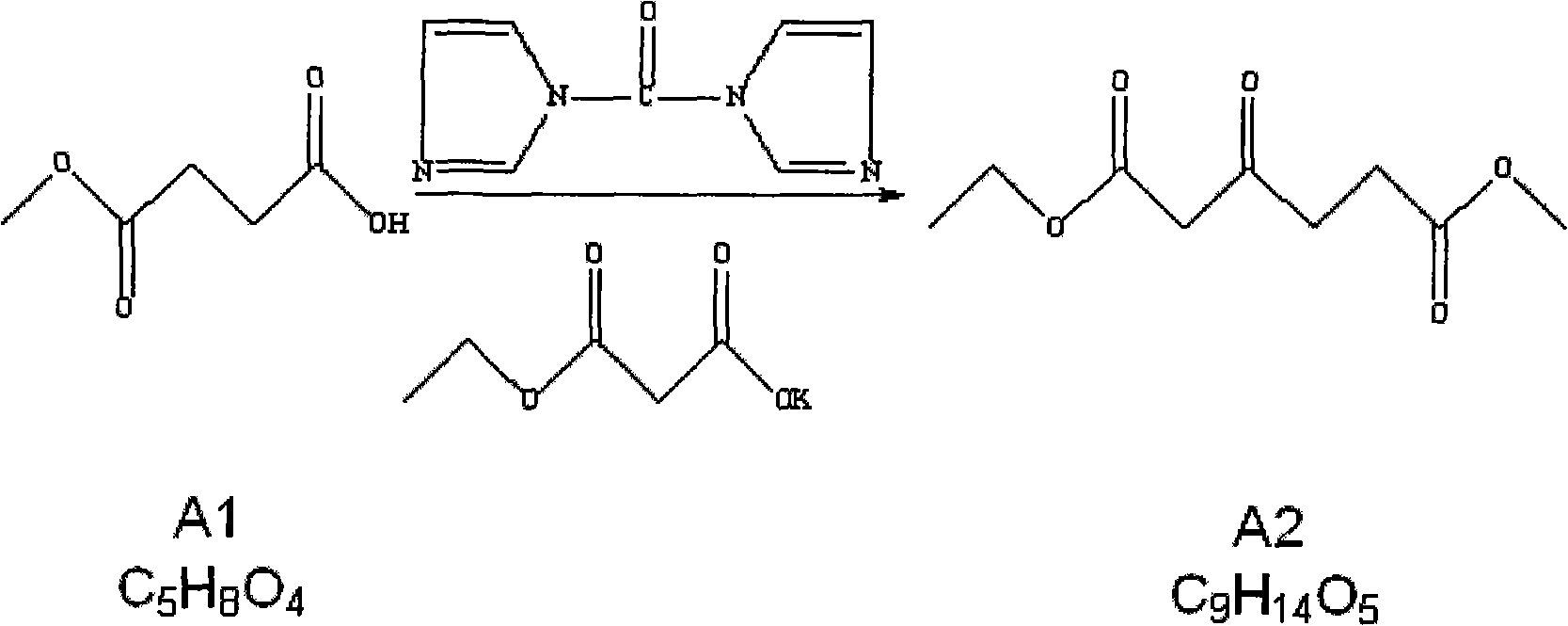
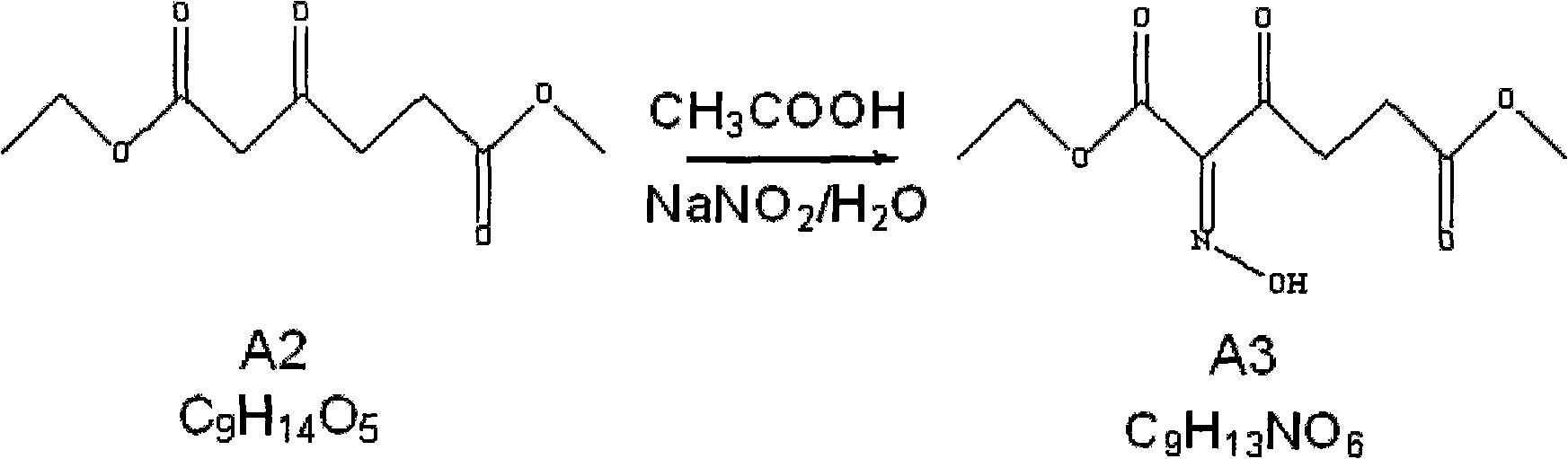
The invention relates to the field of medicaments, in particular to a method for preparing 5-aminolevulinic acid hydrochloride. The method of the invention comprises the following steps of: performing monoester esterification on succinic anhydride serving as a raw material and methanol to obtain a compound A1; performing condensation reaction on the compound A1 and ethyl potassium malonate under the action of N,N-dicarbonylimidazole to obtain a compound A2; performing hydroxylamine amination reaction on the compound A2 and sodium nitrite under the action of glacial acetic acid to obtain a compound A3; reducing the compound A3 by using Zn powder to obtain a compound A4; and hydrolyzing the compound A4 in the solution of hydrochloric acid to obtain the 5-aminolevulinic acid hydrochloride. The method of the invention has the characteristics of readily available raw material, low reaction cost, high yield, safe industrial production and the like.
Owner:XIANDAO CHEM SHANGHAI CO LTD
Method for totally synthesizing natural product (+/-)-rupestine G and resolving enantiomers
ActiveCN107129462AOptically-active compound separationOrganic racemisationLithium hydroxideEnantiomer
The invention relates to a method for totally synthesizing a natural product (+ / -)-rupestine G and resolving enantiomers. The method comprises the following steps: oxidizing the raw material 2-methyl-5-bromopyridine (1) by m-chloroperoxybenzoic acid to obtain a nitrogen oxidation product compound 2, carrying out a Reissert-Henze reaction to perform cyano substitution so as to obtain a compound 3, carrying out a decarboxylation reaction on the compound 3 and potassium monoethyl malonate to obtain beta-ketoester 4, carrying out alkylation under the condition of sodium ethylate to obtain a compound 5, carrying out a coupling reaction to obtain a compound 6, carrying out an intramolecular olefin double replacement reaction on the compound 6 to obtain a key intermediate compound 7, carrying out reduction with sodium borohydride to obtain a compound 8, carrying out a reaction under the condition of pyridine / MsCl to obtain a compound 9, hydrolyzing the compound 9 under the action of lithium hydroxide, carrying out methyl esterification by using iodomethane to obtain a compound 10, carrying out palladium-carbon catalytic hydrogenation to obtain a pair of diastereoisomers 11 and 12, and carrying out resolution by a semi-preparative high performance liquid chromatograph to obtain the natural product rupestine G and three stereoisomers, namely a compound 13, a compound 14 and a compound 15.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
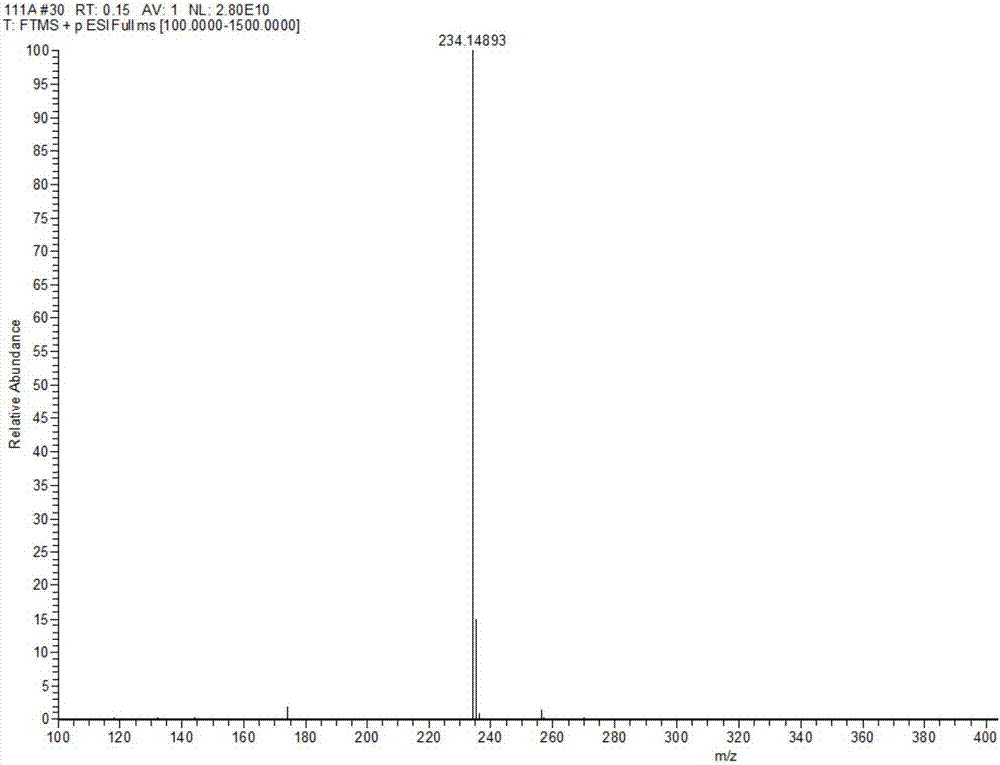
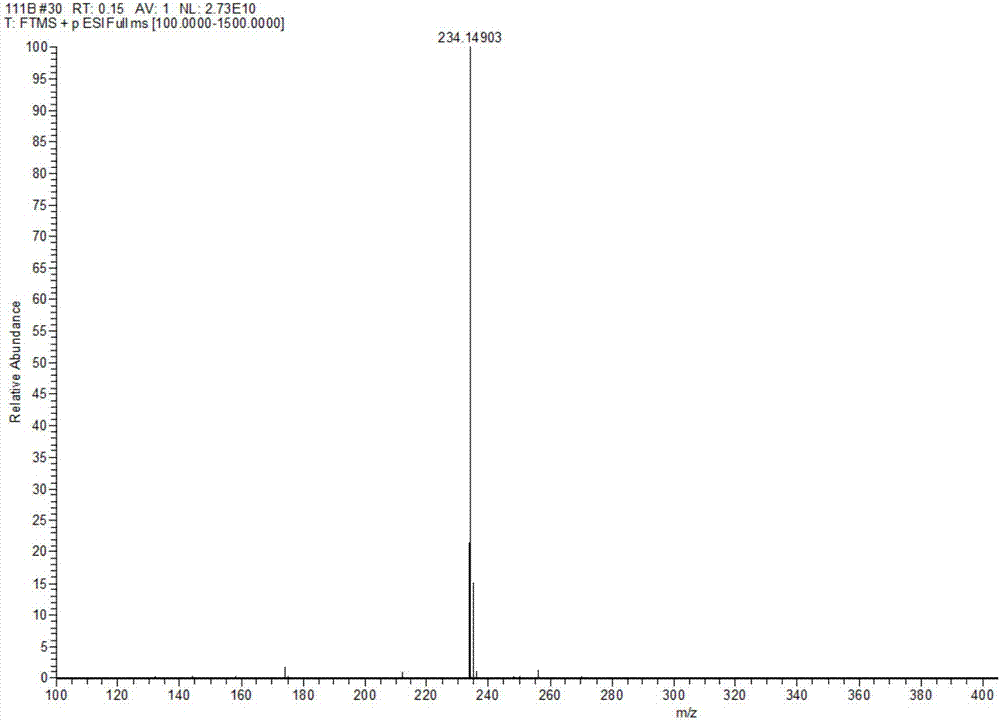
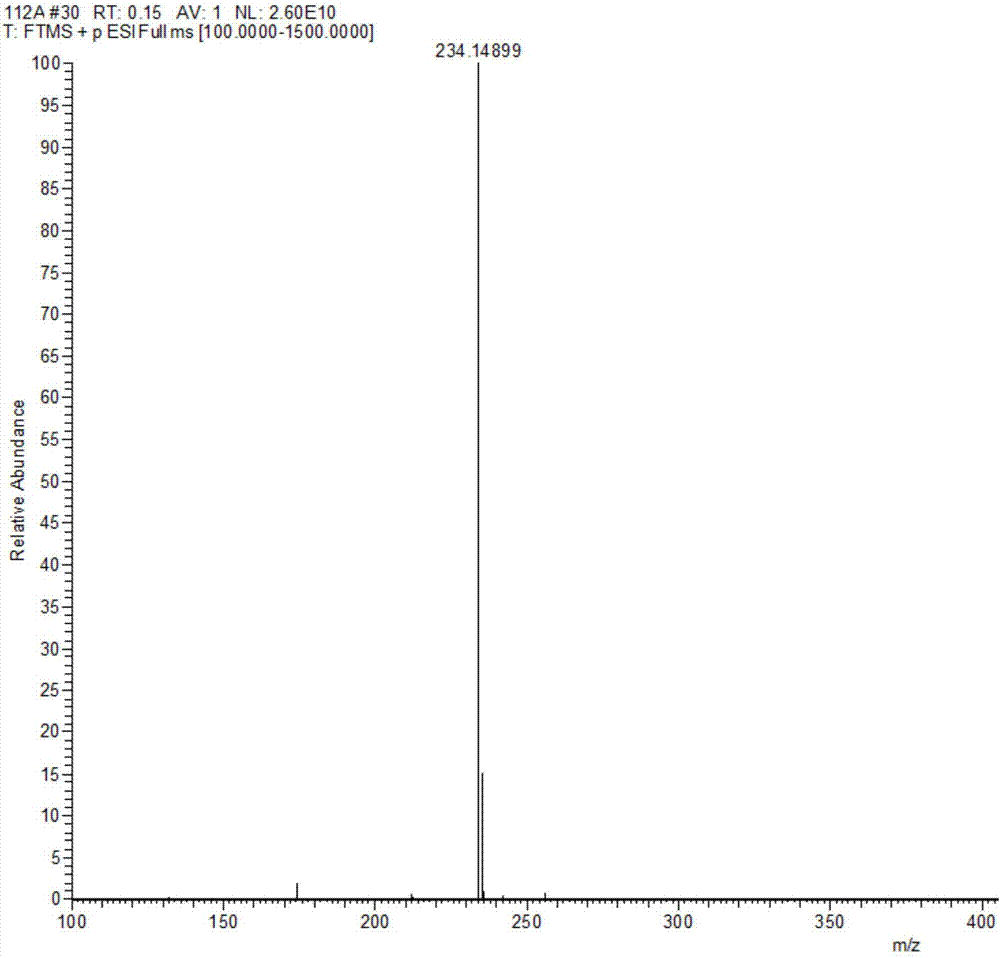
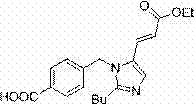
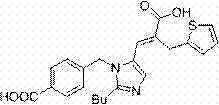
Method for preparing Eprosartan impurity EP12A
The invention discloses a method for preparing an Eprosartan impurity EP12A. The method comprises the step of condensing 4-[[(2-butyl-5-formyl)imidazol-1-yl]methyl]benzoic acid with monoethyl malonate in the presence of a catalyst, so as to obtain the Eprosartan impurity EP12A. The method has the beneficial effects that the reaction conditions are mild, the process is simple, the reaction time is short, side products are few, the yield is good, and the high-purity Eprosartan impurity EP12A can be obtained.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
