Method for preparing benzoxazine intermediate containing triazine structure
A technology for benzoxazine and intermediates, which is applied in the field of preparation of benzoxazine intermediates, can solve the problems of inconvenience in mass synthesis, limited application and promotion, and high synthesis reaction temperature, and achieves advantages of environmental protection, rich performance and high performance. The effect of wide application and reaction temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In this embodiment, the preparation method of a benzoxazine intermediate containing a triazine structure, the process steps are as follows:
[0042] (1) Synthesis reaction
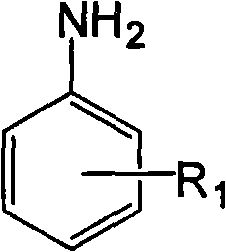
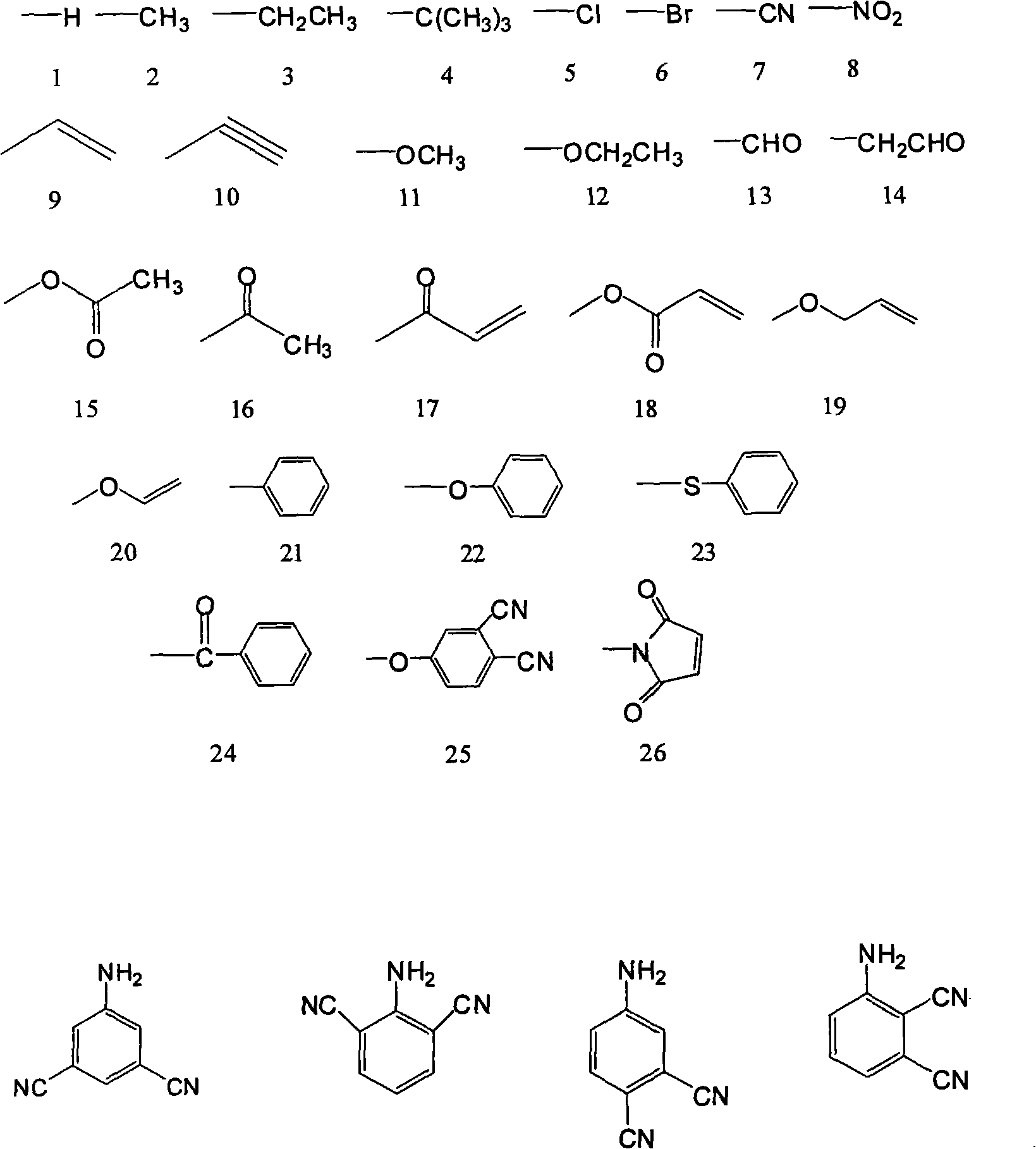
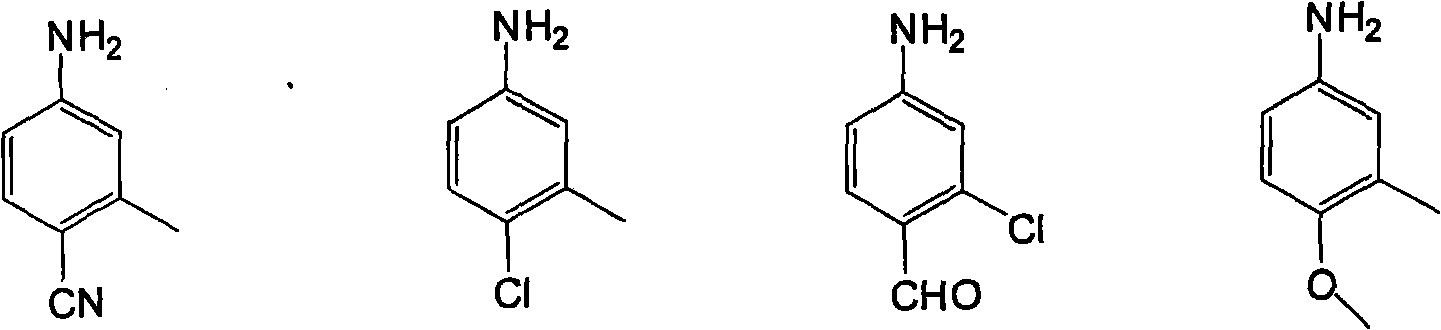
[0043] The raw materials are 150g (1.85mol) of formaldehyde solution with a mass concentration of 37%, 86.2g (0.92mol) of aniline, and the structural formula is compound 1, the R in compound 1 1 The group is the structural formula 1) on page 4 of the description,
[0044] First, at room temperature (15°C) and normal pressure, add 100ml of formaldehyde solution and ethanol into a reaction kettle with a stirrer and mix evenly to prepare a mixed solution with a mass concentration of aldehyde of 24%, and then mix the measured formaldehyde solution with stirring Add aniline dropwise to the reactor, control the rate of addition so that the temperature of the reaction system does not exceed 15°C, start timing after the addition is complete, and react for 2 hours under normal pressure and without heating; ...
Embodiment 2
[0050] In this embodiment, the preparation method of a benzoxazine intermediate containing a triazine structure, the process steps are as follows:
[0051] (1) Synthesis reaction
