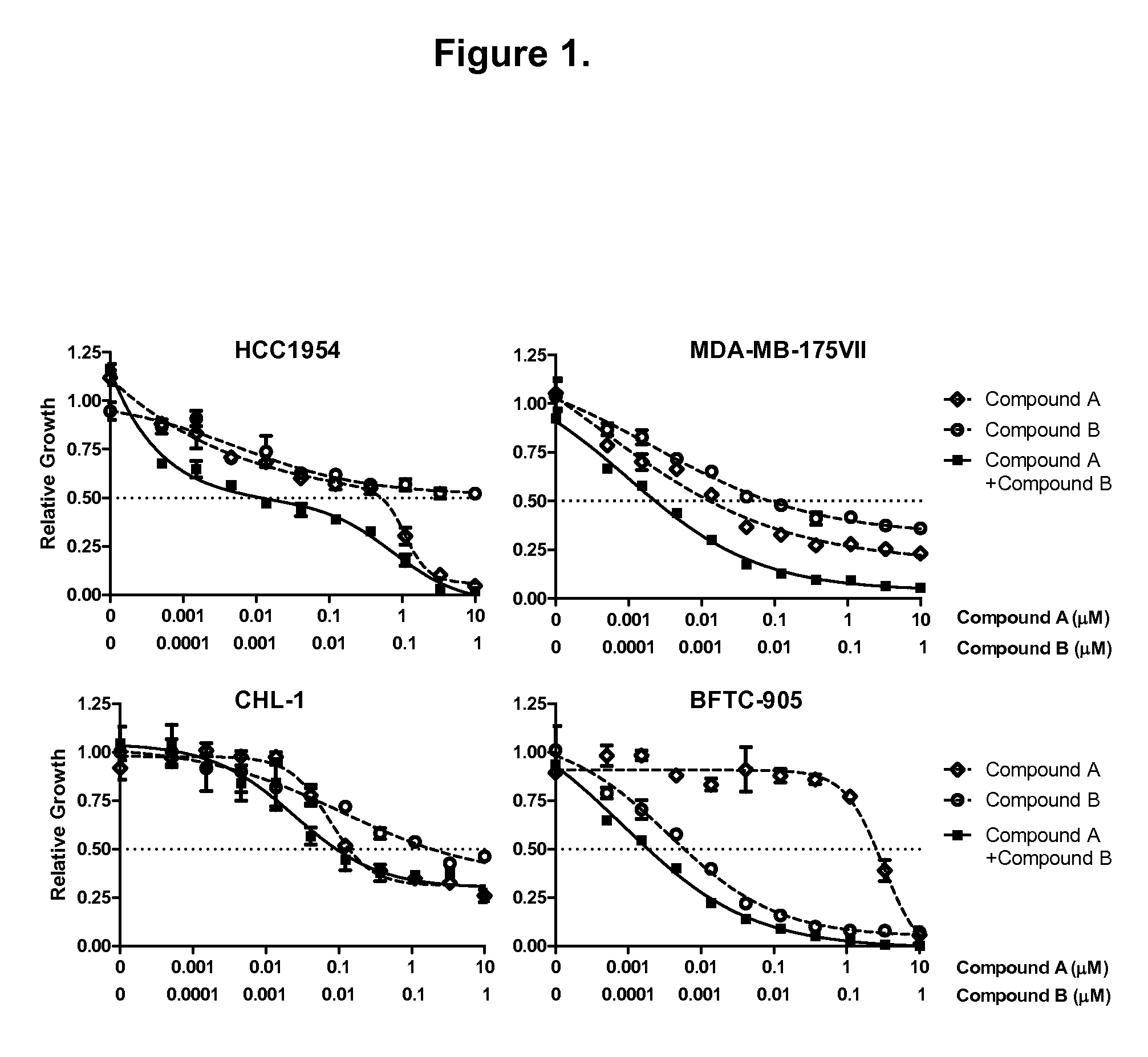
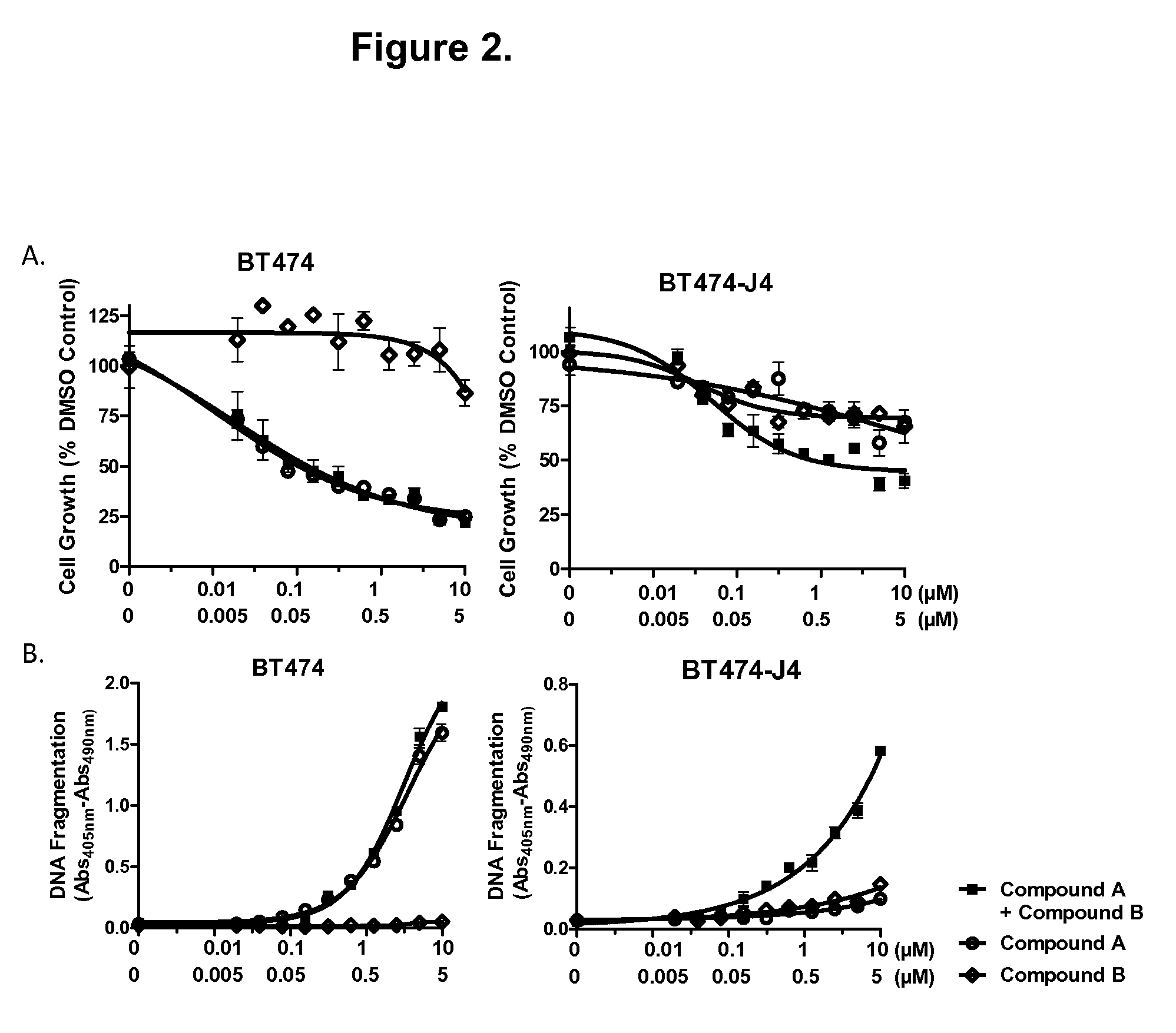
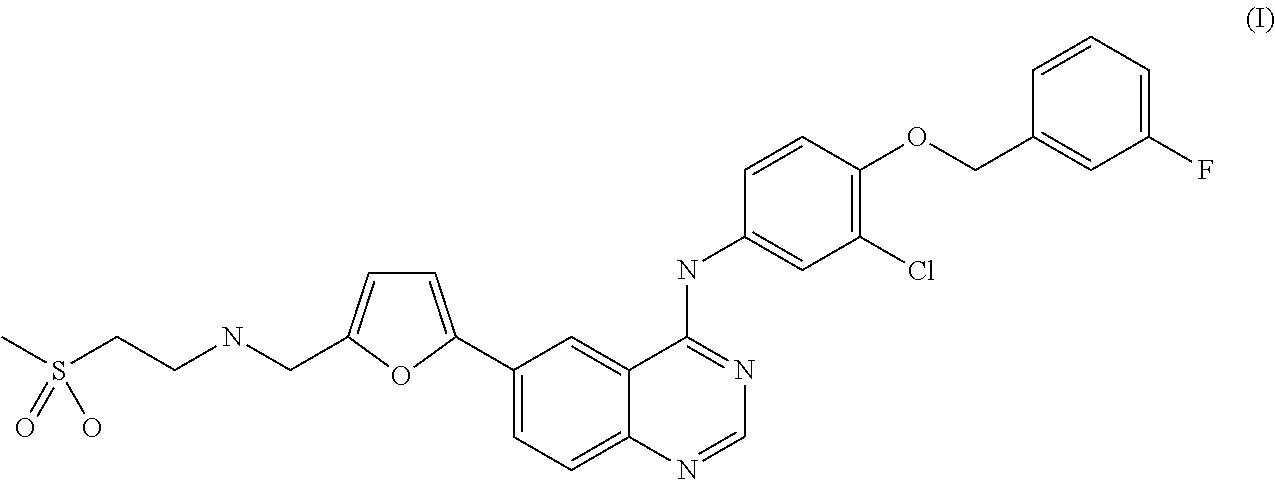
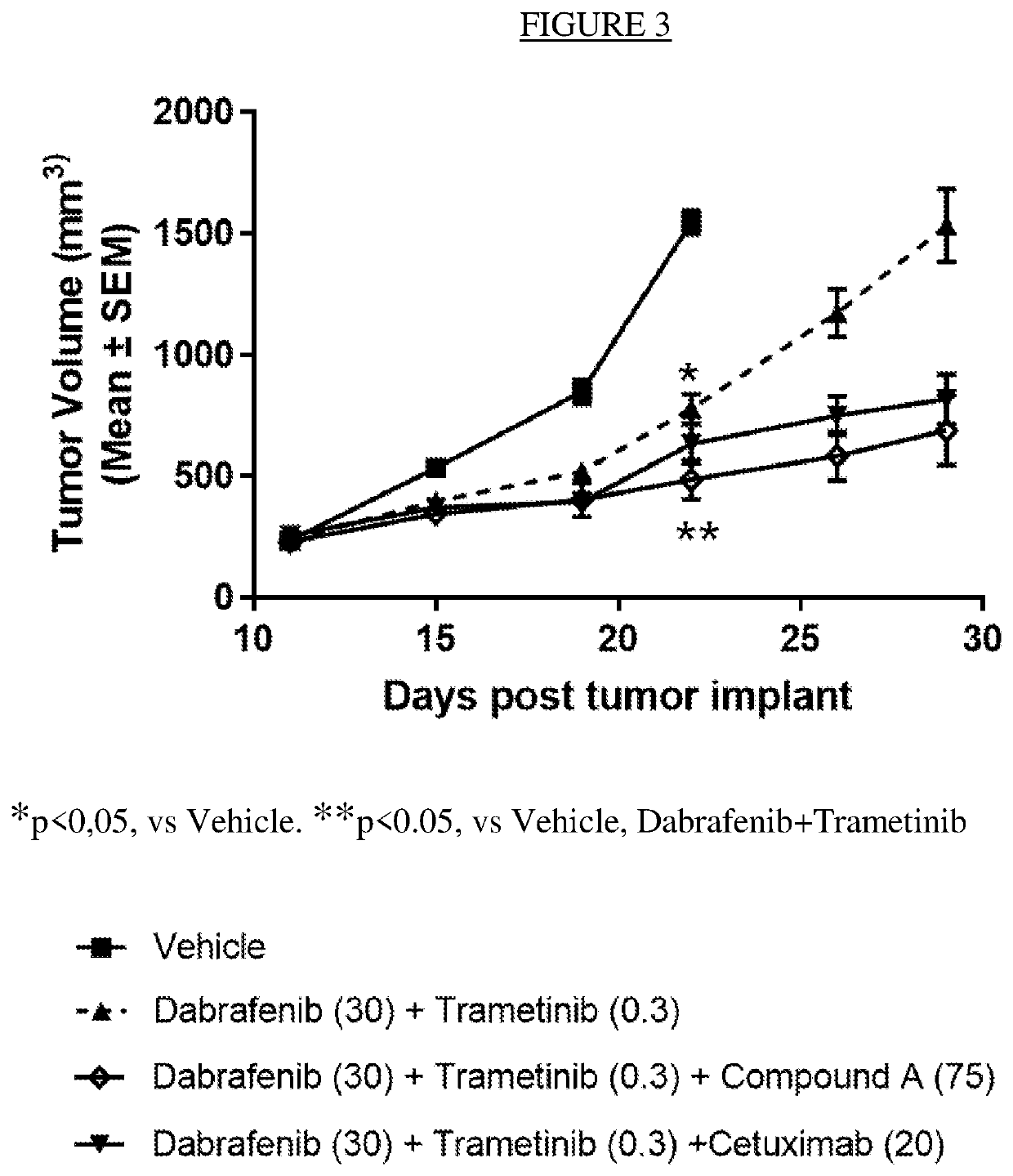
Patents
Literature
38 results about "Trametinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trametinib may be used alone or in combination with another medication (dabrafenib) to treat a type of skin cancer (melanoma). It is also used with dabrafenib to treat thyroid cancer and a type of lung cancer (non-small cell lung cancer-NSCLC).
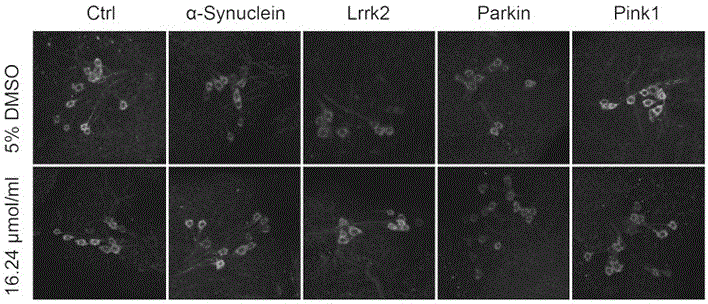
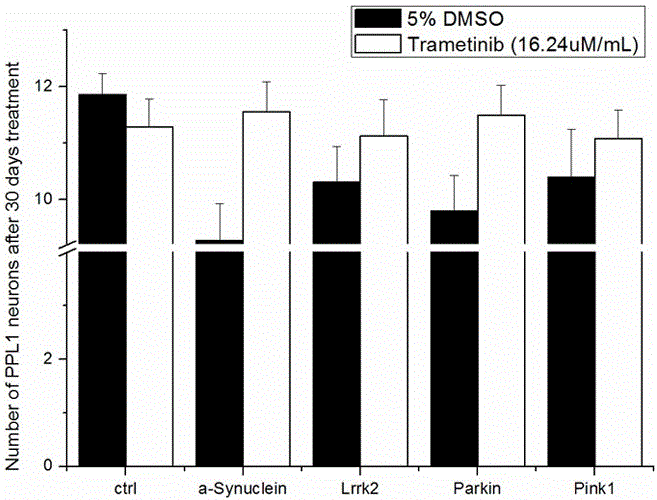
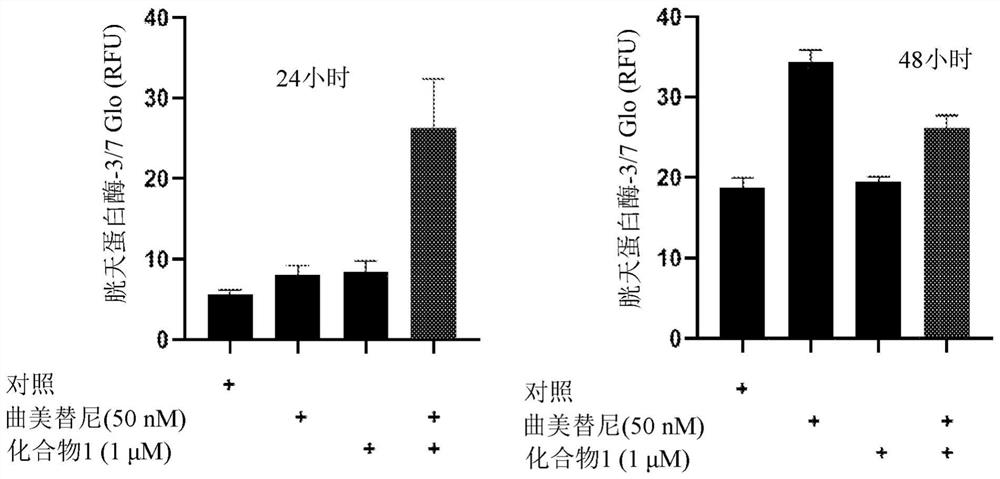
Application of trametinib in preparation of drug for treating Parkinson's disease
ActiveCN105816461ADelay degenerative deathGood curative effectOrganic active ingredientsNervous disorderCurative effectTherapeutic effect
The invention belongs to the technical field of medicine, and discloses application of trametinib in preparation of a drug for treating Parkinson's disease, wherein trametinib is used for preparing the drug to delay the degeneration of dopaminergic neuron. The obtained product can effectively delay the degenerative death of dopaminergic neuron, to treat Parkinson's disease fundamentally; the drug can be taken orally without injection to achieve the treatment effect, has excellent curative effect for Parkinson's disease caused by a variety of reasons, and has important significance for the treatment and curing of Parkinson's disease.
Owner:FUZHOU UNIV
Compounds for treatment of cancer
ActiveUS10022356B2Reduce severityReduce riskDermatological disorderAntineoplastic agentsMEK inhibitorBRAF inhibitor
The present invention relates to pharmaceutical compositions for treating cancer comprising BRAF inhibitors, (e.g. vemurafenib) and / or MEK inhibitor, (e.g. trametinib, RO5068760), in combination with anti-tubulin compounds of the invention or other known tubulin inhibitors, and using such compositions for treating cancer such as melanoma, drug-resistant cancer, and cancer metastasis.