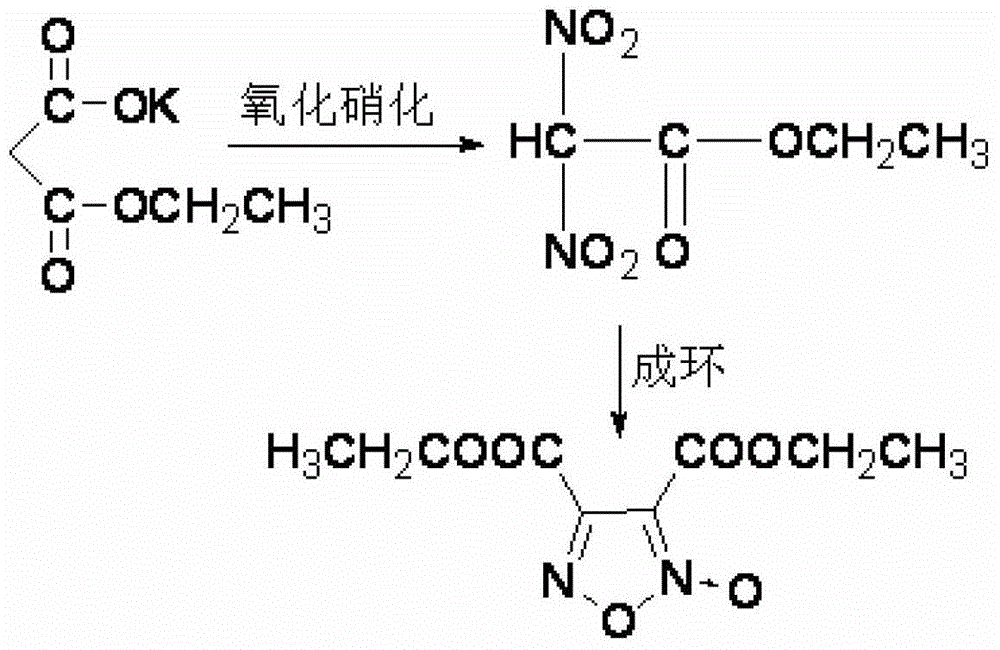
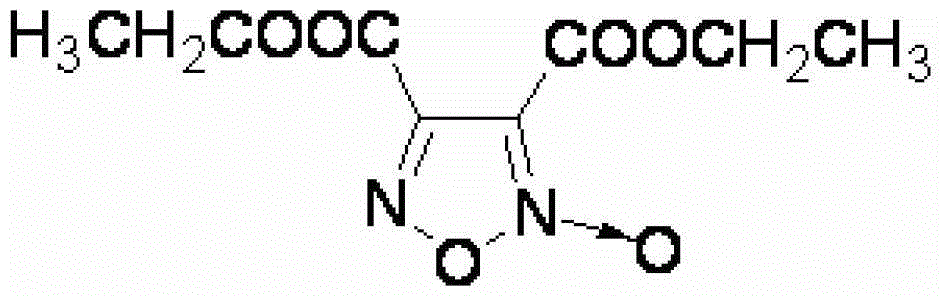
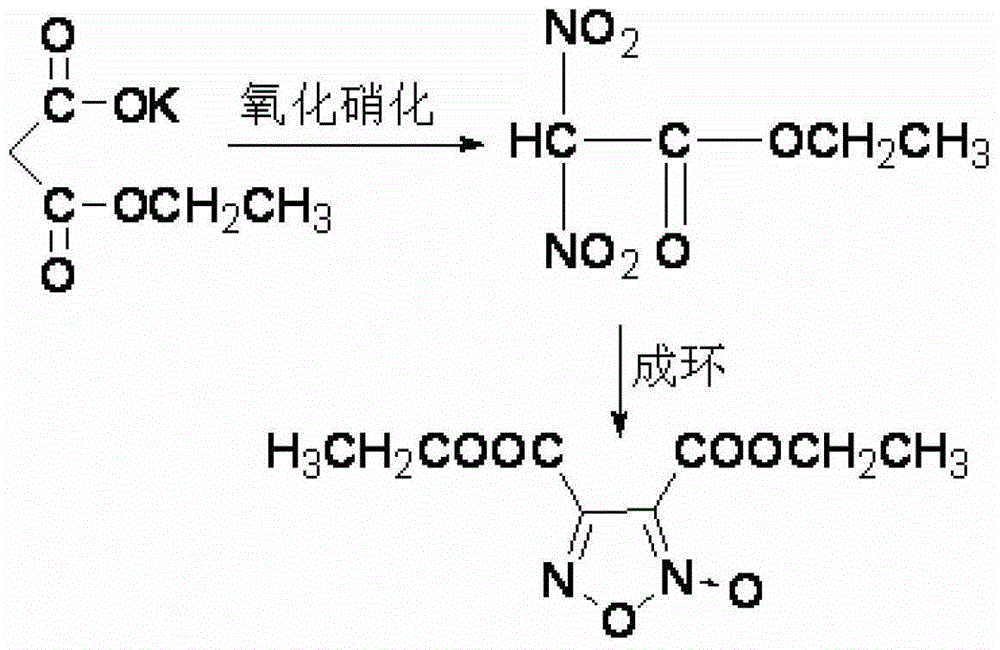
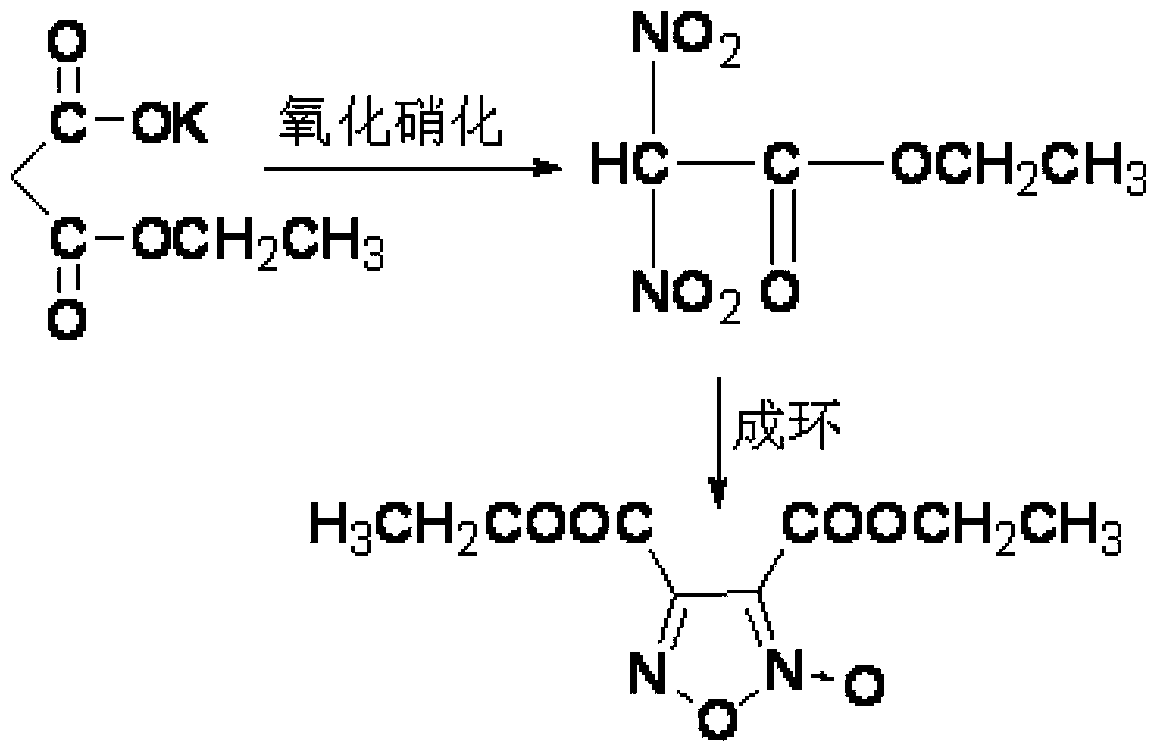
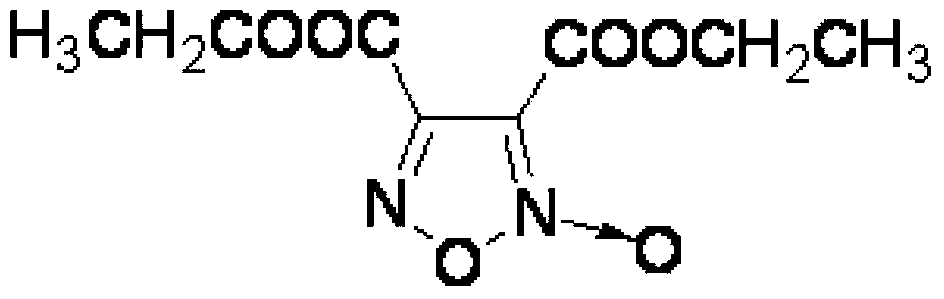
Synthesis method of 3, 4-dicarboxylic acid diethyl ester furoxan
InactiveCN103351356ARaw materials are easy to obtainLow priceOrganic chemistrySynthesis methodsNitration
The invention relates to a furoxan ring, and provides a synthesis method of 3, 4-dicarboxylic acid diethyl ester furoxan. The method adopts a monoethyl malonate potassium salt as a raw material and takes sodium nitrate / nitrite as an oxidizing agent. The substances are subjected to oxidation nitration and a ring formation reaction in a carbon tetrachloride solution, thus obtaining the target compound 3, 4-dicarboxylic acid diethyl ester furoxan with purity up to 98.7% and yield up to 94.2%. The synthesis method provided in the invention has the advantages of high purity and high yield.
Owner:XIAN MODERN CHEM RES INST
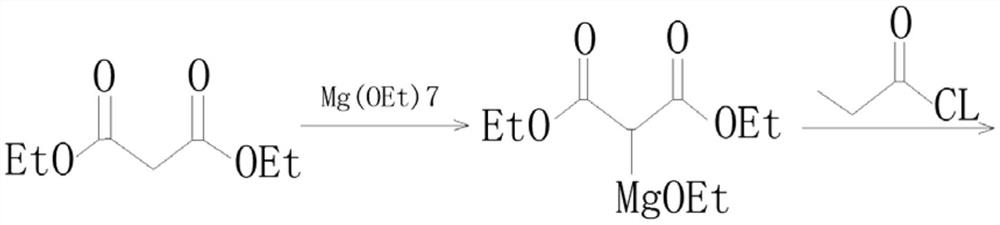
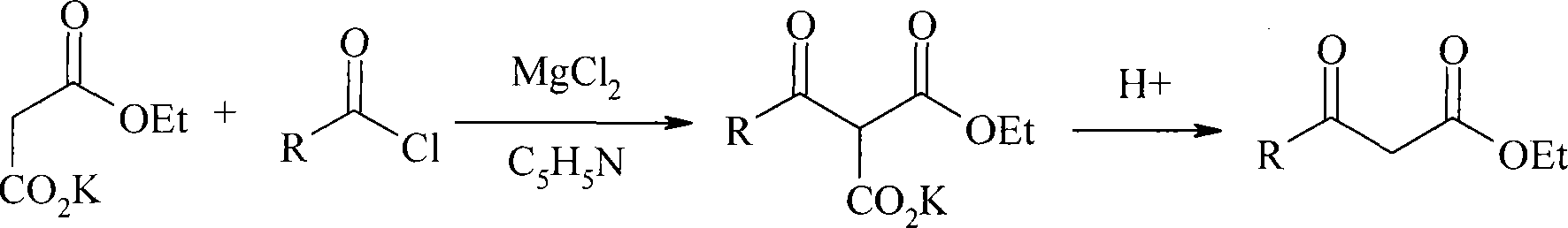
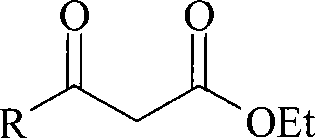
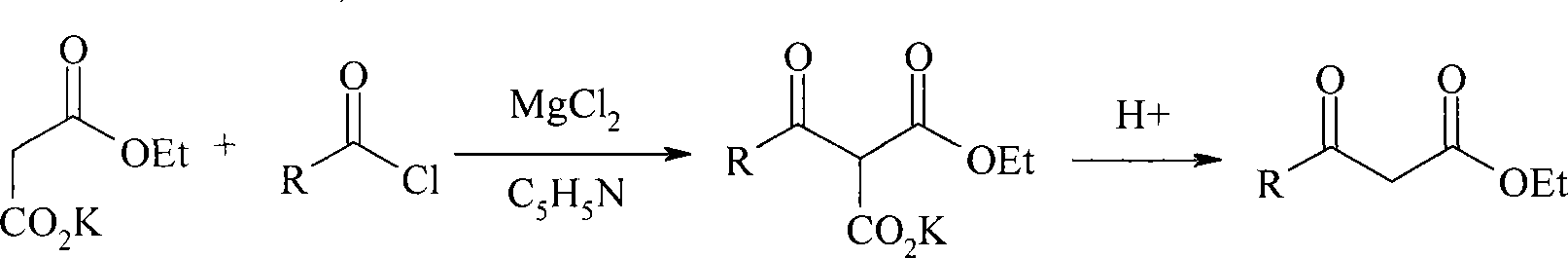
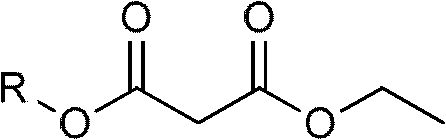
Method for preparing beta-keto acid ethyl ester
InactiveCN101215234AMild reaction conditionsImprove product qualityOrganic compound preparationCarboxylic acid esters preparationEthyl esterChloride
The invention discloses a process for preparing ethyl beta-ketoacid, which utilizes potassium monoethyl malonate as raw material. The raw material is condensed with acyl chloride by the effect of magnesium chloride and pyridine and is hydrochlorinated, then, target products of the invention ethyl beta-ketoacid are obtained, and the purity reaches above 99% (GC). Compared with the prior art, the invention is moderate in conditions in the reaction process, stable in product quality, fine in purity, high in yield coefficient and safe and simple in operation, thereby the invention is suitable for commercial process.
Owner:SHANGHAI CHEM REAGENT RES INST
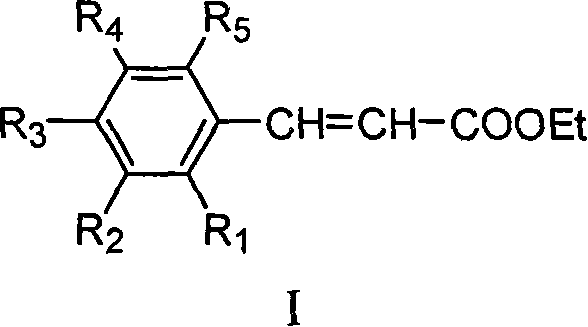
Method for preparing ethyl cinnamate derivative
InactiveCN101121664AThe production process is simple to operateLow costOrganic compound preparationCarboxylic acid esters preparationChemical synthesisEthyl cinnamate
The invention relates to the field of chemical synthesis, particularly relating to a preparation method for the ethyl cinnamate derivatives. The preparation method of the invention is that the malonate diethylester reacts with the potassium hydroxide in the solution to produce the potassium salt of the malonate single-diethyl ester.The ice acetic acid is added to produce the malonate single-diethyl ester; the malonate single-diethyl ester then does the condensation reaction with the cinnamate with the catalysis of the amino acid. The preparation method of the invention is cheap and easy in the obtainment of the raw materials, simple in the operation, and low in the cost.
