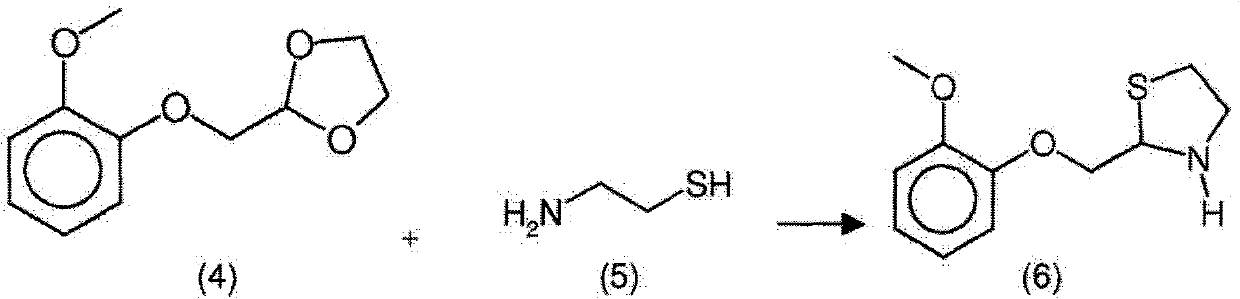
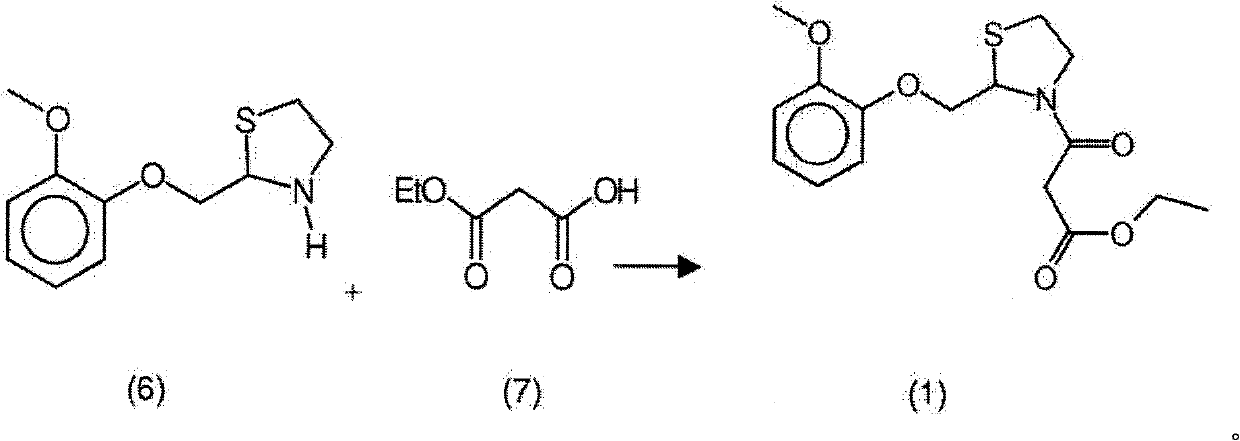
New process for the synthesis of moguisteine
A technology of methyl and dioxolane, applied in the new field of synthesizing mojisteine, can solve problems such as removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
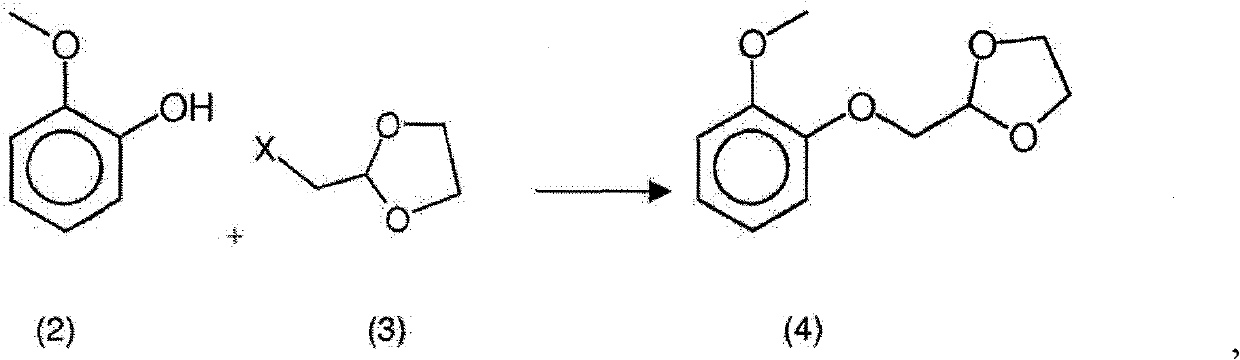
[0070] Step a) Preparation of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane in 1-methoxy-2-propanol Intermediate of ring (4) (4)
[0071] The raw materials were used in the amounts shown in Table 1 below.
[0072] Table 1: Substances and amounts of Example 1
[0073] Raw materials
Density
g
ml
Moore
The molar ratio of
1.129
297.6
263.6
2.40
1.00
1-methoxy-2-propanol
0.920
607.2
660.0
*
*
497.6
3.60
1.50
97% 2-bromomethyl-1,3-dioxide
Pentacyclic
1.628
475.2
291.9
2.76
1.15
50% KOH solution
1.516
84.2
55.5
0.75
0.31
Deionized water, first serving
1.00
660.0
660.0
*
*
Deionized water, second portion
1.00
1320.0
1320.0
*
*
Deionized water for washing
1.00
2x500
2x500
*
*
[0074] Under a nitrogen atmosphere, load potassium carbonate fine powder and 1-methoxy-2-propanol into a completely dry 6-liter flask and place the material under stirrin...
Embodiment 2
[0100] Step a) Preparation of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane in N-methylpyrrolidone Intermediate of ring (4) (4)
[0101] The following raw materials were used in the amounts shown in Table 2.
[0102] Table 2: Substances and amounts used in Example 2
[0103] Raw materials
Density
g
ml
Moore
The molar ratio of
1.129
99.2
87.9
0.80
1.00
N-methylpyrrolidone
1.028
514.0
500.0
*
*
221.14
1.60
2.00
97% 2-bromomethyl-1,3-dioxide
Pentacyclic
1.628
158.4
97.3
0.92
1.15
Deionized water for quenching
1.00
1400.0
1400.0
Toluene for extraction
0.865
692.0
800.0
Deionized water, first wash
1.00
800.0
800.0
30% NaOH solution
1.335
50.0
37.5
Deionized water, second wash
1.00
800.0
800.0
30% NaOH solution
1.335
50.0
37.5
Deionized water, third wash
1.00
800.0
800.0
30% NaOH solution
1.335
50.0
37.5
Carbon
5.0
Tolue...
Embodiment 3-31
[0114] Step a) Intermediate (4) of formula 2-[(2-methoxyphenoxy)methyl]-1,3-dioxolane (4) preparation
[0115] By following the same procedure as shown in Example 1, but using the amount, reaction solvent, time, and temperature shown in Table 3, the yield and purity shown in Table 4 below were used to obtain Intermediate (4).
[0116] Table 3: Reaction conditions of Example 3-31
[0117]
[0118] Example
[0119] Table 4: Yield and purity of intermediate (4) obtained in Example 3-31
[0120]
[0121] Therefore, in all the examples, intermediate (4) was obtained in high yield and with a purity of greater than 99%. According to the present invention, the intermediates of Examples 1, 28, 29 and 31 have the best appearance and quality.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com