Owner:UNIV OF TENNESSEE RES FOUND
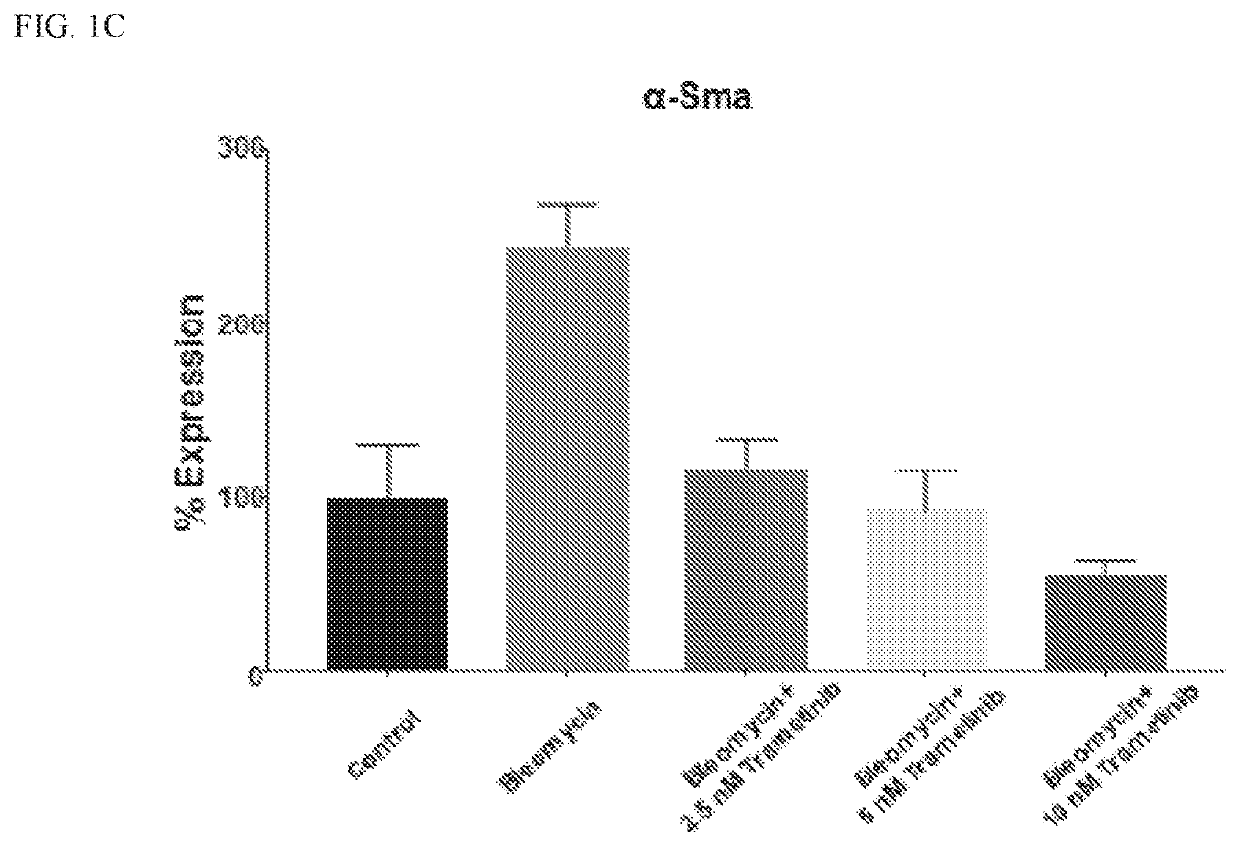
Application of trametinib in preparation of medicine for preventing and/or treating non-alcoholic hepatitis and/or non-alcoholic fatty liver disease
ActiveCN110876751AReduce fatReduced responseOrganic active ingredientsDigestive systemDiseaseAlcohol hepatitis
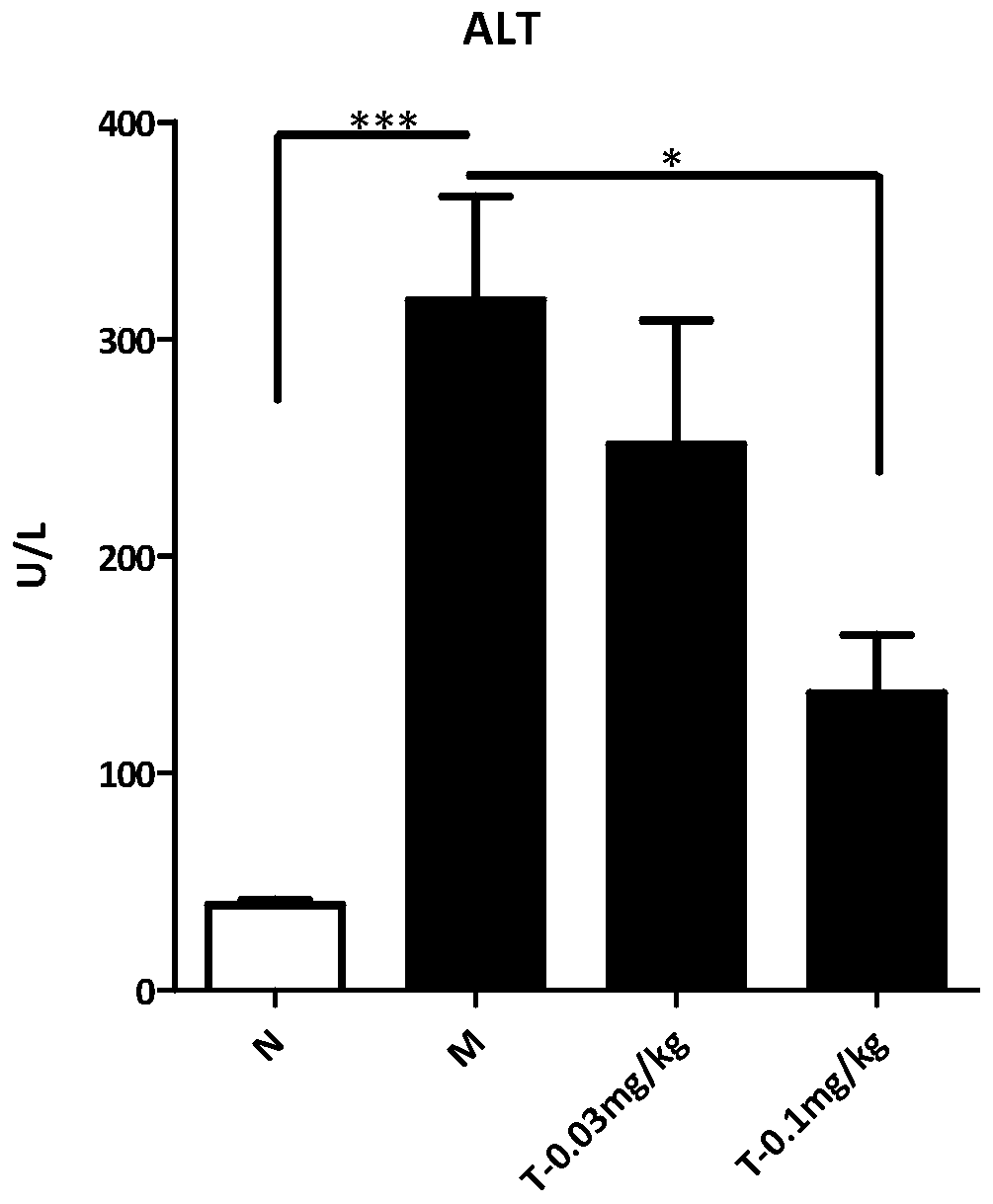
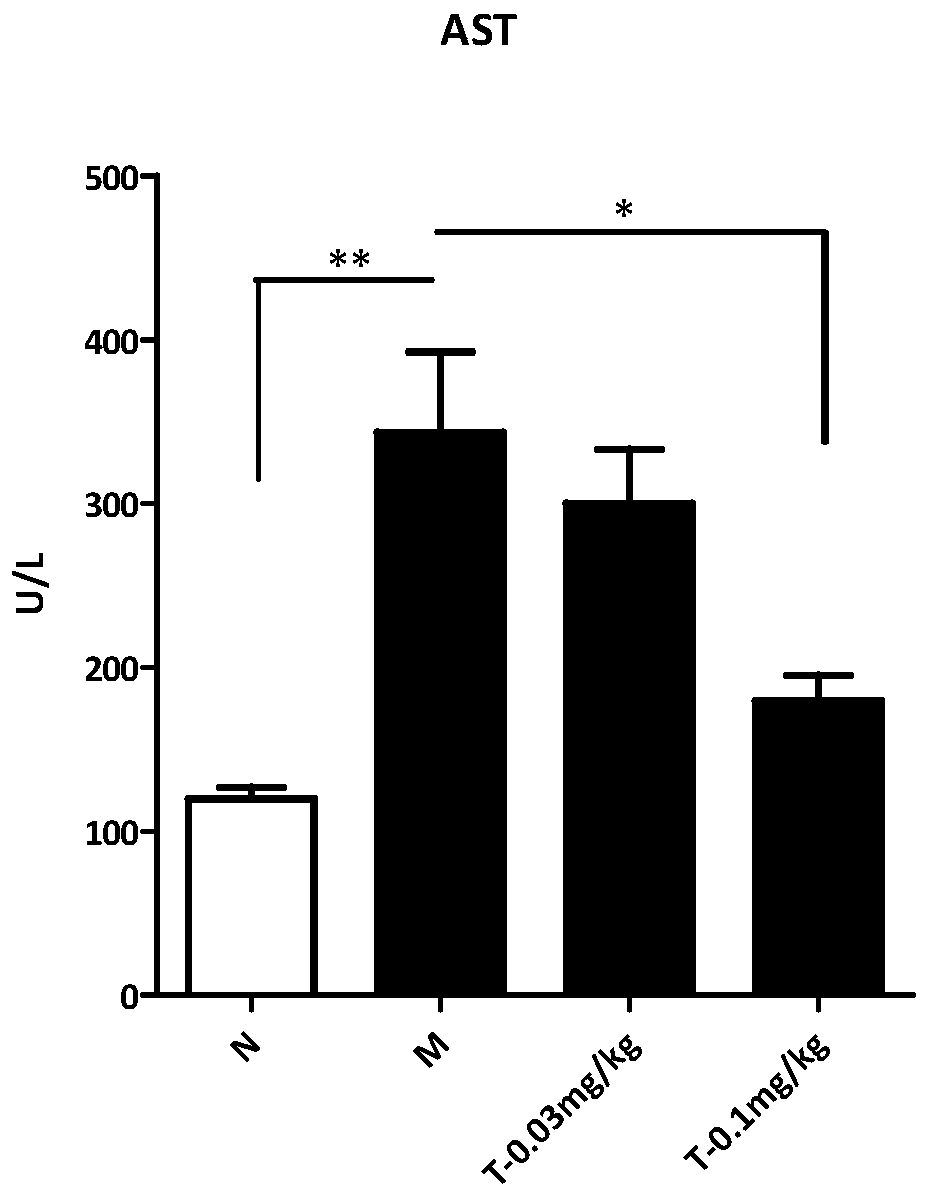
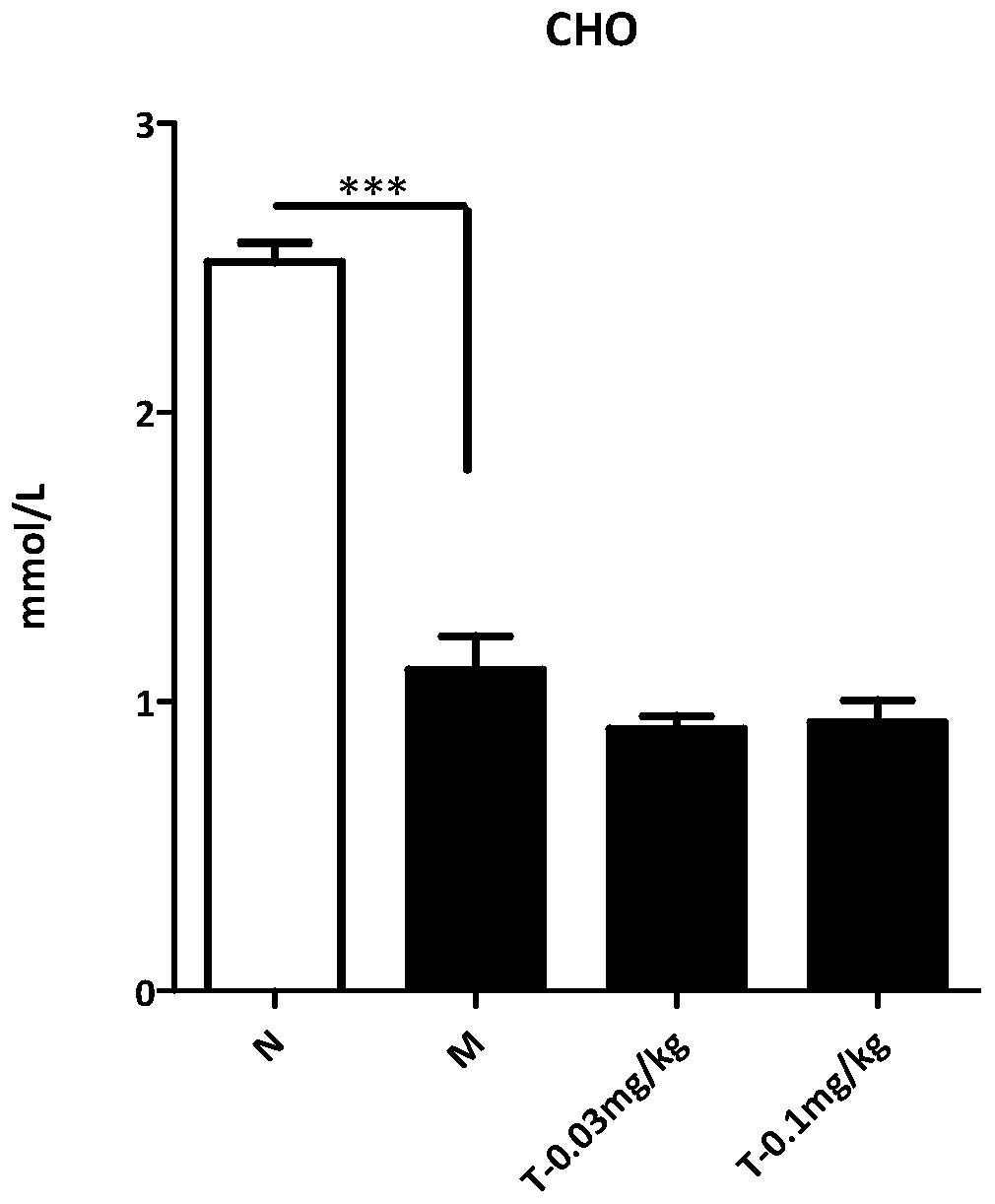
The invention provides an application of trametinib in preparation of a medicine for preventing and / or treating non-alcoholic hepatitis, and also provides an application of trametinib in preparation of a medicine for preventing and / or treating non-alcoholic fatty liver disease. The invention provides the application of trametinib in preparation of the medicine for preventing and / or treating non-alcoholic hepatitis and non-alcoholic fatty liver diseases. The results show that the trimeltinib can effectively reduce ALT, AST, CHO and TG values in an MCD model and reduce liver indexes, the dyeingsurface value of a trimeltinib treatment group is small, fat particles disappear, and inflammatory necrosis is realized; the trimeltinib can effectively reduce fat and inflammatory response in a highfat / fructose model, the dyeing surface value of the trimeltinib treatment group is small, fat granules are obviously reduced, and damage is small.