Owner:曾庆友 +1
Tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method
InactiveCN110563726AHigh yieldThe reaction is easy to scale upOrganic chemistrySodium methoxideFormate
The invention relates to a tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method. A purpose of the present invention is to mainly solve the technical problem that no method is suitable for industrial synthesis in the prior art. The method comprises four steps, and comprises: dissolving a compound 1 in ethanol to generate a compound 2 under the action of ammonium acetate and monoethyl malonate; in the presence of potassium carbonate, making the compound 2 act with ethyl malonyl chloride in a mixed solution of tetrahydrofuran and water to obtain a compound 3; and carrying out a Dieckmann condensation reaction on the compound 3, toluene and sodium methoxide, treating to obtain a compound 4, and carrying out heating decarboxylating on the compound 4 in water and acetonitrile to obtain a compound 5, wherein the reaction formula is defined in the specification. According to the present invention, the obtained compound can be used as the useful intermediates or productsfor synthesis of a plurality of medicaments.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
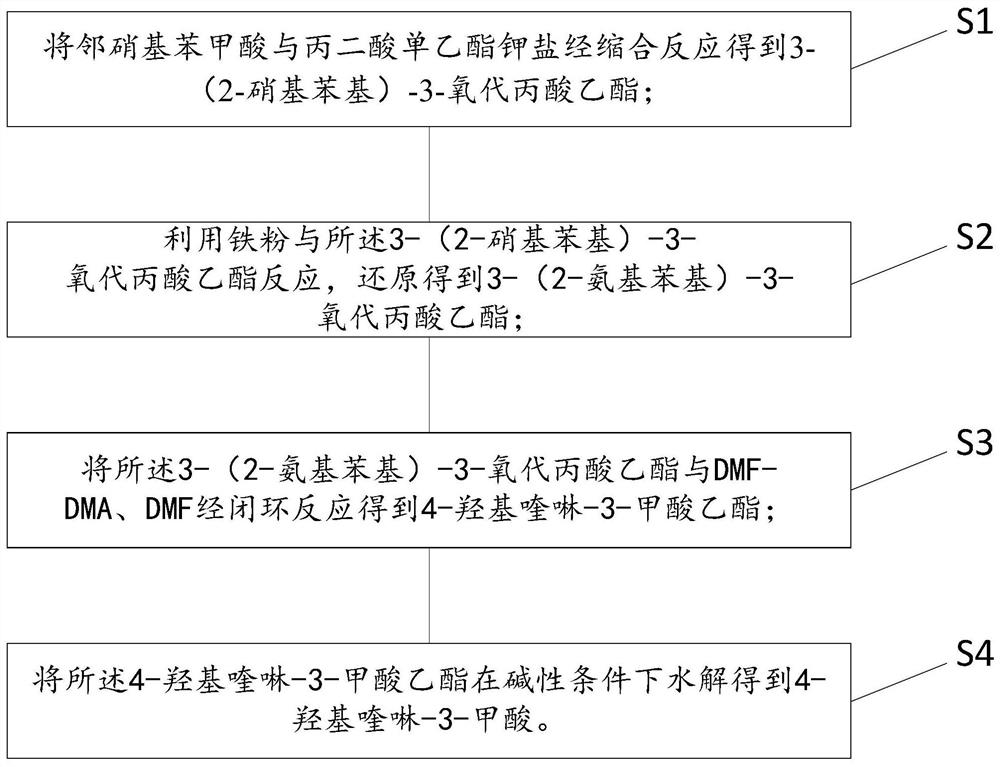
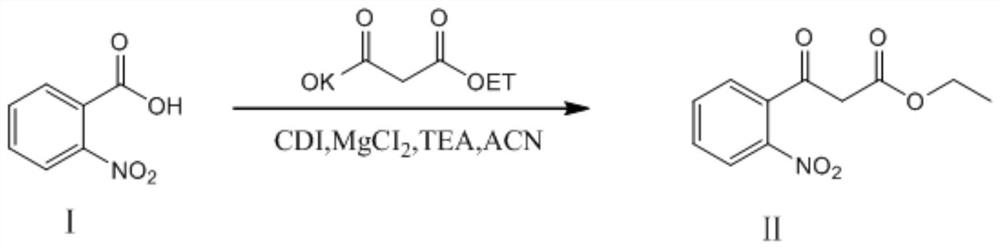
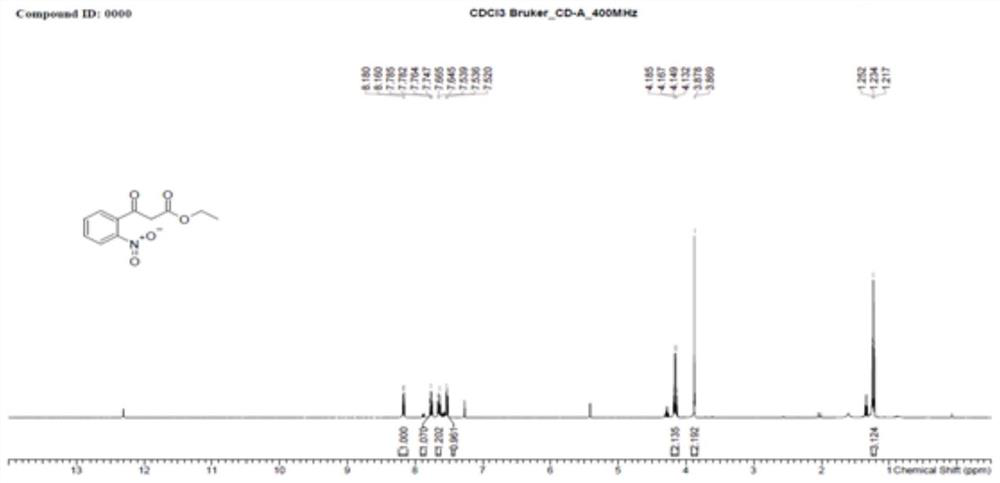
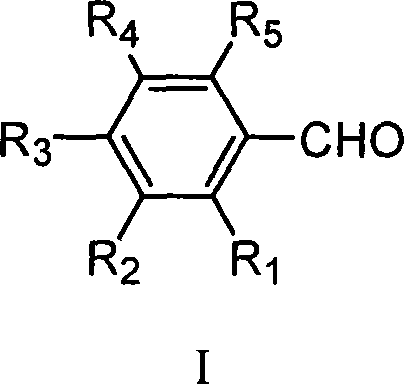
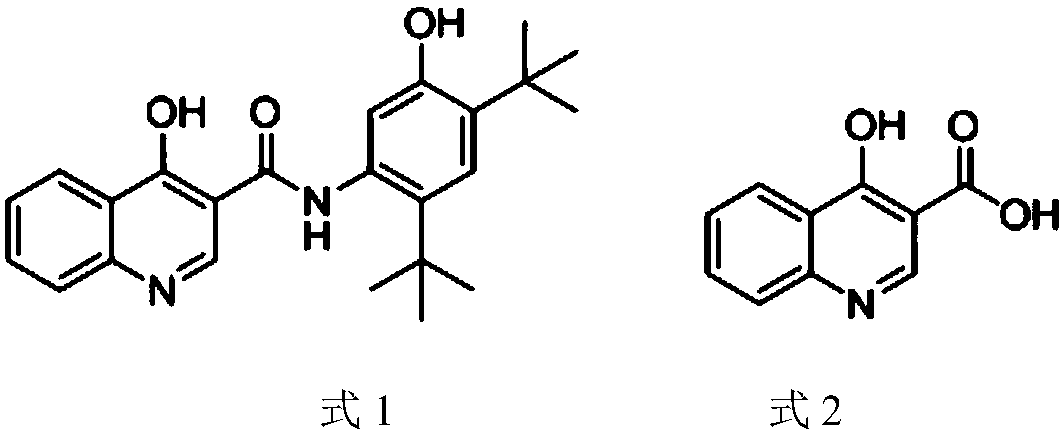
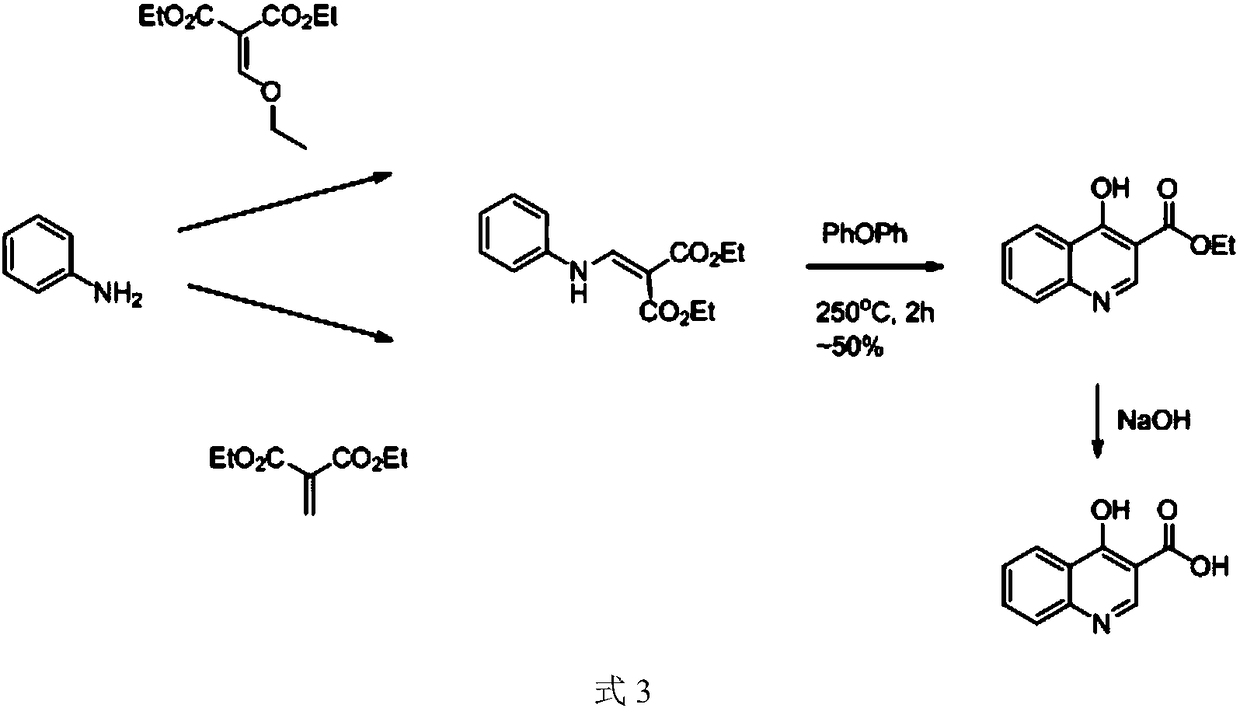
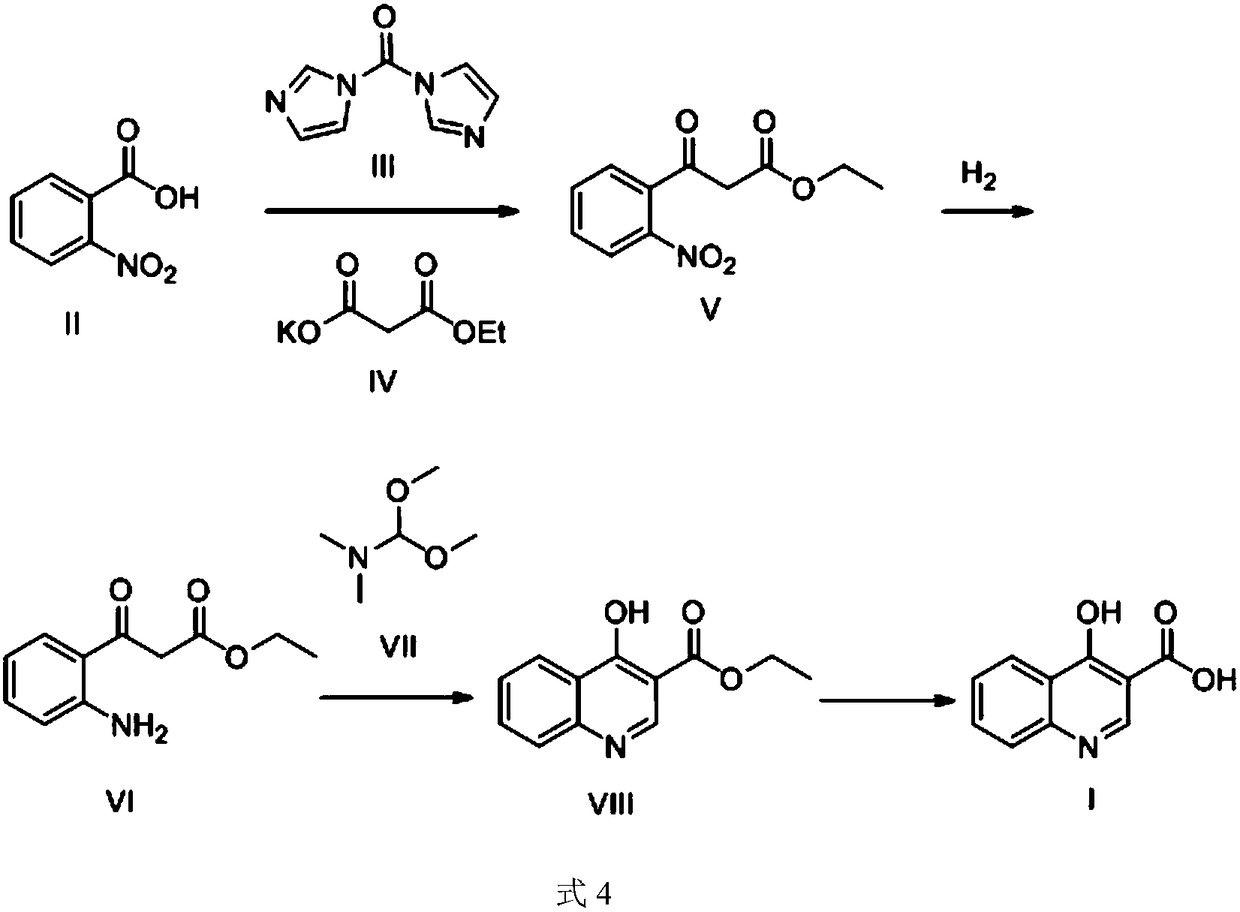
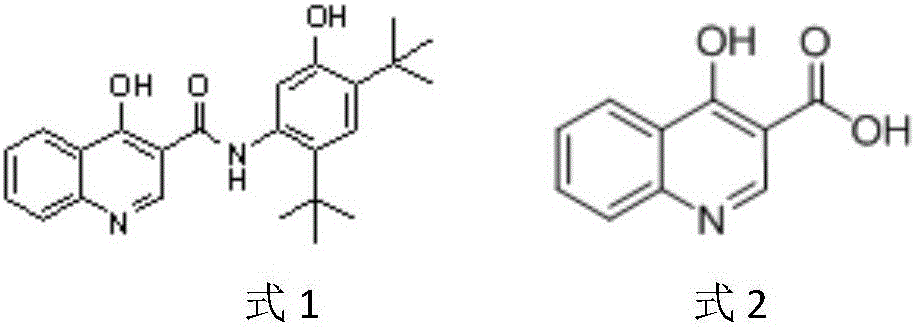
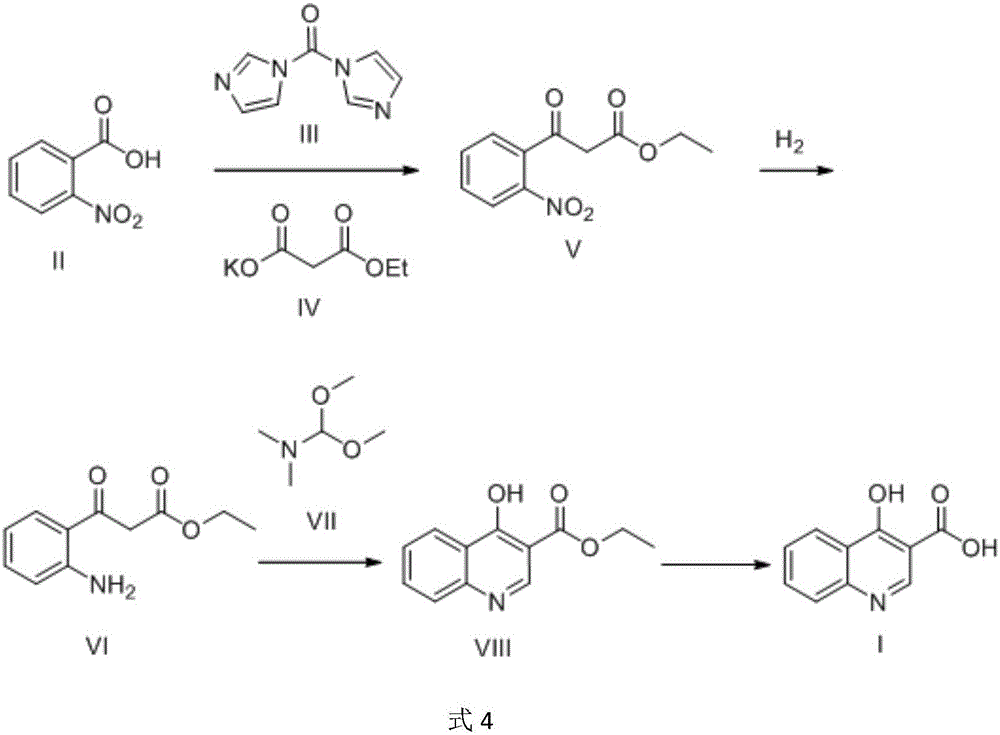
Preparation method of 4-hydroxyquinoline-3-carboxylic acid
ActiveCN106187887ARaw materials are easy to getSimple processOrganic chemistryOrganic synthesisCarboxylic salt
The invention relates to the technical field of organic synthesis and bulk drug intermediates, and concretely relates to a preparation method of a key intermediate 4-hydroxyquinoline-3-carboxylic acid of a new medicine ivacaftor for treating cystic fibrosis. The preparation method comprises the following steps: 1, carrying out a condensation reaction: reacting o-nitrobenzoic acid, potassium monoethyl malonate and N,N-carbonyldiimidazole to prepare ethyl 3-(2-nitrophenyl)-3-oxopropanoate; 2, carrying out a reduction reaction: carrying out catalytic hydrogenation reduction on ethyl 3-(2-nitrophenyl)-3-oxopropanoate to prepare ethyl 3-(2-aminophenyl)-3-oxopropanoate; 3, carrying out a cyclization reaction: carrying out nucleophilic addition and cyclization reaction on ethyl 3-(2-aminophenyl)-3-oxopropanoate and N,N-dimethyl formamide dimethyl acctel to obtain ethyl 4-hydroxyquinoline-3-carboxylate; and 4, carrying out a hydrolysis reaction: carrying out the hydrolysis reaction on ethyl 4-hydroxyquinoline-3-carboxylate to obtain 4-hydroxyquinoline-3-carboxylic acid. The preparation method has the advantages of easily available raw materials, mild reaction conditions, simplicity and convenience in post-treatment, suitableness for amplified preparation, and high yield.