[0052] The raw material is 30g (1mol) of paraformaldehyde, 107.15g (1mol) of p-toluidine, the structural formula is compound 1, the R in compound 1 1 The group is the structural formula 2 on page 4 of the instruction manual, and the position on the benzene ring is a para-position substitution),
[0053] First, at room temperature (20°C) and normal pressure, add 200ml of measured paraformaldehyde and ethanol into a reaction kettle with a stirrer and mix evenly to prepare a mixed solution with an aldehyde mass concentration of 16%, and then mix the measured Add p-toluidine to the reaction kettle, control the feeding rate so that the temperature of the reaction system does not exceed 30°C, start timing after the feeding is completed, and react at normal pressure and 30°C for 1 hour;
[0054] (2) Recy...
Embodiment 3
[0059] In this embodiment, the preparation method of a benzoxazine intermediate containing a triazine structure, the process steps are as follows:
[0060] (1) Synthesis reaction
[0061] The raw material is 150g (1.85mol) of formaldehyde solution with a mass concentration of 37%, 89.67g (0.74mol) of o-ethylaniline, the structural formula is compound 1, and R in compound 1 1 The group is the structural formula 3 on page 4 of the instruction manual, and the position on the benzene ring is ortho-substituted),
[0062] First, at room temperature (30°C) and normal pressure, add 200ml of formaldehyde aqueous solution and methanol into a reaction kettle with agitator and mix evenly to prepare a mixed solution with an aldehyde mass concentration of 18%, and then mix the measured formaldehyde solution under stirring. Add o-ethylaniline to the reaction kettle, control the feeding rate so that the temperature of the reaction system does not exceed 40°C, start timing after the feeding i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com