Owner:BEIJING GIGACEUTICALS TECH CO LTD
Method of Adjuvant Cancer Treatment
InactiveUS20170202842A1Improve survivalOrganic active ingredientsDermatological disorderAdjuvantHuman patient
The present invention provides a method of providing adjuvant treatment to a human patient which comprises administering to such a patient therapeutically effective doses of debrafenib and trametinib for a time period sufficient to increase relapse-free survival (RFS).
Owner:NOVARTIS AG
Method of blocking transmission of malarial parasite
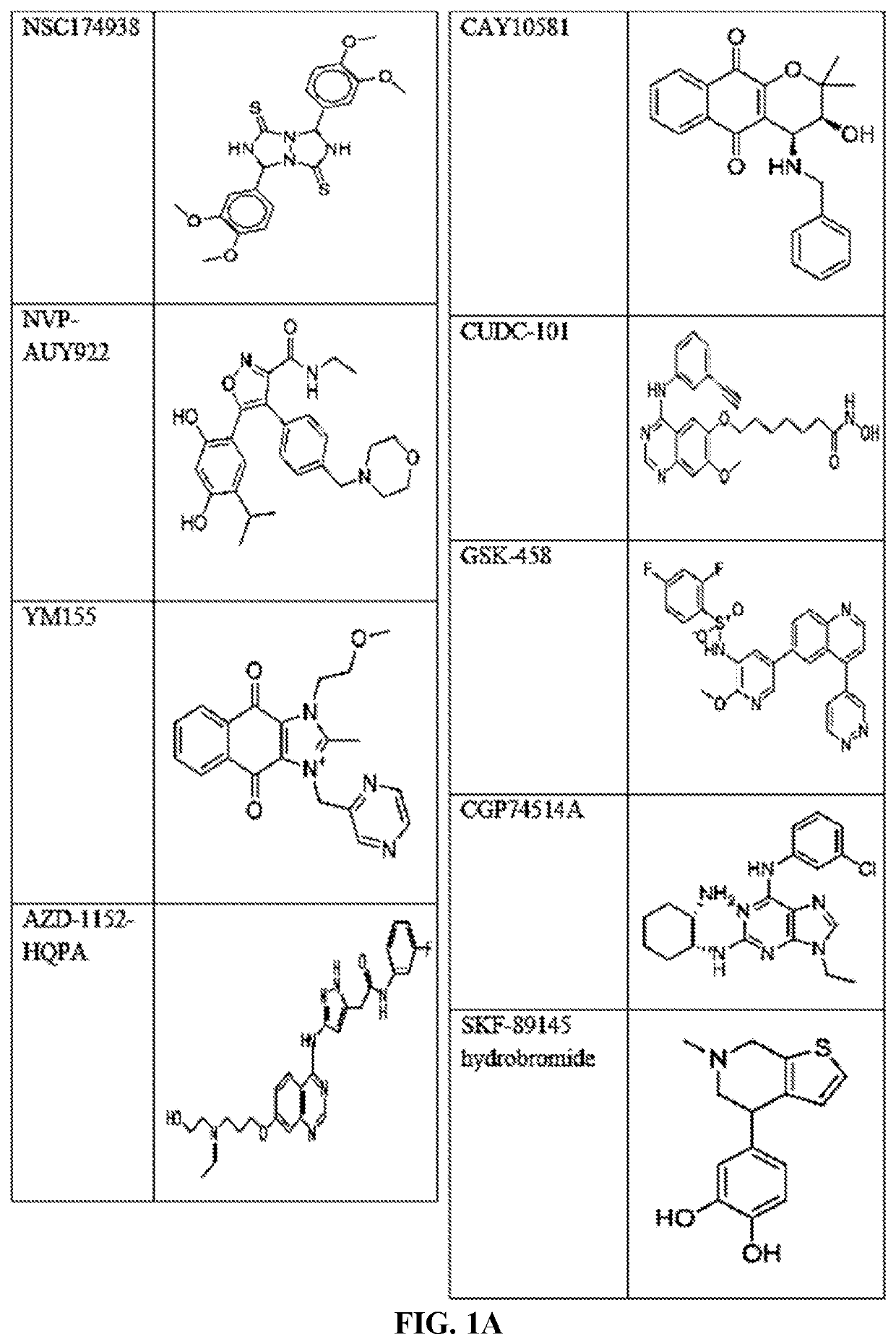
The invention provides a method of blocking transmission of a Plasmodium parasite and a method of treating or preventing malaria comprising administering to an animal an effective amount of a first compound of formula I: wherein A, B, R1, R2, R10, and R11 are described herein, either alone or in combination with a second compound selected from elesclomol, NSC 174938, NVP-AUY922, Maduramicin, Narasin, Alvespimycin, Omacetaxine, Thiram, Zinc pyrithione, Phanquinone, Bortezomib, Salinomycin sodium, Monensin sodium, Dipyrithione, Dicyclopentamethylene-thiuram disulfide, YM155, Withaferin A, Adriamycin, Romidepsin, AZD-1 152-HQPA, CAY10581, Plicamycin, CUDC-101, Auranofin, Trametinib, GSK-458, Afatinib, and Panobinostat.