Owner:SHANGHAI UNIV OF ENG SCI
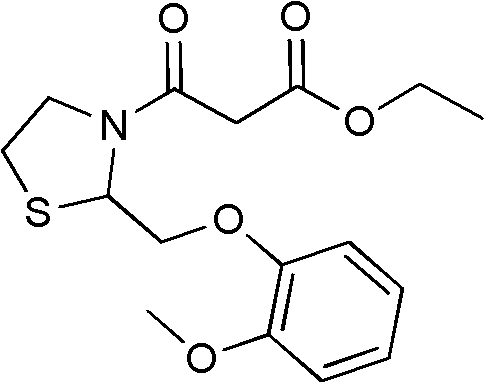
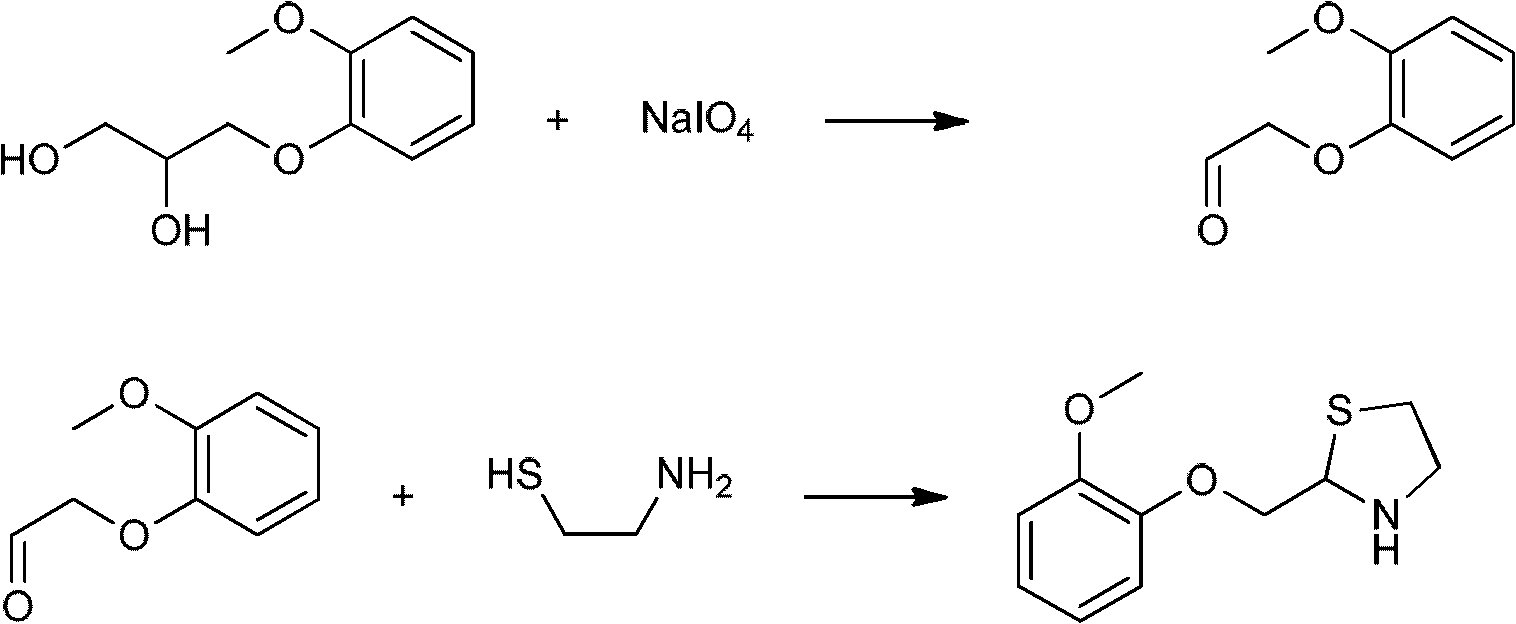
Moguisteine synthesis process
InactiveCN102653527ASynthetic operation is simpleHigh purityOrganic chemistryOrganic layerMethyl group
The invention relates to the technical field of medicines, in particular to a moguisteine synthesis process. The moguisteine synthesis process provided by the invention comprises the following steps of: generating ethyl malonyl chloride by adopting acyl chloride synthesis reaction; layering and then acquiring an organic layer containing a dichloromethane solution of acyl chloride as a dichloromethane solution of acyl chloride; and dropping the dichloromethane solution of the acyl chloride of the organic layer into a dichloromethane solution of (+ / -)-2-((2-methoxyphenoxy)methyl) thiazolidine for reacting to obtain moguisteine. The moguisteine synthesis operation is simplified, the equipment is simple, the operation is easy, the prepared moguisteine has higher purity, and the production cost of the moguisteine is lowered.
Owner:FOSHAN DAYI TECH LTD
Synthesis method of 2-indolone-3-carboxylate derivatives
ActiveCN103524549AEasy to operateMild reaction conditionsGroup 4/14 element organic compoundsSodium acetrizoateSynthesis methods
The invention discloses a synthesis method of 2-indolone-3-carboxylate derivatives (I), which comprises the following steps: reacting substituted aniline (II) used as a raw material and triethylamine as an acid-binding agent with ethyl malonyl chloride (III) to generate ethyl malonyl chloride (IV); reacting the ethyl malonyl chloride (IV) with acetyl chloride in an acetonitrile-tetrahydrofuran mixed solution under the action of sodium hydride to generate an enol form compound (V); carrying out intramolecular cyclization on the enol form compound (V) under the action of iodosobenzene diacetate to remove acetyl group, thereby obtaining 2-indolone-3-carboxylate; and reacting the 2-indolone-3-carboxylate with triisopropyl trifluoromethanesulfonates under the action of triethylamine to generate the 2-indolone-3-carboxylate derivatives (I). The synthesis method is simple to operate, and has the advantages of mild reaction conditions, accessible reaction raw materials and reaction reagents, no need of toxic metal reagents, environment friendliness and the like.
Owner:BIRDO (SHANGHAI) PHARM R&D CO LTD
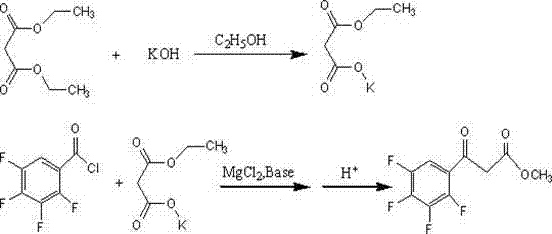
Preparation method of ethyl (2,3,4,5-tetrafluorobenzoyl) acetate
InactiveCN102249922AOvercoming constraintsSave energyOrganic compound preparationCarboxylic acid esters preparationOrganic basePotassium hydroxide
The invention relates to a preparation method of ethyl (2,3,4,5-tetrafluorobenzoyl) acetate, comprising the following process steps of: 1: reacting diethyl malonate as a raw material and absolute ethyl alcohol as a solution with potassium hydroxide to prepare potassium ethyl malonate; 2: reacting 2,3,4,5-tetrafluorobenzoyl chloride with the potassium ethyl malonate in a solvent under the condition of the coexistence of magnesium chloride and organic base; and 3: hydrolyzing a reaction product, purifying and crystallizing. According to the preparation method disclosed by the invention, the raw material used for an original synthesizing process is mainly changed so that reaction can be fast carried out at normal temperature, and therefore, energy sources are saved, the defects of the original synthesizing process on the restriction of the moisture of a reaction environment are overcome and then reaction is not influenced by the environment; in addition, according to the ethyl (2,3,4,5-tetrafluorobenzoyl) acetate prepared by the invention, technical indexes can be achieved as follows: appearance is white powder, purity is higher than 98.5 percent, and yield is 78-95 percent.
Owner:GANSU JINDUN CHEM
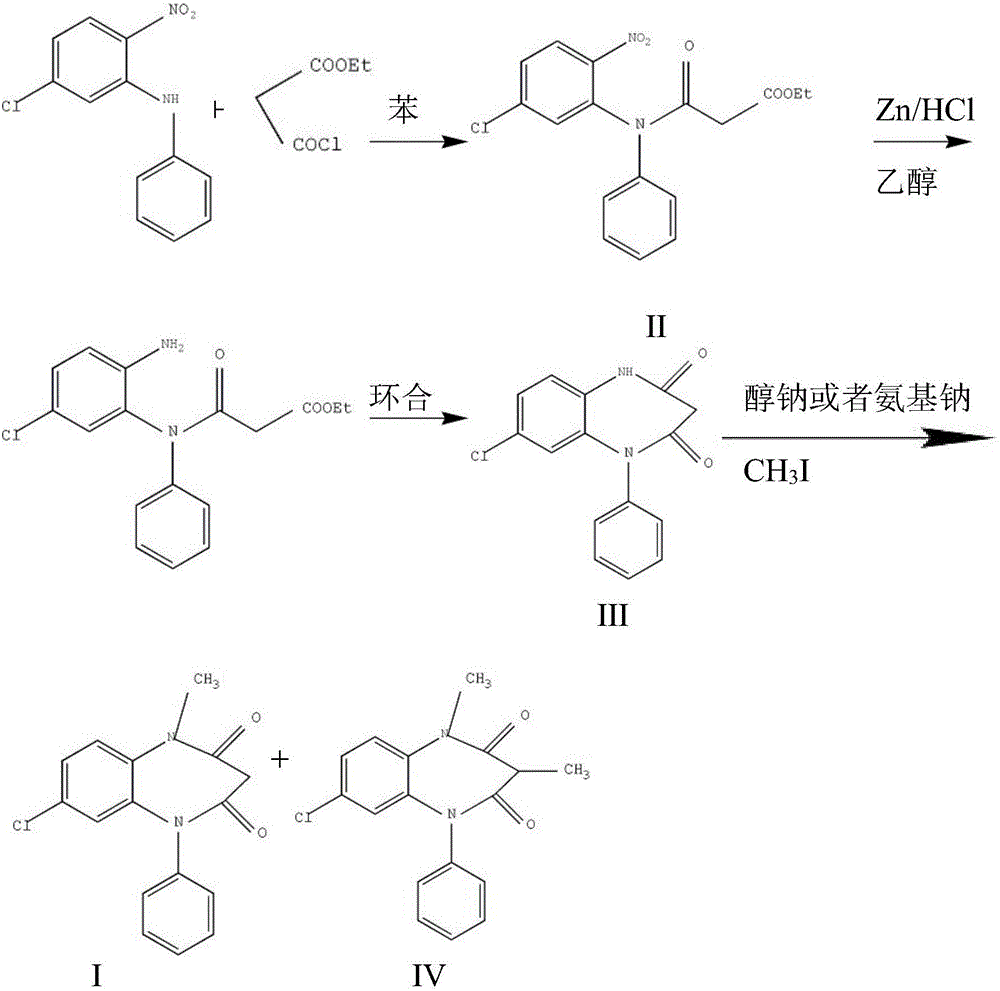
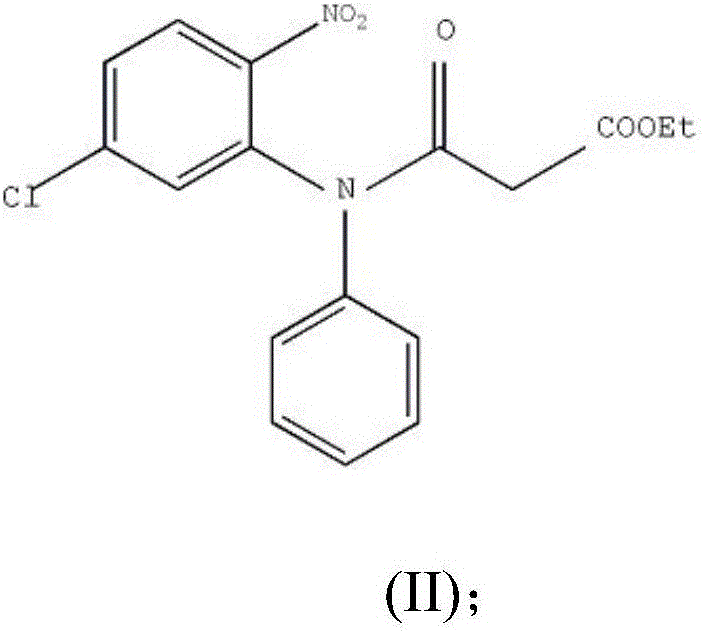
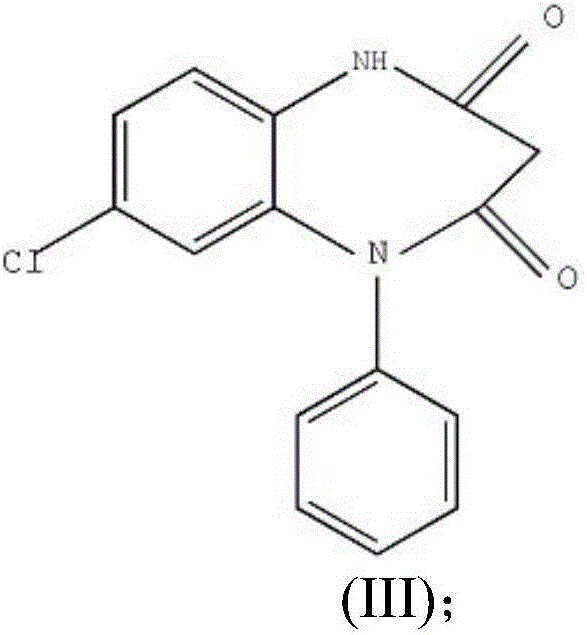
Preparation method of clobazam
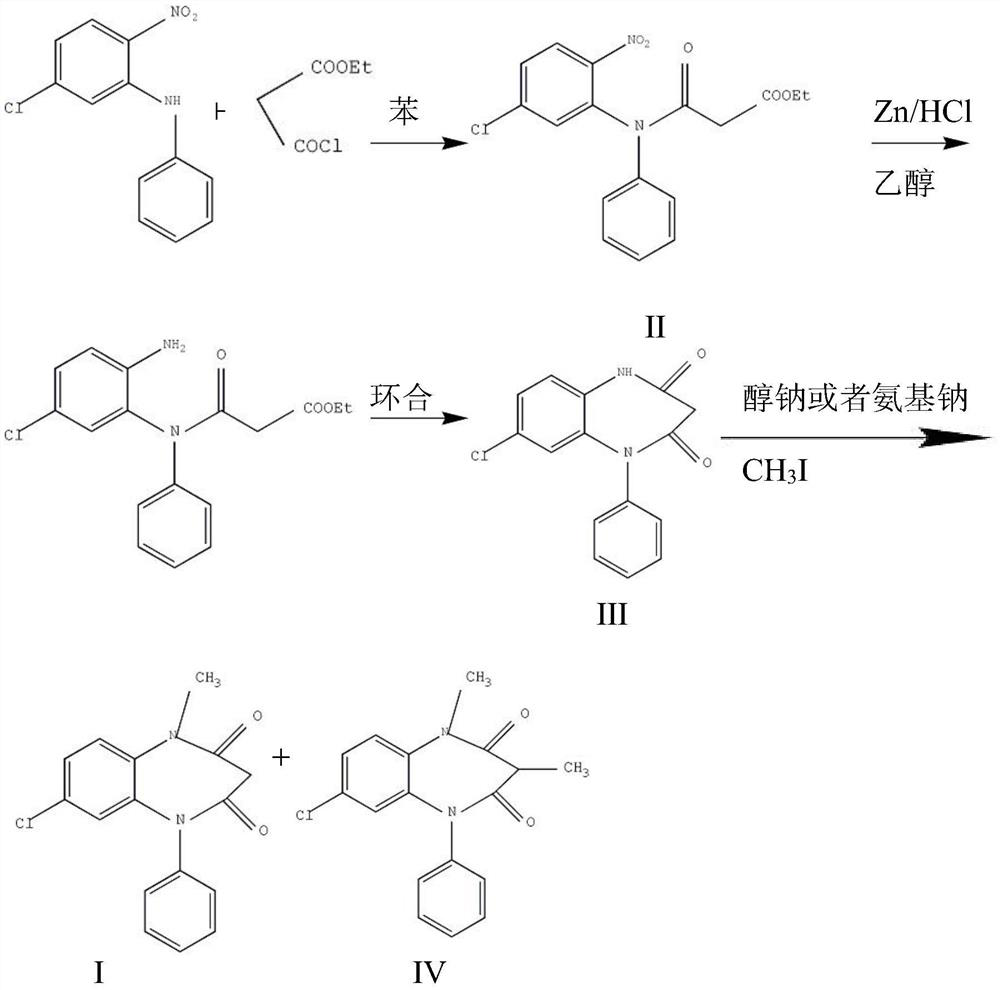
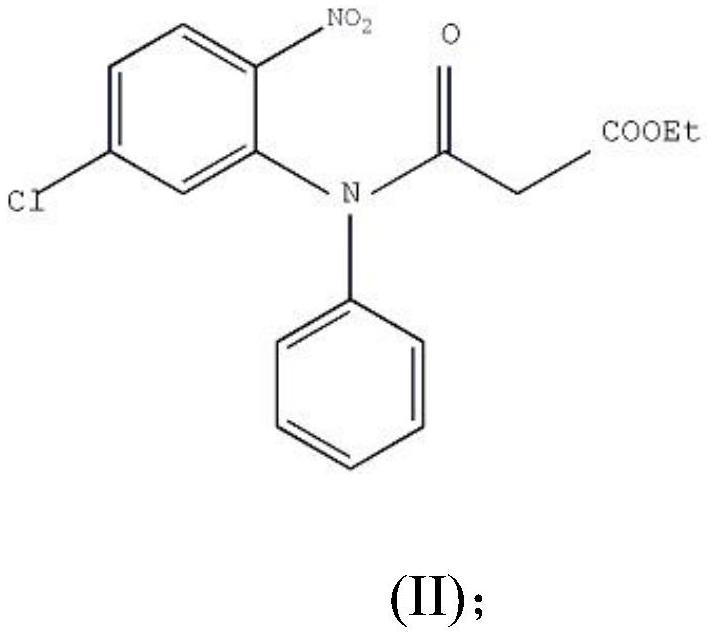
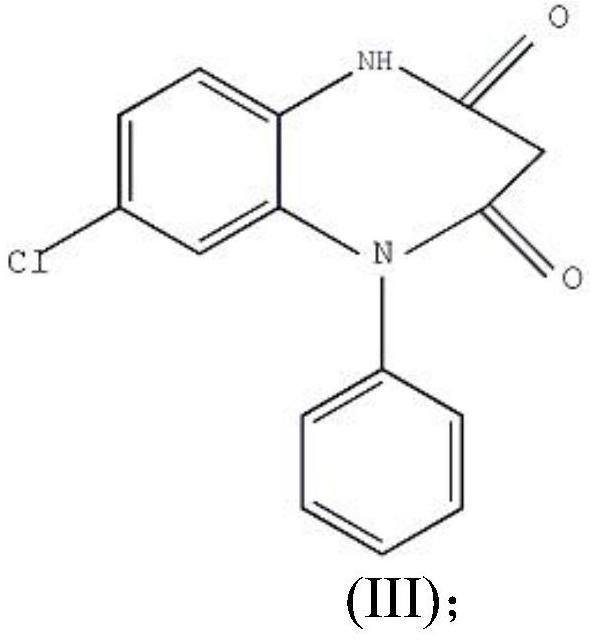
ActiveCN106749052AAvoid it happening againHigh purityOrganic chemistryLithium hydroxidePotassium hydroxide
The invention provides a preparation method of clobazam. The preparation method comprises the following steps: (1), using 2-nitro-5-chlorodiphenylamine and ethyl malonyl chloride as raw materials, performing a reflow reaction in an organic solvent, after the reaction, decompressing the organic solvent in the reaction system, then performing evaporation to dryness, and adding a refining solvent for refining treatment so as to obtain a compound as shown in a formula II; (2), performing zinc powder reduction on the compound as shown in the formula II, and performing ammonolysis cyclization so as to obtain a compound as shown in a formula III; (3) enabling the compound as shown in the formula III to react with methyl iodide in an alkaline alcohol solution so as to obtain the clobazam, wherein alkali is one or more of sodium hydroxide, lithium hydroxide and potassium hydroxide. The clobazam prepared by the method is few in impurities and high in purity.
Owner:济南科汇医药科技有限公司
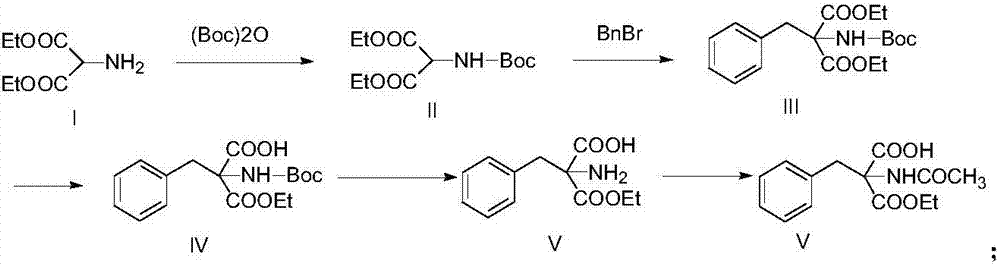
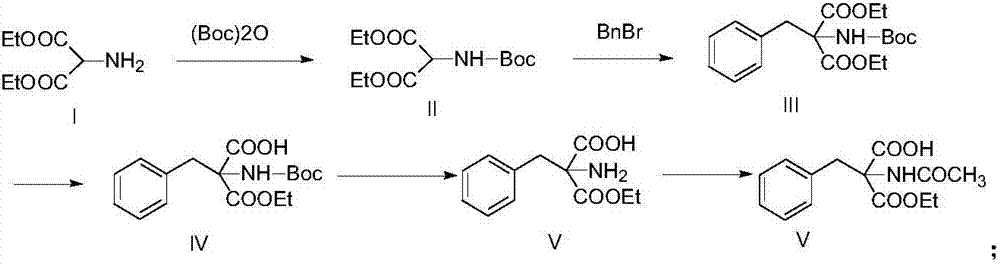
Synthetic method of monoamino inhibitor intermediate monoethyl 2-acetylamino-2-benzylmalonate
ActiveCN106946724ARaw materials are easy to getLow costCarbamic acid derivatives preparationOrganic compound preparationBenzoyl bromideTert-Butyloxycarbonyl protecting group
The invention discloses a synthetic method of a monoamino inhibitor intermediate monoethyl 2-acetylamino-2-benzylmalonate. The method comprises the following steps: 1, carrying out amino protection on a compound I diethyl aminomalonate and di-tert-butyl dicarbonate to obtain a compound II diethyl-2-Boc-aminomalonic acid; 2, reacting the compound II with benzyl bromide to obtain a compound III diethyl 2-(N-(tertbutyloxycarbonyl)amino)-2-benzylmalonate; 3, removing monoester from the compound III to obtain a compound IV monoethyl 2-(N-(tertbutyloxycarbonylamino)-2-benzyl-malonate; 4, carrying out amino deprotection on the compound IV to obtain a compound V monoethyl 2-amino-2-benzyl-malonate; and 5, carrying out an acetylation reaction on the compound V to prepare a compound VI monoethyl 2-(N-acetylamino)-2-benzyl-malonate. The method has the advantages of simple reactions, easily available raw materials, simple post-treatment, high yield, low cost, high operationality, and suitableness for industrial production.
Owner:苏州汉德创宏生化科技有限公司
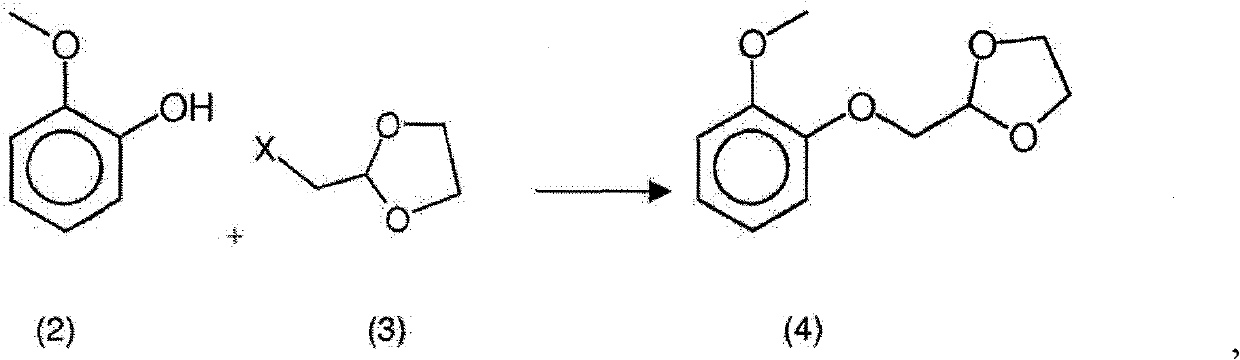
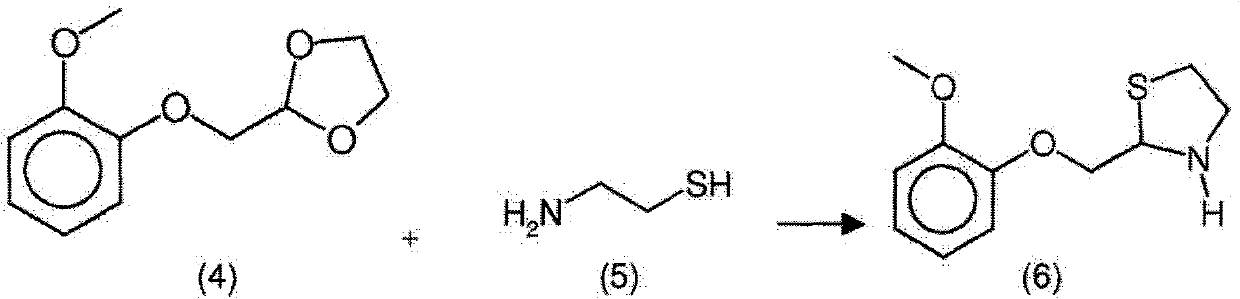
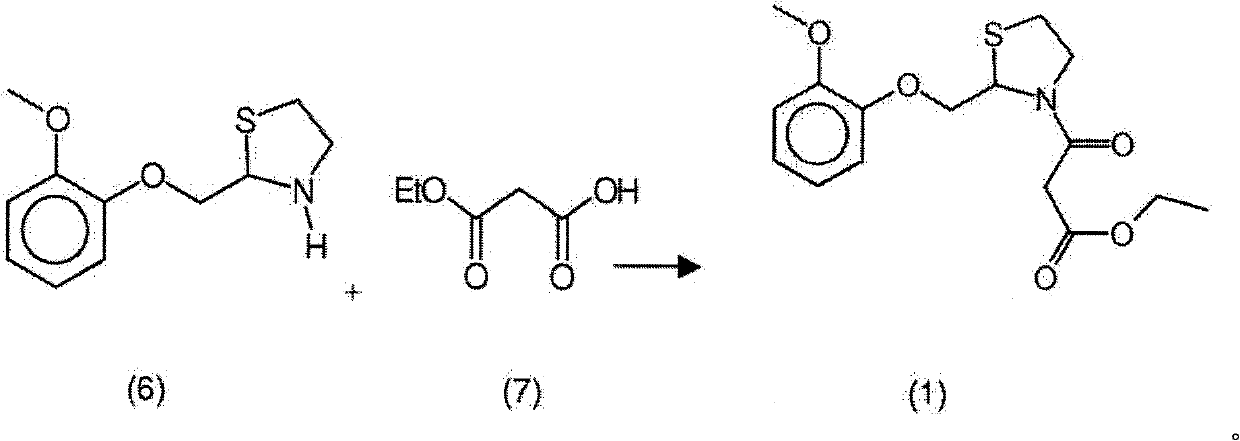
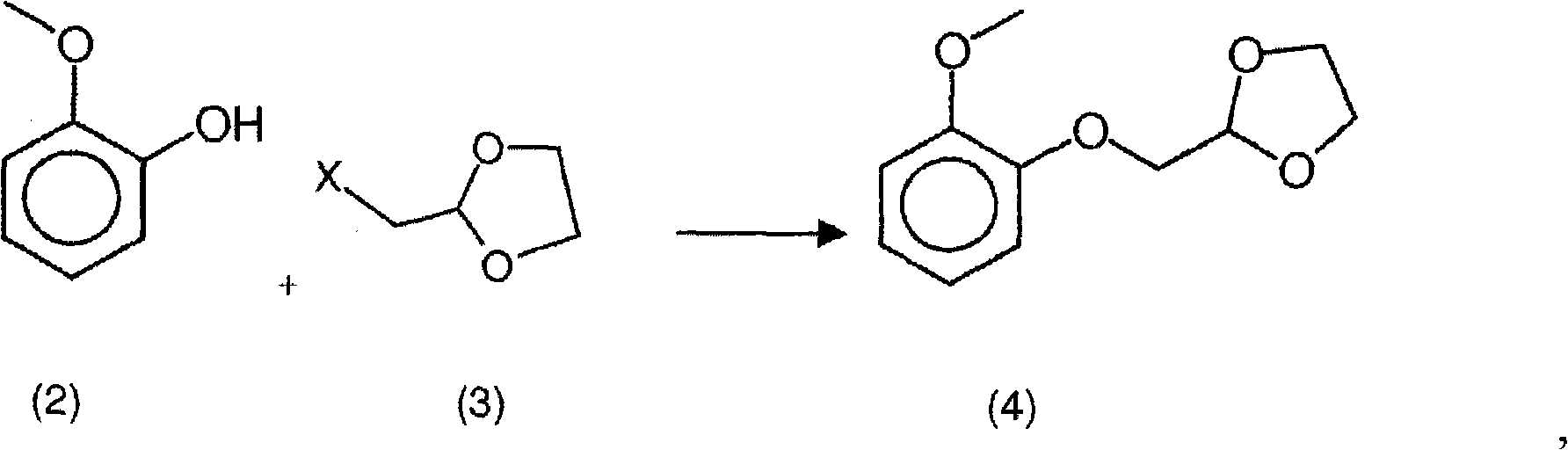
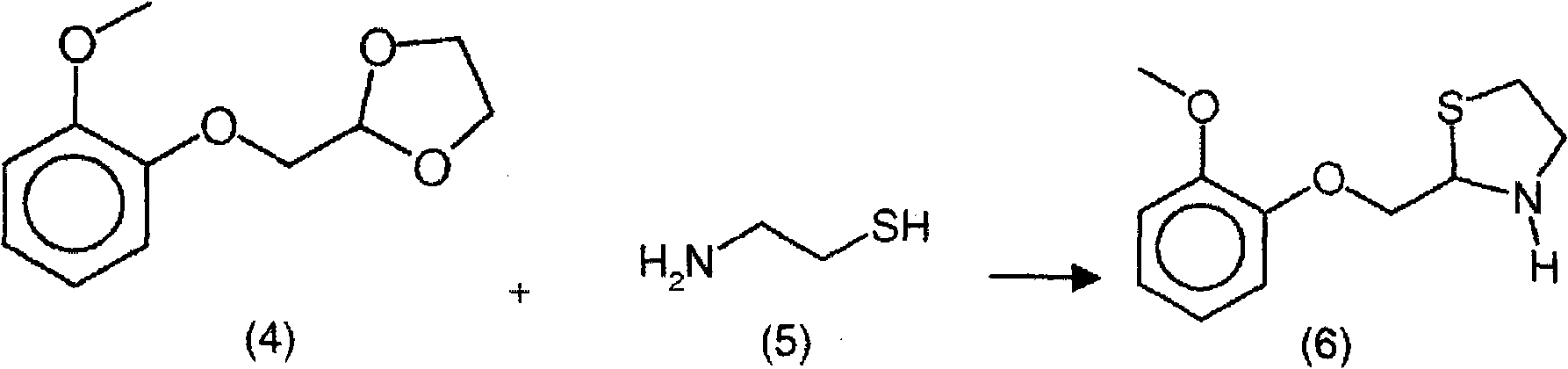
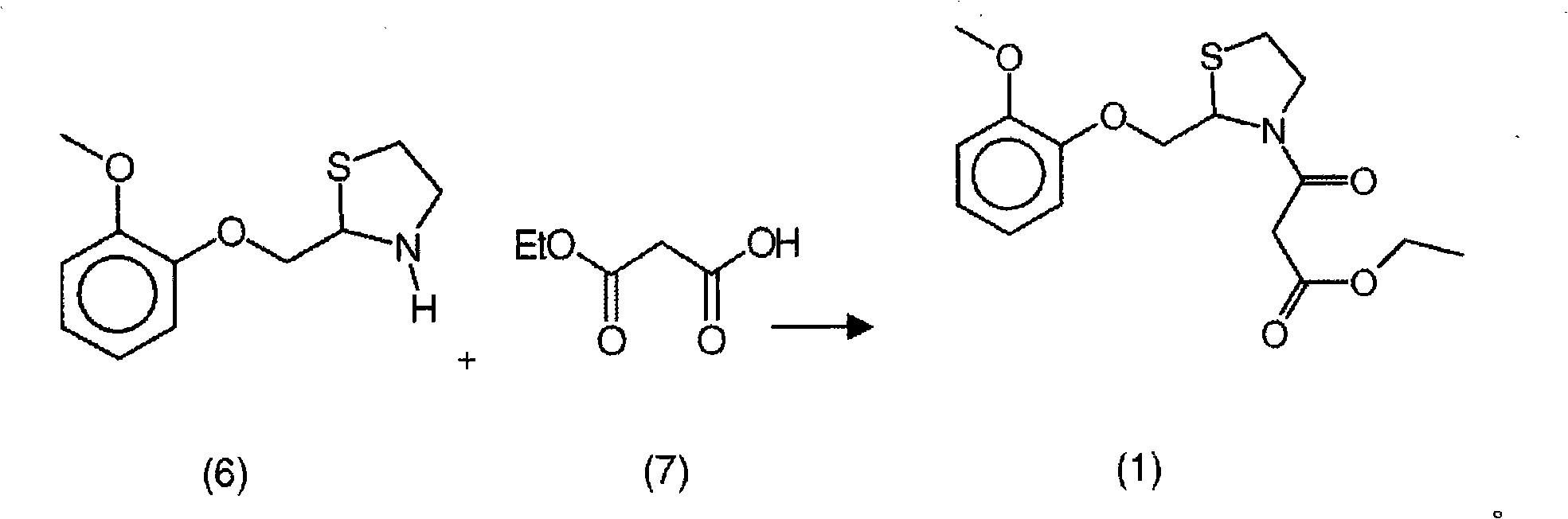
New process for the synthesis of moguisteine
The invention relates to a process for the synthesis of moguisteine that is ethyl ester of (R,S)-3-[2-[(2-methoxyphenoxy)methyl]-1,3-thiazolidin-3-yl]-3-oxypropanoic acid which comprises the steps of forming a new cyclic intermediate of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane (4), forming (R,S)-2-[(2-methoxyphenoxy)methyl]-1,3-thiazolidine (6) and reacting this latter with monoethylmalonic acid (7) or a salt thereof. The moguisteine of the invention is obtained in high yield and purity.