Owner:UNITED STATES OF AMERICA +1
Applications of trametinib to preparation of drug for treating pulmonary inflammatory diseases and drug for promoting Tfh cell differentiation
InactiveCN111529532AMediated maturationMediates antibody productionOrganic active ingredientsAntiviralsDiseaseAntiendomysial antibodies
The invention relates to applications of trametinib to preparation of a drug for treating pulmonary inflammatory diseases and promotion of Tfh cell differentiation. The chemical formula is C26H23FIN5O4. The applications include an application of trametinib to promotion for Tfh cell differentiation and an application of trametinib to preparation of the drug for treating the pulmonary inflammatory diseases. The trametinib has beneficial effects as follows: cytokine storm caused by activation of T cells and mononuclear macrophages can be better inhibited, and meanwhile, Tfh differentiation, B cell maturation mediation and antibody production are promoted; and as abroad-spectrum immune regulation drug, the trametinib has an effect of promoting antibody production in the aspects of other viralpneumonia, virus infection or seedling inoculation.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Compounds and method for blocking transmission of malarial parasite
Owner:UNITED STATES OF AMERICA +1
Methods for treating colorectal cancer
PendingUS20220160714A1Immunoglobulins against growth factorsAmide active ingredientsColon rectal cancerMethiodide
In one aspect, provided herein are methods for treating colorectal cancer in a human subject, the methods comprising administering to the human subject a composition comprising a mitogen-activated protein kinase kinase (MEK) inhibitor and a composition comprising bisphosphonate. In a particular aspect, provided herein is a method for treating colorectal cancer in a human subject, the method comprising administering to the human subject trametinib dimethyl sulfide or a composition thereof and zoledronic acid or a composition thereof.
Owner:MT SINAI SCHOOL OF MEDICINE
Method of Adjuvant Cancer Treatment
InactiveUS20150216868A1Increase survivalImprove survivalBiocideOrganic active ingredientsAdjuvantHuman patient
The present invention provides a method of providing adjuvant treatment to a human patient which comprises administering to such a patient therapeutically effective doses of dabrafenib and trametinib for a time period sufficient to increase relapse-free survival (RFS).
Owner:NOVARTIS AG
Combination of lapatinib and trametinib
Owner:NOVARTIS AG
Inducer for inducing mesenchymal stem cells to differentiate into islet cells
ActiveCN113106054AFew induction stepsShort induction timePancreatic cellsCulture processIslet cellsMesenchymal stem cell
The invention belongs to the field of biomedicine, and relates to an inducer for inducing mesenchymal stem cells to differentiate into islet cells. The invention discloses the inducer for inducing mesenchymal stem cells to be differentiated into islet cells. The inducer is prepared from the following components: GLP-1, parathyroid hormone, acetaminophen, rapamycin, icariin, trametinib, EPO and VEGF. According to the inducer for inducing and differentiating the mesenchymal stem cells into the islet cells, all the components are safe and non-toxic, the number of steps needed by induced differentiation is small, the time is short, and the induction efficiency is high.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Medicine for treating lung cancer
ActiveCN110664818AGrowth inhibitionGrowth inhibitory TAK1 signaling effectively preventsOrganic active ingredientsAntineoplastic agentsPharmaceutical drugOncology
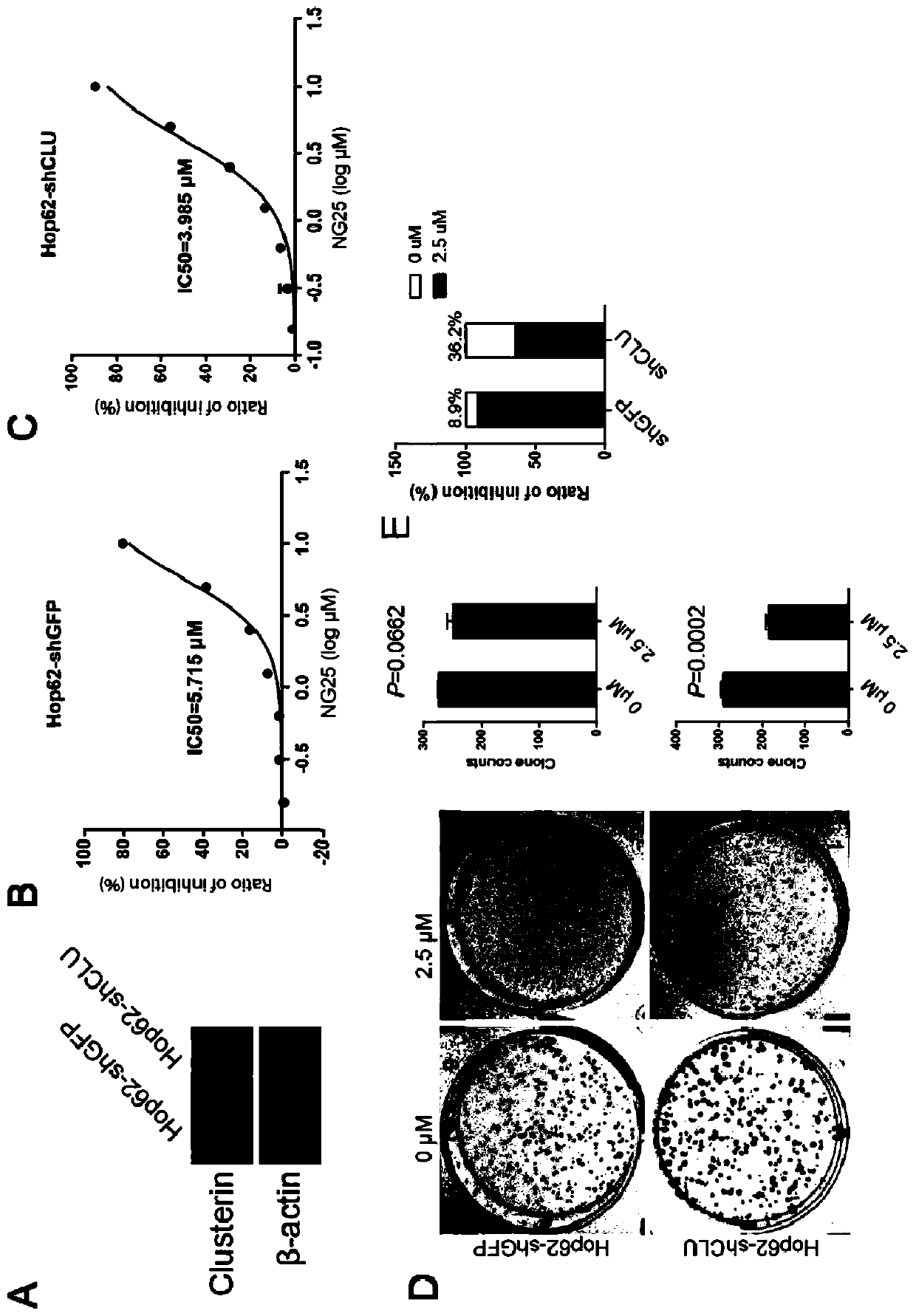
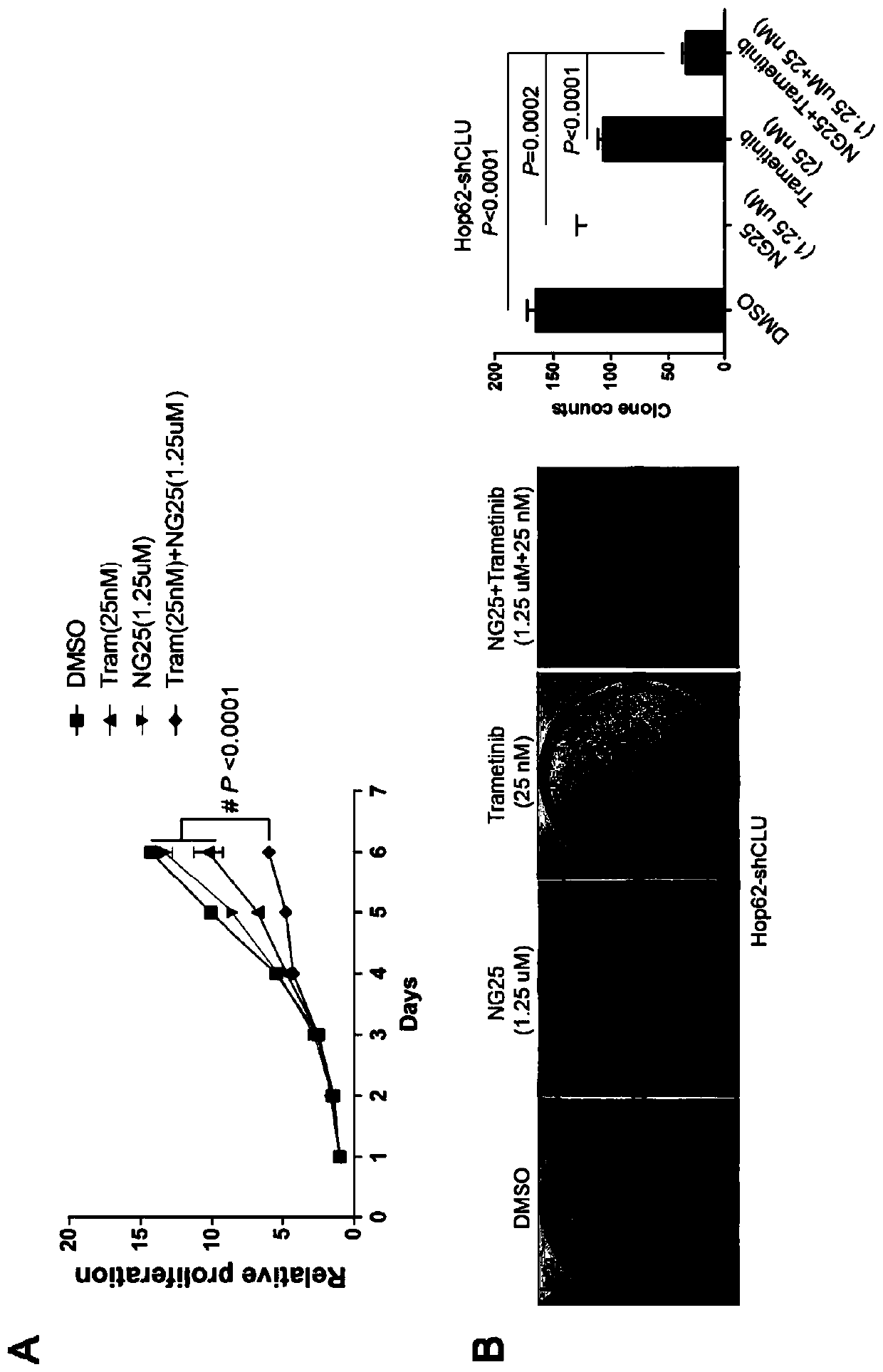
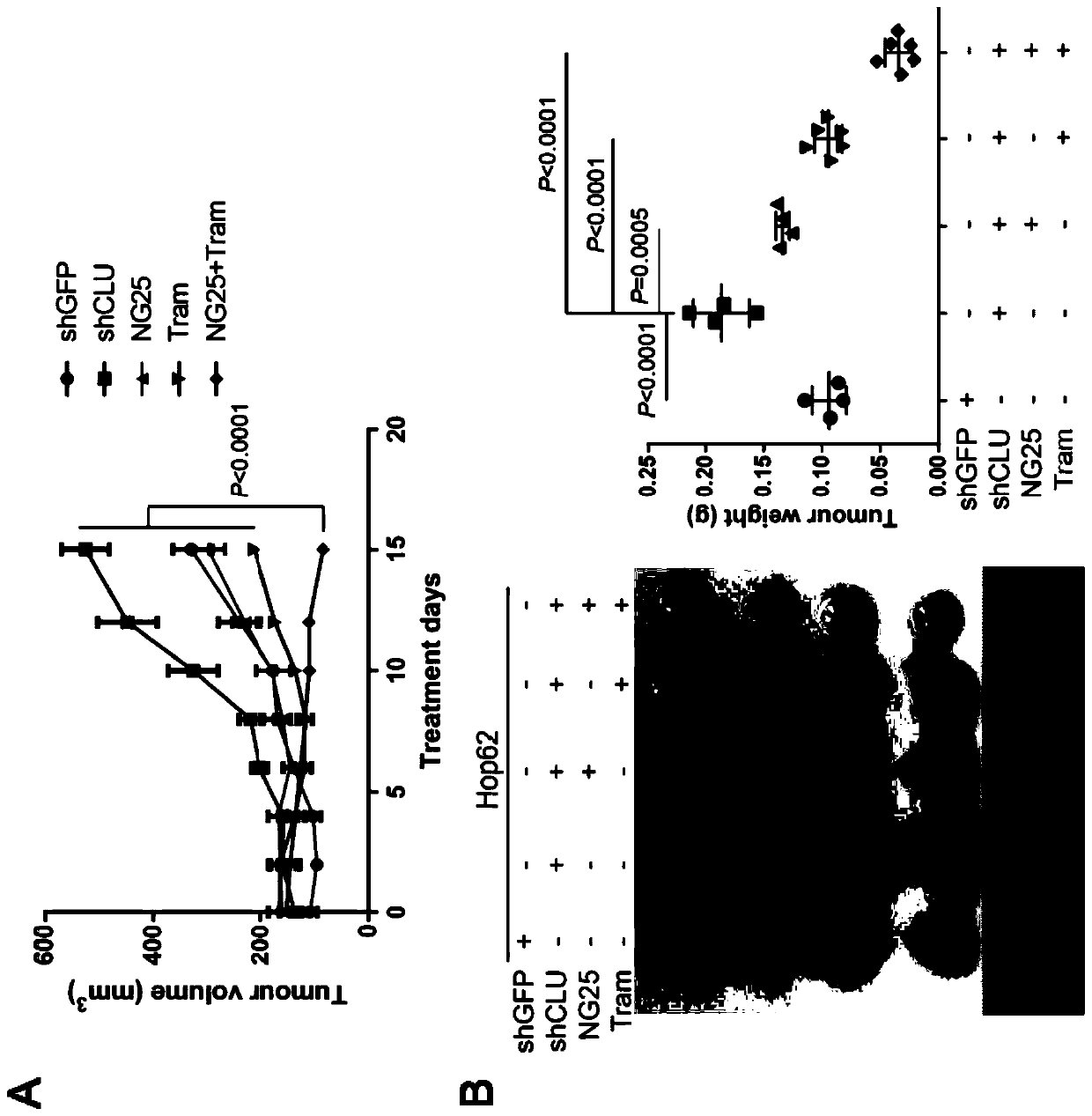
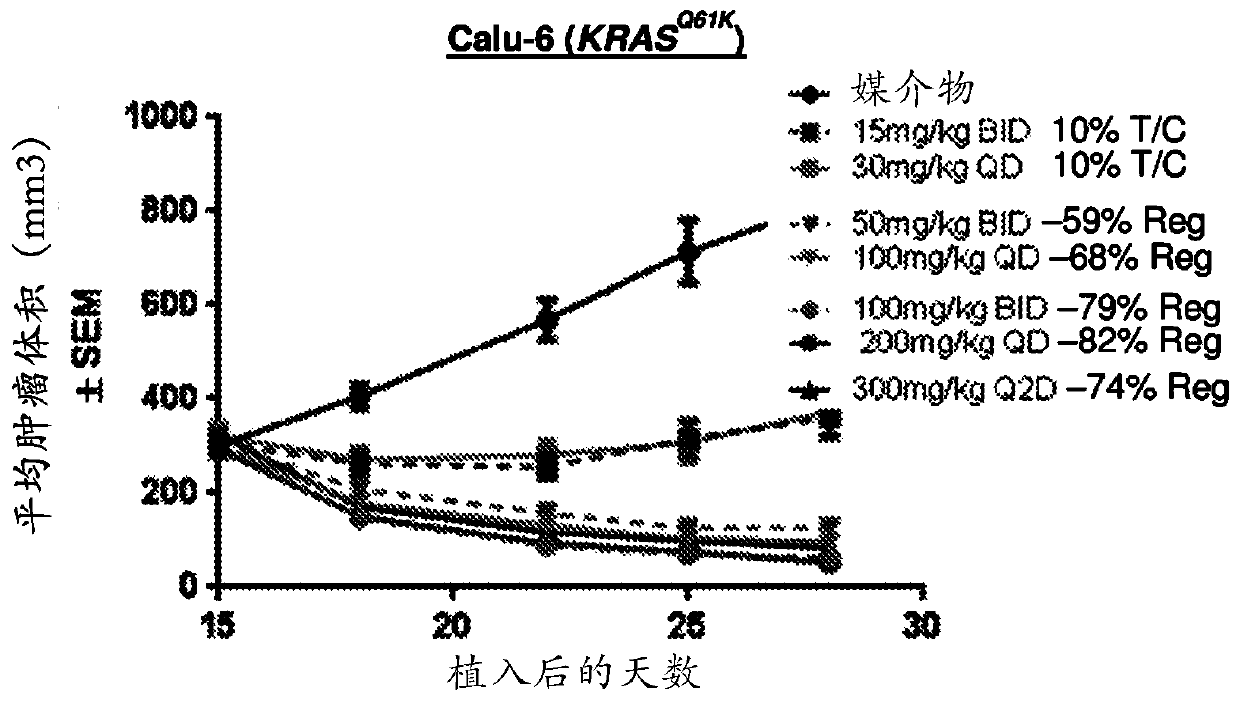
The invention discloses a medicine for treating lung cancer. The medicine is composed of NG25 and trametinib at a mass ratio of 4 to 1; and the lung cancer is a lung cancer in which the function of CLU genes is absent or down-regulated. Further, the lung cancer is a lung cancer in which the function of the CLU genes is absent or down-regulated and is driven by KRASG 12D oncogenes. In vitro experiments show that CLU knock-down lung cancer cells are more sensitive to NG25, compared with controls, the NG25 can significantly inhibit the growth of the lung cancer cells, and while after the cells are treated by combining with the Trametinib, it is found that the inhibition on the lung cancer cells is even more significant. Similarly, in animal models, a TAK1 inhibitor NG25 can inhibit the growthof CLU knock-down cell transplantation tumors and CLU knock-out orthotopic lung cancer, the treatment combined with the Trametinib can significantly shrink the tumor, and the purpose of treating thetype of the lung cancer is achieved.