Owner:DOMPE FARM SPA
Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof
ActiveCN113278021AImprove responseEasy to controlOrganic compound preparationCarboxylic acid salt preparationNonaneMannich reaction
The invention relates to the field of synthesis of medical intermediates, and particularly discloses tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof. The preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate comprises the following steps: by taking N-tert-butyloxycarbonyl-4-piperidone, ethyl hydrogen malonate and ammonium acetate as initial raw materials, sequentially carrying out Mannich reaction, reduction reaction, halogenation, cyclization reaction and purification to obtain purified tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate. The preparation method disclosed by the invention is simple to operate and suitable for industrial mass production.
Owner:TIANJIN QUANHECHENG TECH
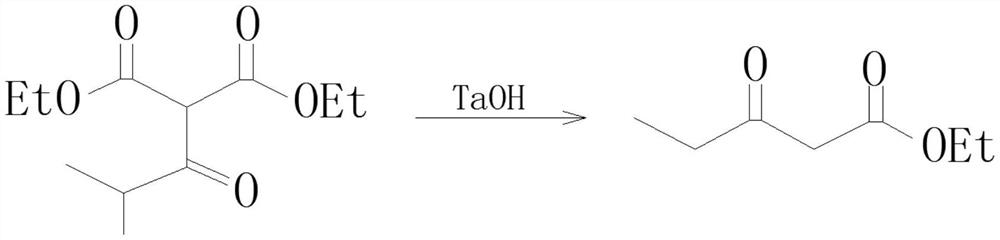
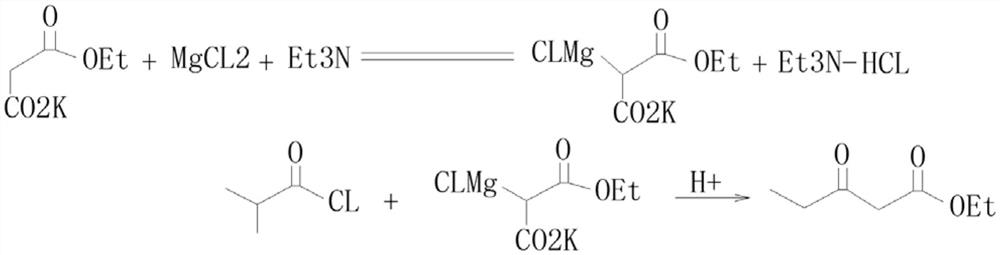
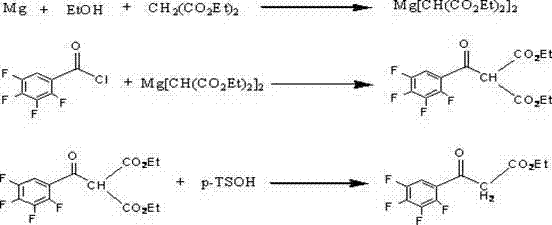
Preparation process of isobutyryl ethyl acetate
InactiveCN112358396AAdvantages of preparation processSolve the problem of low productivityOrganic compound preparationCarboxylic acid esters preparationSodium bicarbonateDistillation
The invention provides an isobutyryl ethyl acetate preparation process which is characterized by comprising the following specific steps: step one, adding 125 mL of ethyl acetate and 13.6 g (80 mmol)of potassium mono-ethyl malonate into a three-neck flask, stirring and cooling to 0-5 DEG C, then sequentially adding 9.12 g (96 mmol) of anhydrous magnesium chloride and 27.8 mL (0.2 mol) of triethylamine, heating to 65 DEG C within 0.5 h, and stirring for 6 hours at the temperature of 65 DEG C; step two, cooling to 0 DEG C, dropwise adding 6 mL (57 mmol) of isobutyryl chloride within one hour, and carrying out reactions at the room temperature for 12 hours; step three, cooling to 0 DEG C, carefully adding 70 mL of 13% hydrochloric acid, keeping the temperature not higher than 20 DEG C in theprocess; step four, separating out an organic phase, extracting a water layer with toluene (40 mL*3), merging the organic phase, washing with a saturated sodium bicarbonate solution until the organicphase is neutral, and washing with 25 mL of saturated edible salt water, and carrying out reduced pressure distillation to remove the solvent; and step five, carrying out reduced pressure distillation on the crude product to obtain 5.5 g of colorless liquid. The method provided by the invention solves the problems of low productivity and low purity during production and manufacturing of isobutyryl ethyl acetate at present.
Owner:门希国
New process for the synthesis of moguisteine
The invention relates to a process for the synthesis of moguisteine that is ethyl ester of (R,S)-3-[2-[(2-methoxyphenoxy)methyl]-1,3-thiazolidin-3-yl]-3-oxypropanoic acid which comprises the steps of forming a new cyclic intermediate of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane (4), forming (R,S)-2-[(2-methoxyphenoxy)methyl]-1,3-thiazolidine (6) and reacting this latter with monoethylmalonic acid (7) or a salt thereof. The moguisteine of the invention is obtained in high yield and purity.
Owner:DOMPE FARM SPA
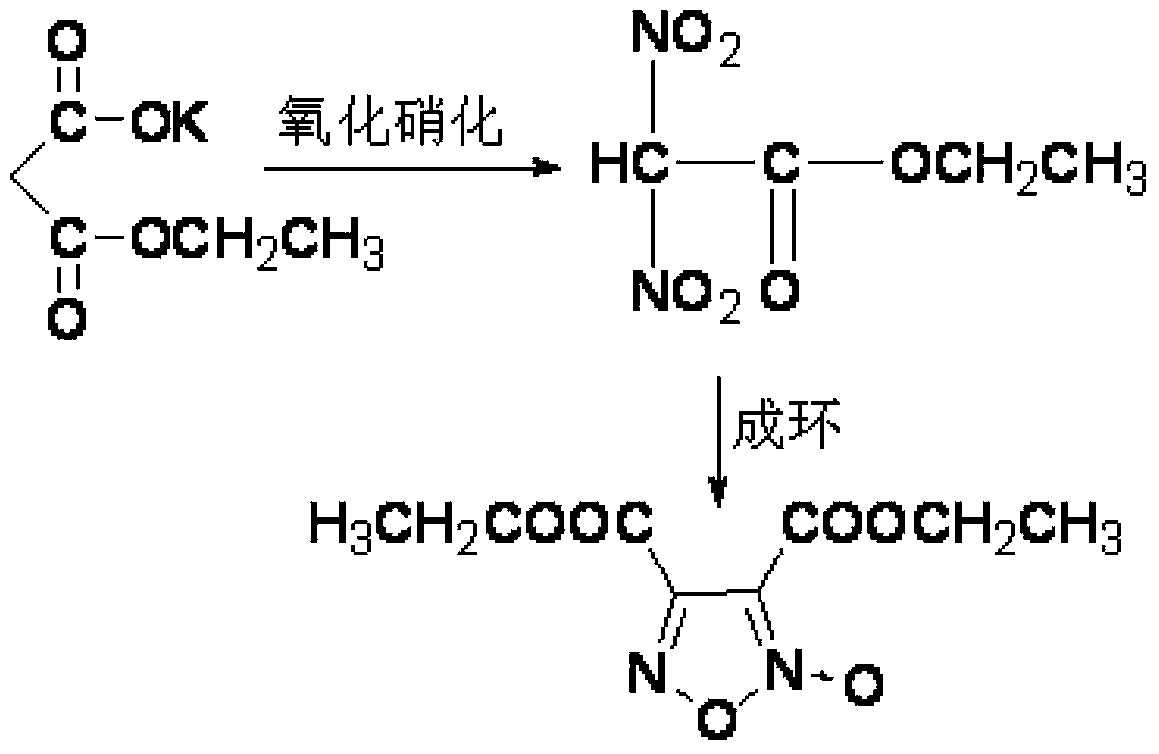
Synthesis method of 3,4-dicarboxylic acid diethyl ester furoxan
InactiveCN103351356BRaw materials are easy to obtainLow priceOrganic chemistrySynthesis methodsNitration
The invention relates to a furoxan ring, and provides a synthesis method of 3, 4-dicarboxylic acid diethyl ester furoxan. The method adopts a monoethyl malonate potassium salt as a raw material and takes sodium nitrate / nitrite as an oxidizing agent. The substances are subjected to oxidation nitration and a ring formation reaction in a carbon tetrachloride solution, thus obtaining the target compound 3, 4-dicarboxylic acid diethyl ester furoxan with purity up to 98.7% and yield up to 94.2%. The synthesis method provided in the invention has the advantages of high purity and high yield.
Owner:XIAN MODERN CHEM RES INST
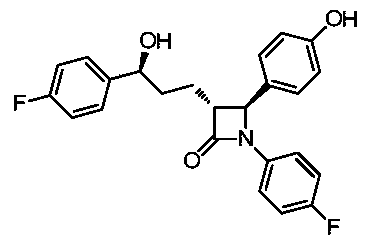
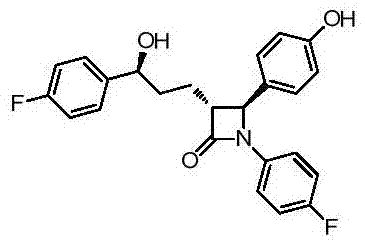
A kind of synthetic technique of ezetimibe intermediate
ActiveCN105439929BReduce typesReduce manufacturing costOrganic chemistryPhenyl groupTitanium tetrachloride
Owner:JIANGSU HANSYN PHARMA
Synthesis method and device for 4-hydroxyquinoline-3-formic acid
PendingCN113402459ALow priceShort reaction timeOrganic chemistryChemical/physical/physico-chemical processesBenzoic acidPropanoic acid
The invention relates to the technical field of medicine preparation, and particularly discloses a synthesis method and device for 4-hydroxyquinoline-3-formic acid. The method comprises the following steps: subjecting o-nitrobenzoic acid and potassium monoethyl malonate to a condensation reaction to obtain ethyl 3-(2-nitrophenyl)-3-oxo-propionate; subjecting iron powder to reacting with ethyl 3-(2-nitrophenyl)-3-oxo-propionate and conducting reducing to obtain ethyl 3-(2-aminophenyl)-3-oxo-propionate; carrying out a ring-closure reaction on ethyl 3-(2-aminophenyl)-3-oxo-propionate, DMF-DMA and DMF to obtain ethyl 4-hydroxyquinoline-3-formate; and subjecting ethyl 4-hydroxyquinoline-3-formate to hydrolysis under an alkaline condition so as to obtain 4-hydroxyquinoline-3-formic acid. In the synthesis process of the 4-hydroxyquinoline-3-formic acid, cost is low, reaction time is short, and post-treatment is relatively simple.
Owner:贵州中医药大学
Method for preparing ethyl cinnamate derivative
InactiveCN101121664BThe production process is simple to operateLow costOrganic compound preparationCarboxylic acid esters preparationChemical synthesisEthyl cinnamate
The invention relates to the field of chemical synthesis, particularly relating to a preparation method for the ethyl cinnamate derivatives. The preparation method of the invention is that the malonate diethylester reacts with the potassium hydroxide in the solution to produce the potassium salt of the malonate single-diethyl ester.The ice acetic acid is added to produce the malonate single-diethyl ester; the malonate single-diethyl ester then does the condensation reaction with the cinnamate with the catalysis of the amino acid. The preparation method of the invention is cheap and easy in the obtainment of the raw materials, simple in the operation, and low in the cost.