Owner:JINAN UNIVERSITY
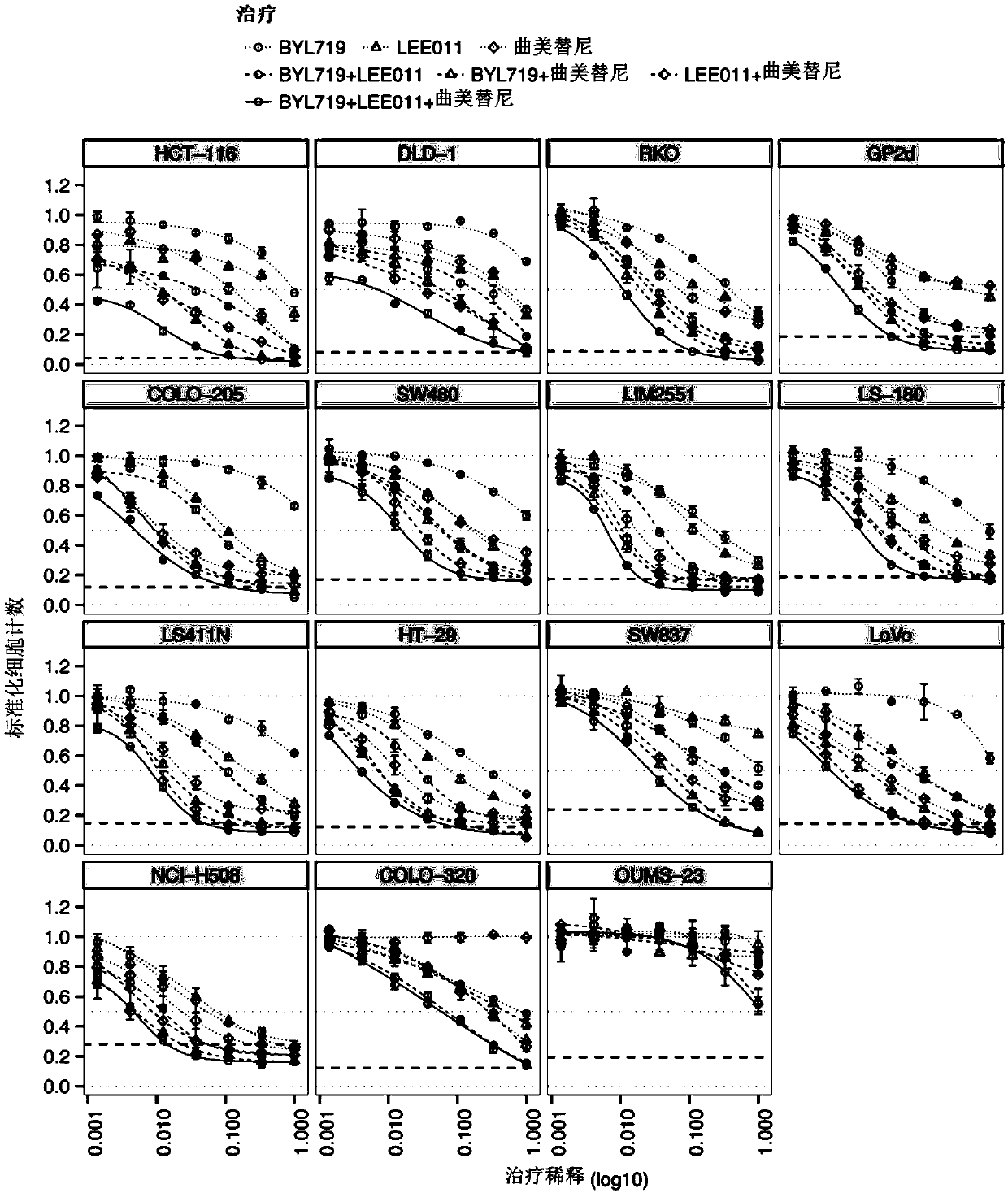
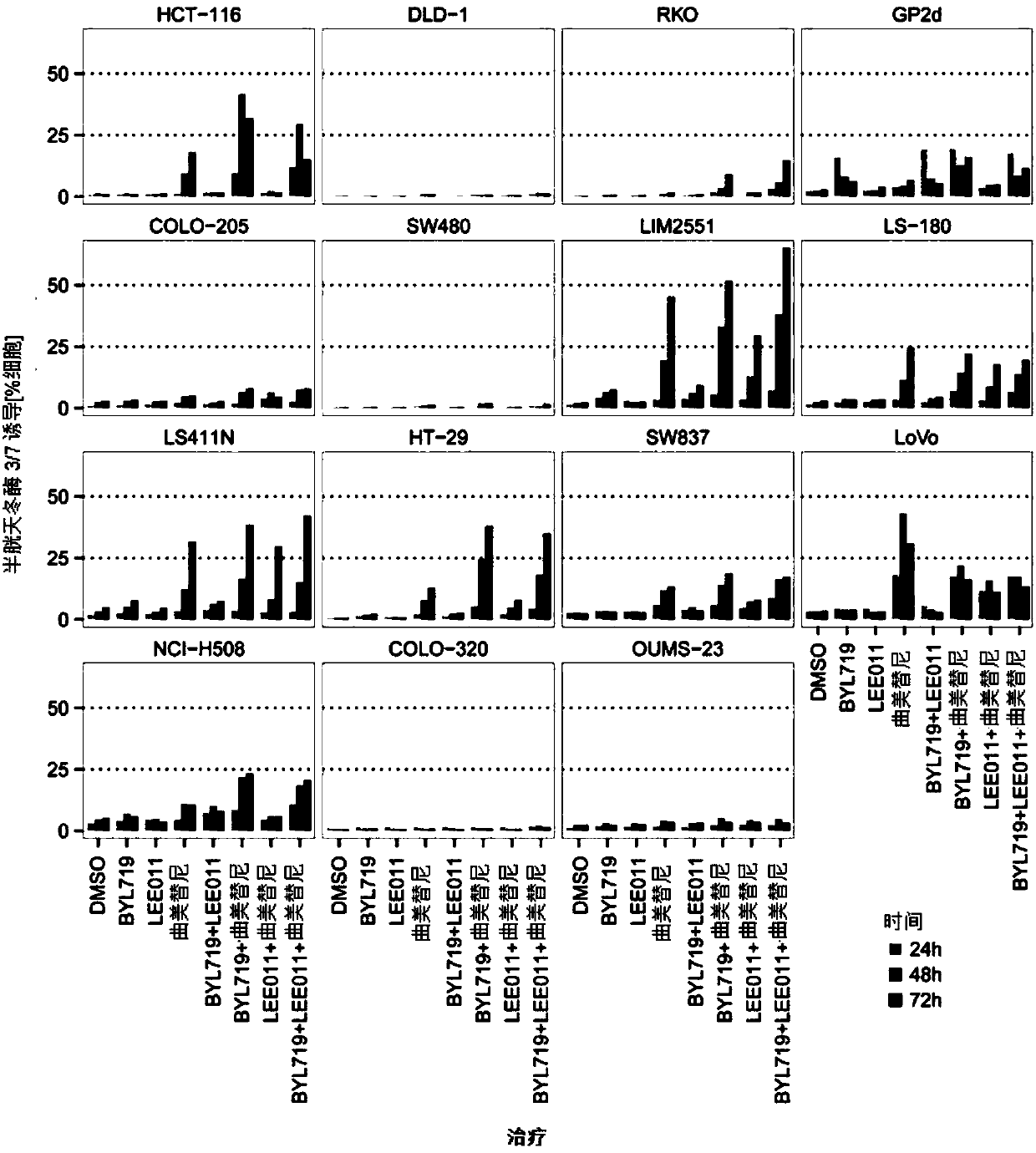
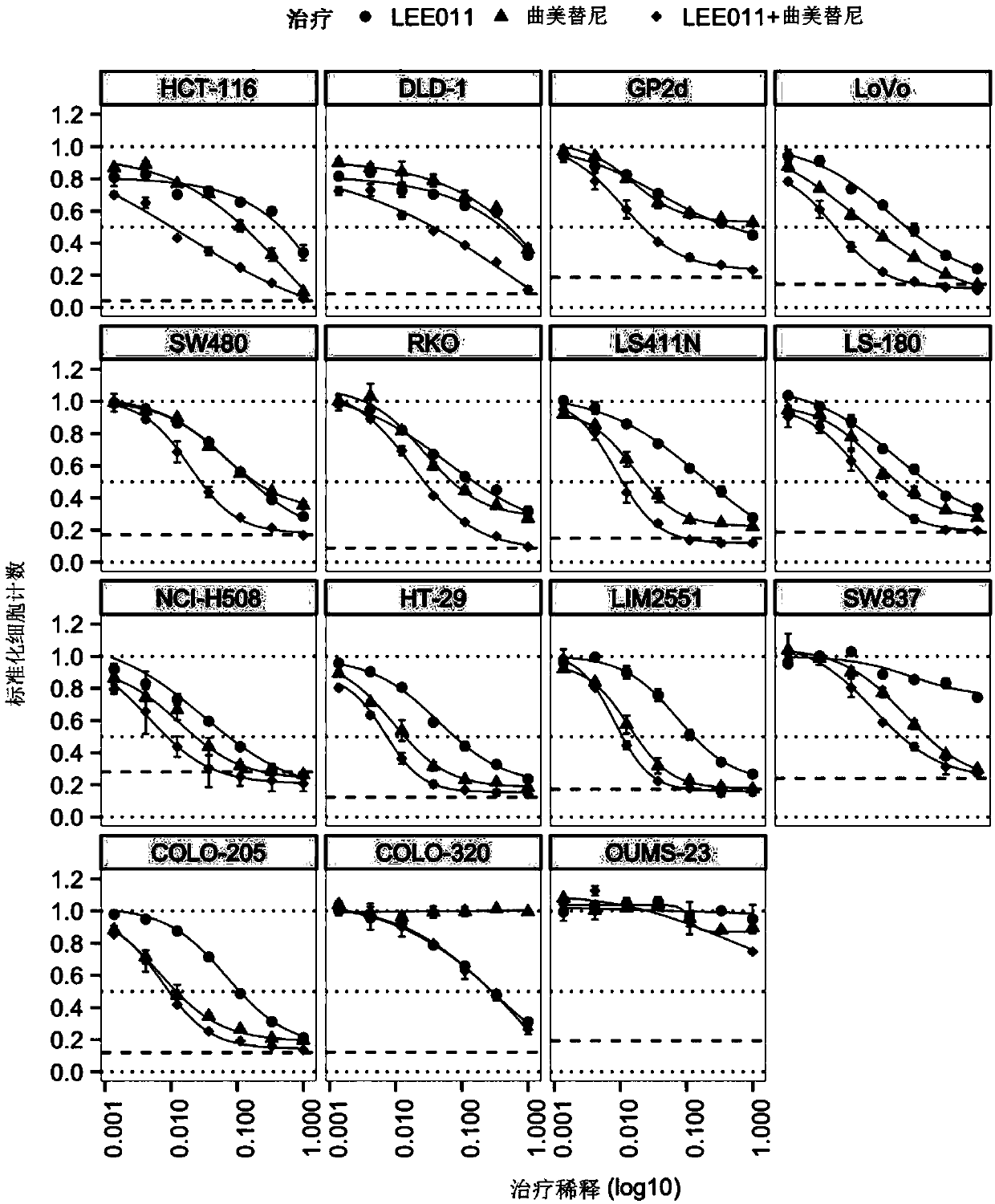
Combinations of cdk4/6 inhibitor lee011 and mek1/2 inhibitor trametinib, optionally further comprising pi3k inhibitor byl719 to treat cancer
The present disclosure relates to pharmaceutical combinations comprising a cyclin dependent kinase 4 / 6 (CDK4 / 6) inhibitor