Owner:曾庆友 +1
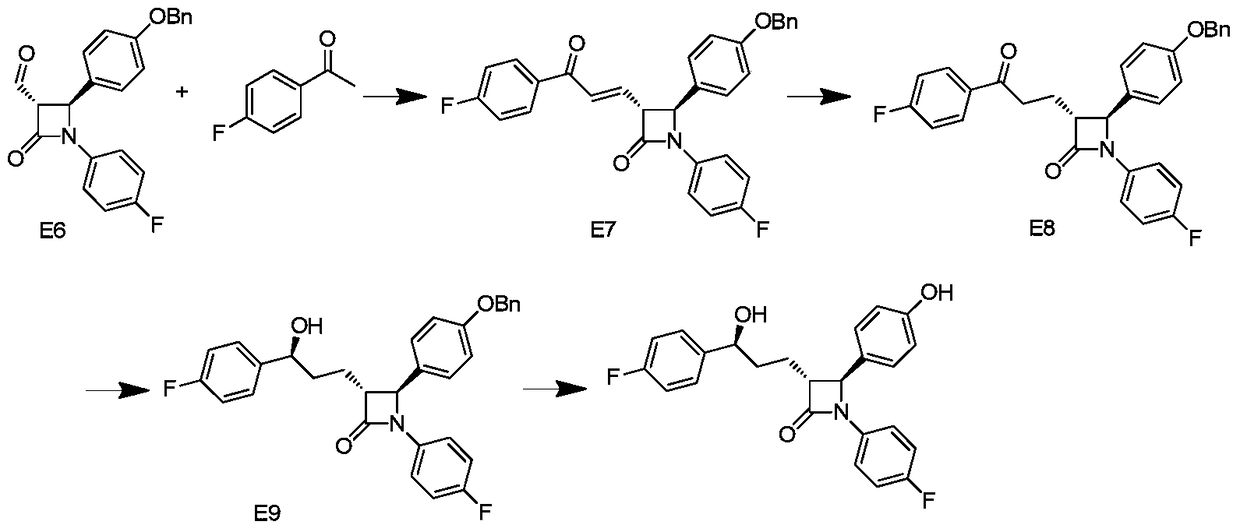
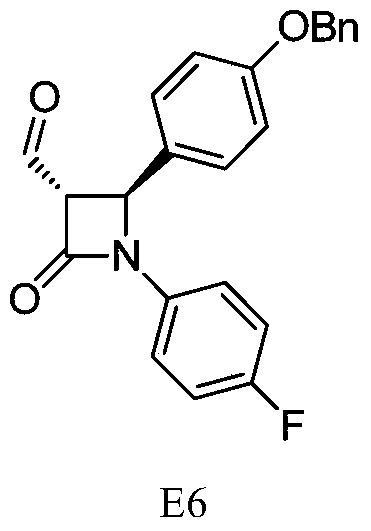
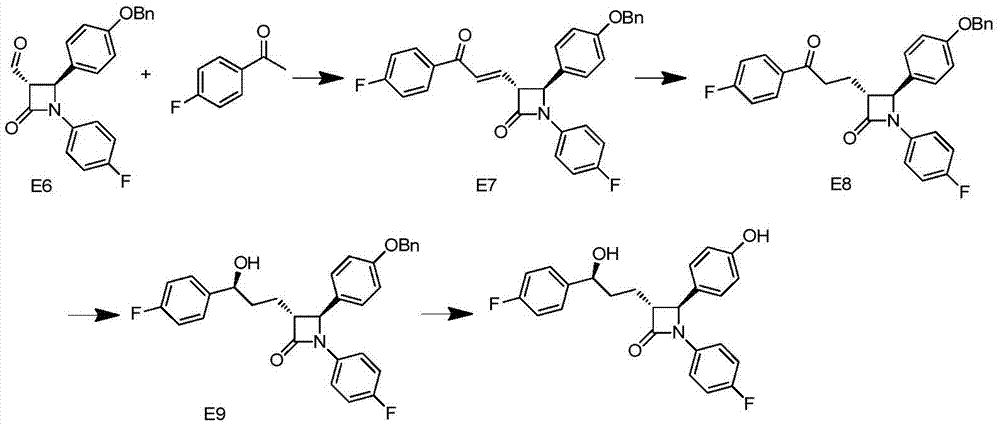
Synthesis process of ezetimibe intermediate
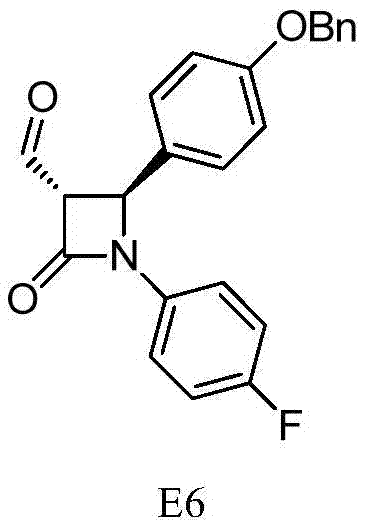
The invention discloses a synthesis process of an ezetimibe intermediate which is (2S,3S)-2-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-4-oxoazetidine-3-carboxaldehyde (an intermediate E6); (s)-4-phenyl-2-oxazolone and ethyl malonyl chloride as raw materials are subjected to condensation through condensation catalysis of TMSCl and TBAF to obtain an intermediate E3, the intermediate E3 and N-(4-fluorophenyl)-4-benzyloxybenzylidene amine are subjected to condensation under catalysis of titanium tetrachloride to obtain an intermediate E4, the intermediate E4 is subjected to cyclization under catalysis of BSA and FBAF to produce beta-lactam intermediate E5, and then the intermediate E5 is reduced by DIBALH into the aldehyde intermediate E6. The raw materials adopted by the process are cheap and easy to obtain, a solvent is single, the reaction time is short, the production cost is low, the yield is high, the operation of production units is simple, and the synthesis process is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
The preparation method of clobazam
ActiveCN106749052BAvoid it happening againHigh purityOrganic chemistryLithium hydroxidePotassium hydroxide
The invention provides a preparation method of clobazam. The preparation method comprises the following steps: (1), using 2-nitro-5-chlorodiphenylamine and ethyl malonyl chloride as raw materials, performing a reflow reaction in an organic solvent, after the reaction, decompressing the organic solvent in the reaction system, then performing evaporation to dryness, and adding a refining solvent for refining treatment so as to obtain a compound as shown in a formula II; (2), performing zinc powder reduction on the compound as shown in the formula II, and performing ammonolysis cyclization so as to obtain a compound as shown in a formula III; (3) enabling the compound as shown in the formula III to react with methyl iodide in an alkaline alcohol solution so as to obtain the clobazam, wherein alkali is one or more of sodium hydroxide, lithium hydroxide and potassium hydroxide. The clobazam prepared by the method is few in impurities and high in purity.
Owner:济南科汇医药科技有限公司
The preparation method of 4-hydroxyquinoline-3-formic acid
ActiveCN106187887BRaw materials are easy to getSimple processOrganic chemistryOrganic synthesisCarboxylic salt
The invention relates to the technical field of organic synthesis and bulk drug intermediates, and concretely relates to a preparation method of a key intermediate 4-hydroxyquinoline-3-carboxylic acid of a new medicine ivacaftor for treating cystic fibrosis. The preparation method comprises the following steps: 1, carrying out a condensation reaction: reacting o-nitrobenzoic acid, potassium monoethyl malonate and N,N-carbonyldiimidazole to prepare ethyl 3-(2-nitrophenyl)-3-oxopropanoate; 2, carrying out a reduction reaction: carrying out catalytic hydrogenation reduction on ethyl 3-(2-nitrophenyl)-3-oxopropanoate to prepare ethyl 3-(2-aminophenyl)-3-oxopropanoate; 3, carrying out a cyclization reaction: carrying out nucleophilic addition and cyclization reaction on ethyl 3-(2-aminophenyl)-3-oxopropanoate and N,N-dimethyl formamide dimethyl acctel to obtain ethyl 4-hydroxyquinoline-3-carboxylate; and 4, carrying out a hydrolysis reaction: carrying out the hydrolysis reaction on ethyl 4-hydroxyquinoline-3-carboxylate to obtain 4-hydroxyquinoline-3-carboxylic acid. The preparation method has the advantages of easily available raw materials, mild reaction conditions, simplicity and convenience in post-treatment, suitableness for amplified preparation, and high yield.
Owner:WUDI REACTION PHARMA & CHEM
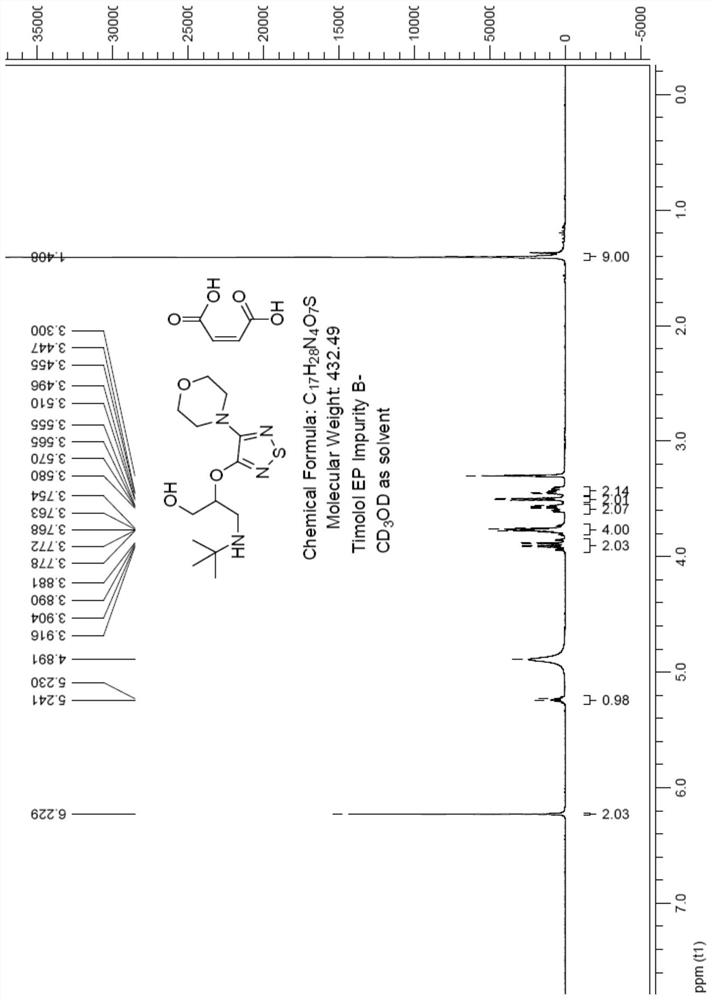
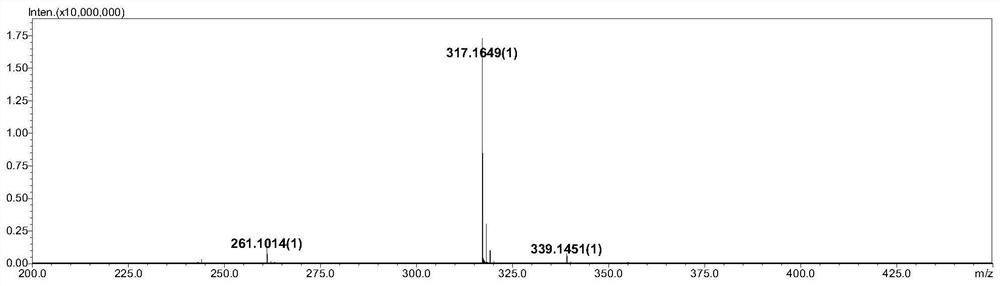
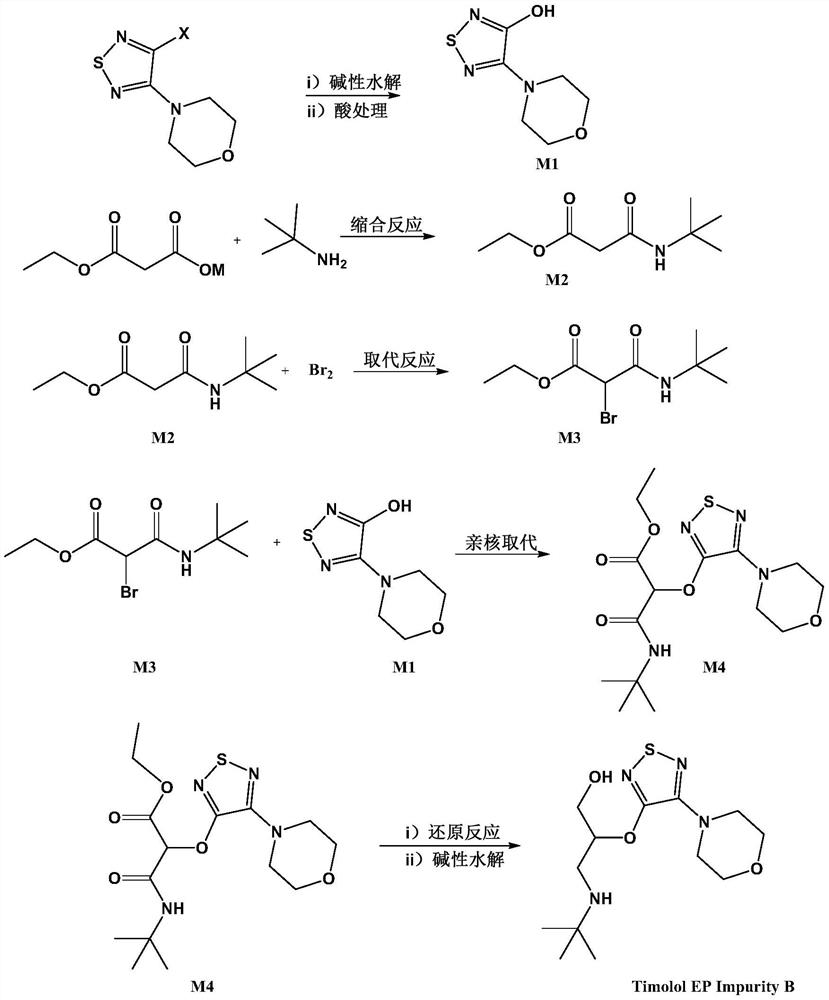
Preparation method of timolol impurity
The invention discloses a timolol impurity preparation method, and belongs to the technical field of timolol impurity synthesis. 3-halo-4-morpholinyl-1,2,5-thiadiazole is used as an initial raw material to prepare an intermediate M1 through alkaline hydrolysis and acid treatment, potassium ethyl malonate and tert-butyl amine are subjected to a condensation reaction to prepare an intermediate M2, the intermediate M2 and liquid bromine are subjected to a substitution reaction to prepare an intermediate M3, the intermediate M3 and the intermediate M1 are subjected to a nucleophilic substitution reaction to prepare an intermediate M4, and the intermediate M4 is subjected to a reduction reaction and alkaline hydrolysis to prepare a target product. The method has the characteristics of reasonable synthetic route, easily available raw materials, simple and easy operation, high yield and high purity. The prepared timolol can be used as a reference substance for qualitative and quantitative research of impurities in timolol quality research, the content of related substances in bulk drugs is controlled, and the quality of the bulk drugs is guaranteed.
Owner:成都摩尔生物医药有限公司
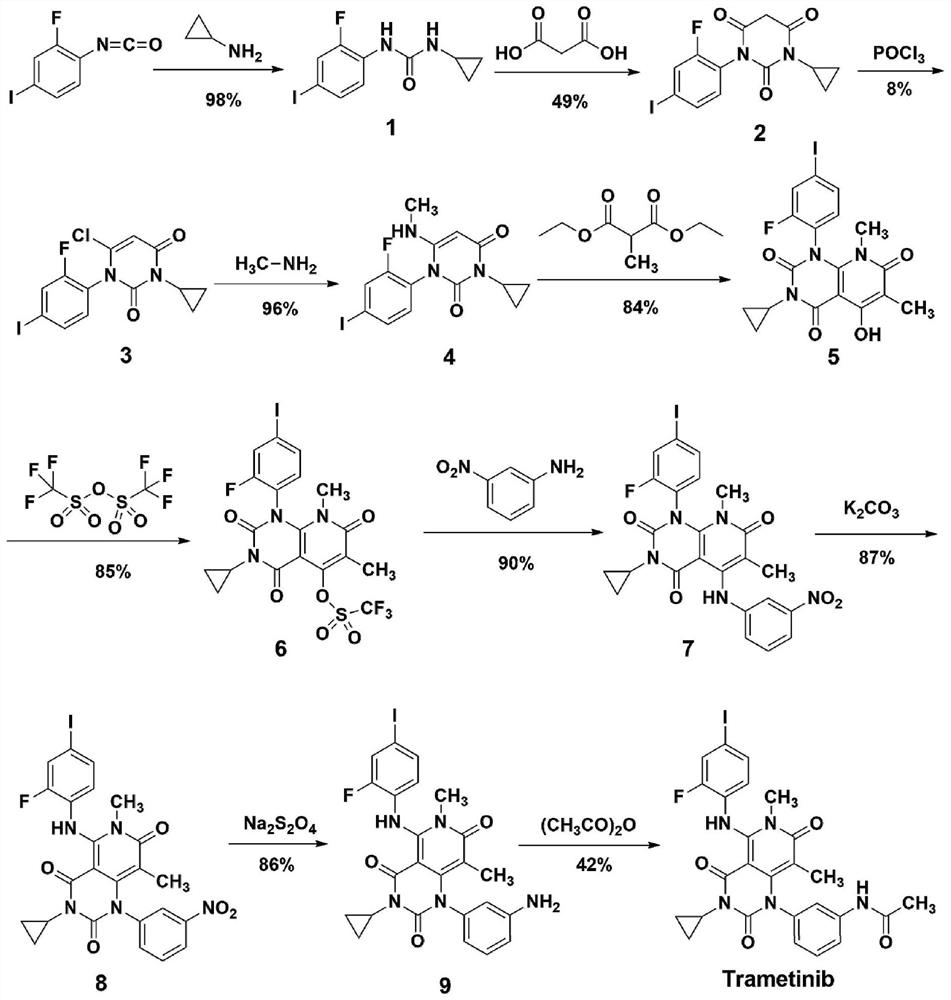
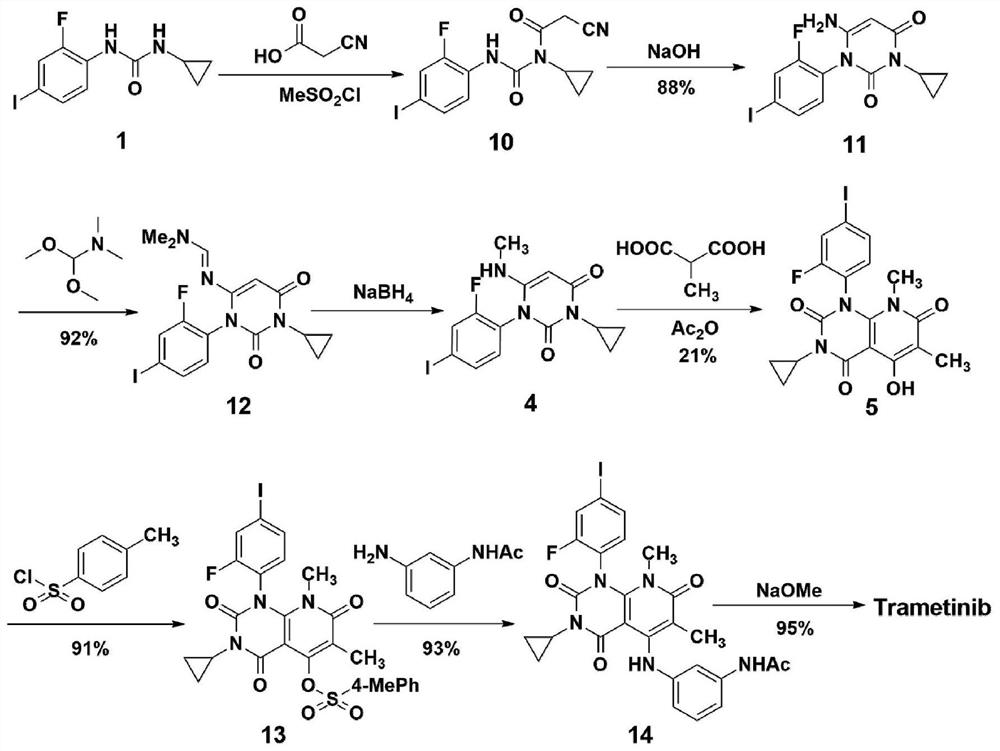
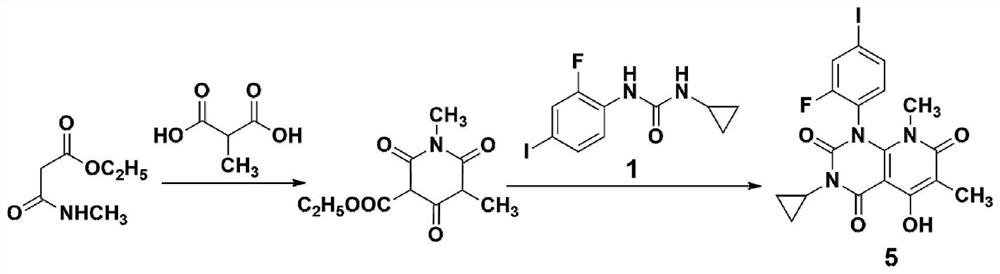
A method for synthesizing trametinib key intermediate
ActiveCN109336884BShort method stepsPotential for pilot scale-upOrganic chemistryMethylmalonic acidTrametinib
The invention discloses a method for synthesizing a key intermediate of trametinib, which uses monoformamide monoethyl malonate and methylmalonic acid to complete the cyclization reaction to obtain a crude pyridinetrione compound; the obtained pyridinetrione The crude compound is directly cyclized with N-(2-fluoro-4-iodophenyl)-N'-cyclopropylurea to obtain the key intermediate of trametinib. The present invention adopts monoethyl malonate and methylmalonic acid to complete the cyclization reaction to obtain a pyridinetrione compound, which is directly cyclized with a urea compound without purification to obtain a key intermediate for synthesizing trametinib. The method steps are short, the total yield is 47.3%, and it has the potential of scale-up in pilot scale, which provides a new scheme for the synthesis of trametinib.
Owner:安庆奇创药业有限公司
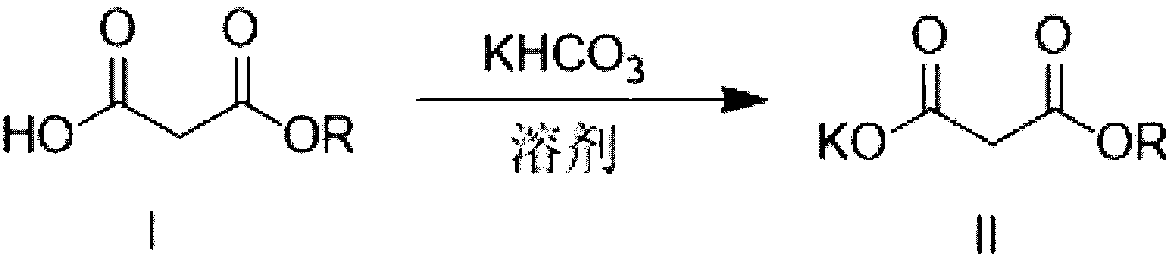
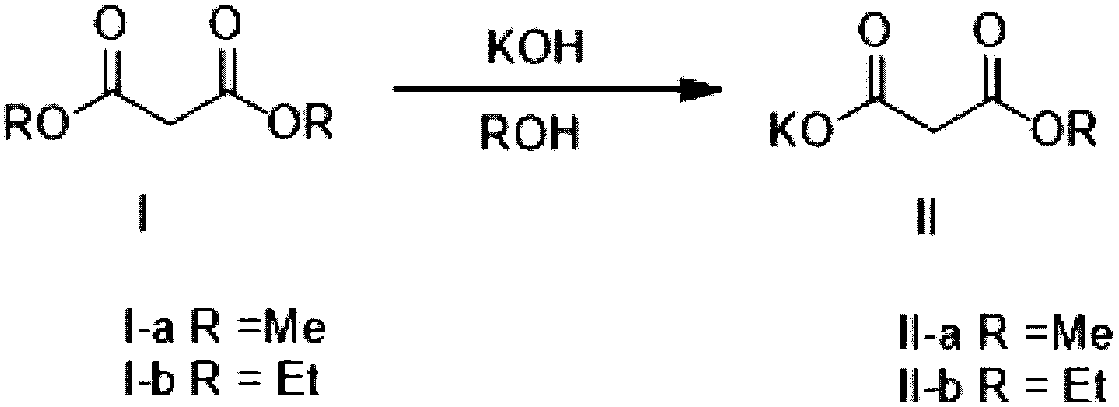
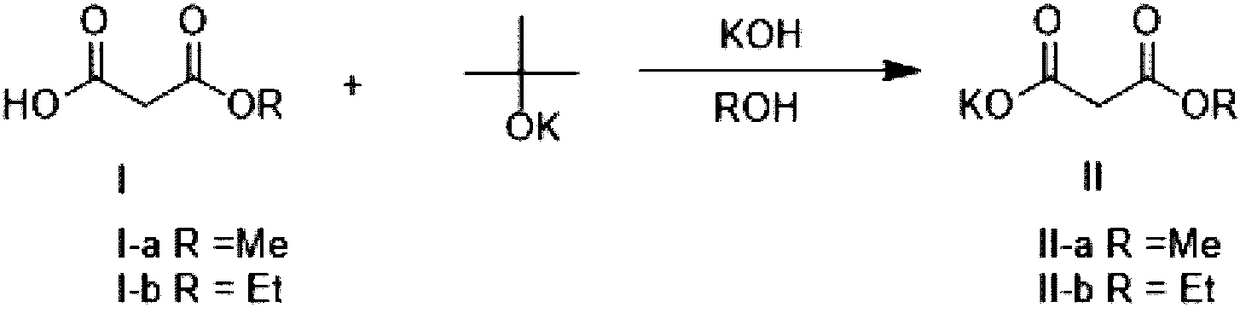
Method for preparing potassium monoethyl malonate
InactiveCN109354572AHigh purityImprove stabilityOrganic compound preparationCarboxylic acid esters preparationPotassium hydrocarbonateMonoethyl malonate
The invention provides a method for preparing potassium monoethyl malonate. According to the method, a compound in a malonate monoester series is used as a raw material to react with potassium bicarbonate to obtain the potassium monoethyl malonate. The invention opens up a new way to prepare potassium monoethyl malonate series under a very mild condition in a safe and environment-friendly mode, and the method is suitable for industrial production and meets the market demands of the compound.
Owner:成都海杰亚医药科技有限公司
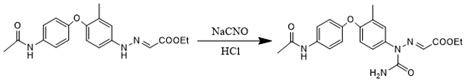
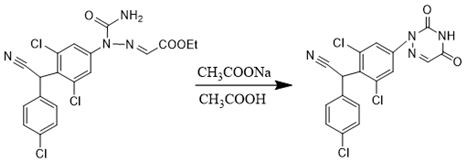
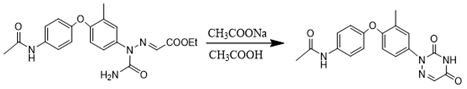
Preparation method of triazinone ring
A preparation method of a triazinone ring relates to the technical field of veterinary drugs, and comprises the following steps: carrying out a diazo condensation reaction on a condensation compound aniline and potassium ethyl malonate, carrying out an amidation reaction with sodium cyanate under an acidic condition, adding acetic acid and sodium acetate, heating, heating, and carrying out a cyclization reaction to obtain a triazinone compound. According to the method, operations such as filtration and separation are not needed before the finished product is obtained, the product loss is reduced, the method has the advantages of simple process and high yield, the yield of the salmizuril can reach 89.65%-94.81%, and the yield of the diclazuril can reach 80.20%-94.11%; and the problem of odor generation of decarboxylation reaction in the production process is also solved, and the method is beneficial to environmental protection and suitable for industrial production.
Owner:SHANDONG GUOBANG PHARMA +1
Synthetic method of royaljelly acid
The invention discloses a synthetic method of royaljelly acid, comprising steps of: firstly, performing a reaction between 8-alkanoyloxy octanal (4) and monoethyl malonate in the presence of DMAP in a solvent, and collecting (E)-10-alkanoyloxy-2-decenoic acid alkyl ester (5) from the reaction product; secondly, performing a saponification reaction of (E)-10-alkanoyloxy-2-decenoic acid alkyl ester(5) in an aqueous solution of alkaline materials, and collecting the target product royaljelly acid from the saponification product. According to the invention, the synthesis of royaljelly acid is designed again, avoiding problems of difficult product purification and low yield in traditional synthesis technology of the compound and greatly reducing production cost. Any other method can not accomplish the result in the invention. According to the invention, reagents used in the whole reaction are all easily available. The process route provided by the invention is of great innovation and convenient for industrial enforcement.
Owner:上海灏翔生物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
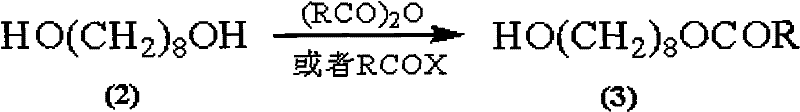
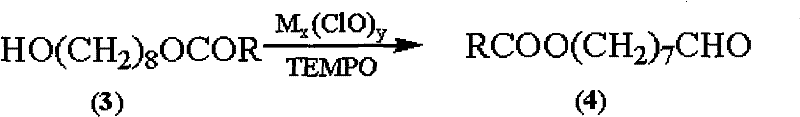

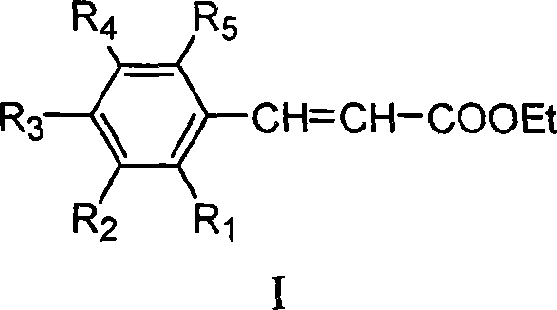
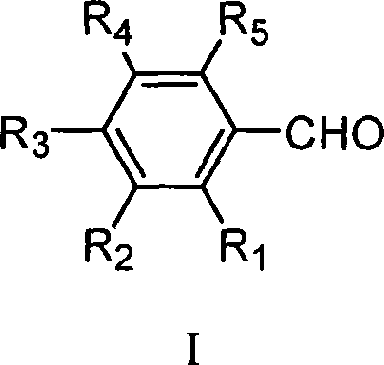
![Tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method Tert-butyl-7,9-dioxo-2,6-diazaspiro[4.5]decane-2-formate preparation method](https://images-eureka.patsnap.com/patent_img/2b60eb42-ee6d-4535-bfa9-adbcd291e04d/33093DEST_PATH_S.png)
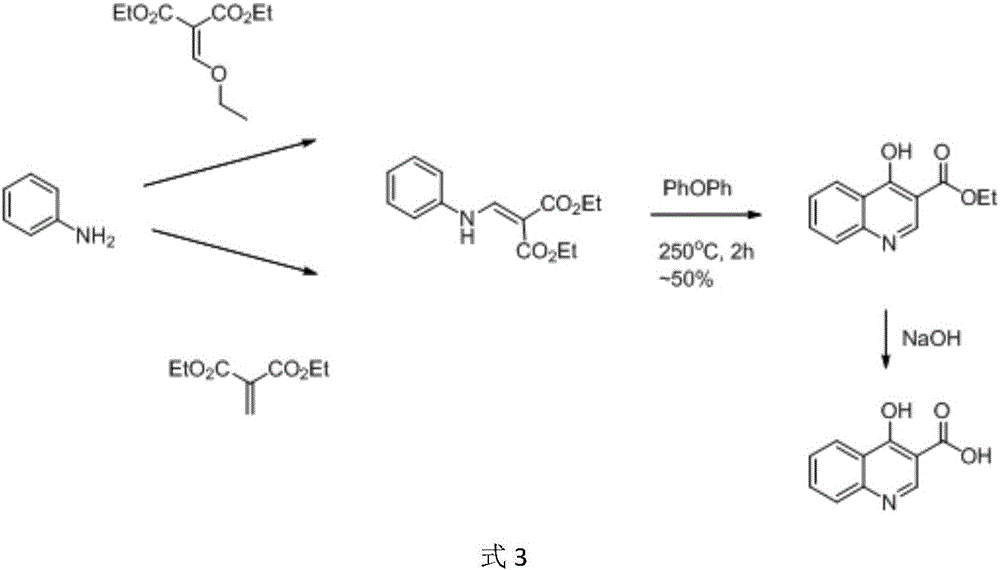
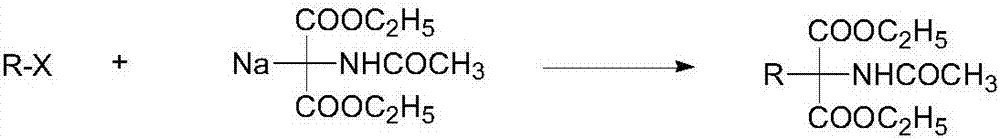
![Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof](https://images-eureka.patsnap.com/patent_img/4142969a-6a68-4d60-b7f6-8072e39bed50/HDA0003091136050000011.png)
![Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof](https://images-eureka.patsnap.com/patent_img/4142969a-6a68-4d60-b7f6-8072e39bed50/DEST_PATH_IMAGE006A.png)
![Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof Preparation method of tert-butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate and oxalate thereof](https://images-eureka.patsnap.com/patent_img/4142969a-6a68-4d60-b7f6-8072e39bed50/DEST_PATH_IMAGE007.png)