compound, (b) a mitogen activated protein kinase (MEK) inhibitor compound, andoptionally (c) an alpha-isoform specific phosphatidylinositol 3-kinase (PI3K) inhibitor compound, for the treatment or prevention of cancer, as well as related pharmaceutical compositions, uses, andmethods of treatment or prevention of cancer.
Owner:NOVARTIS AG
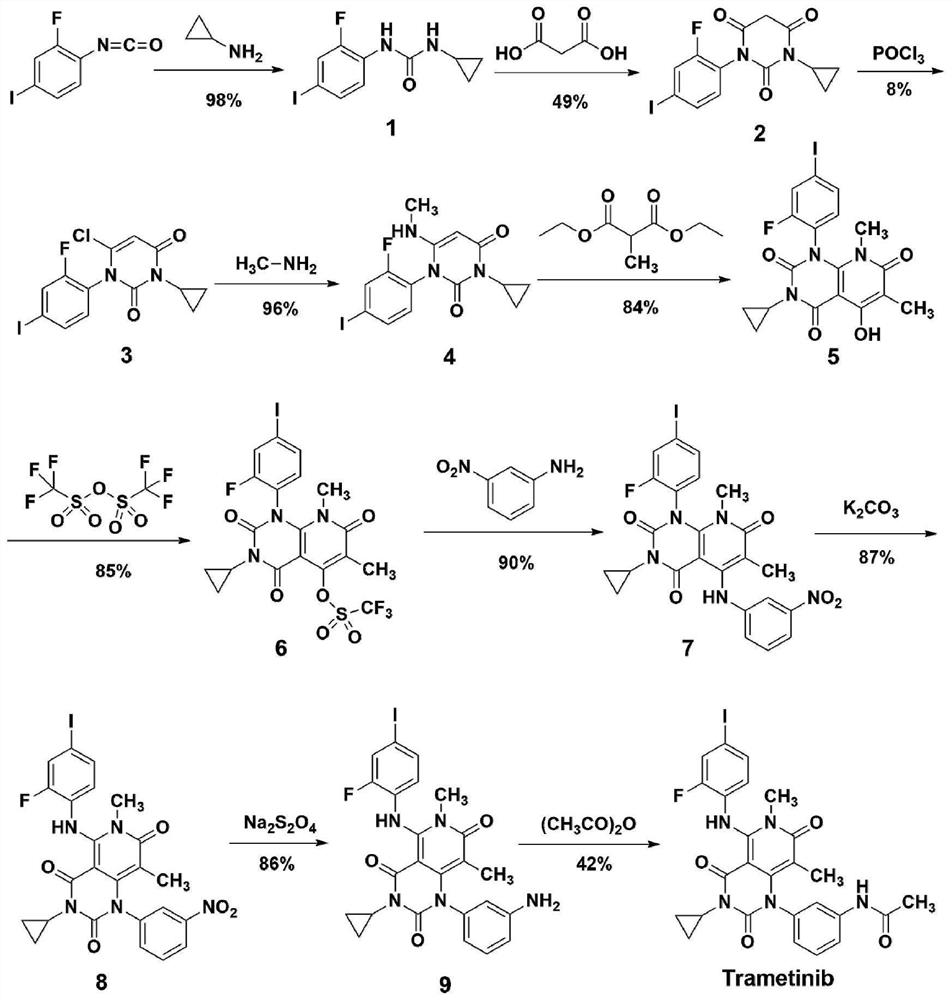
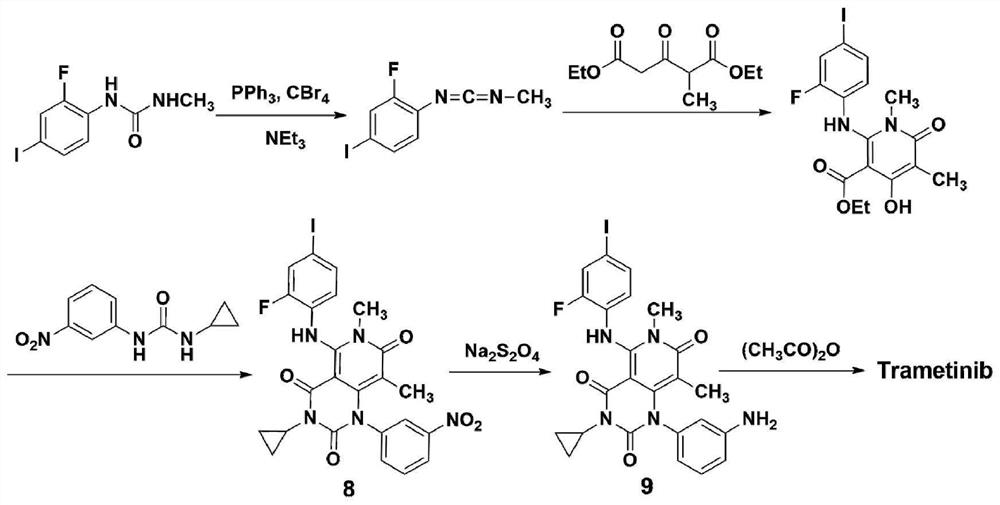
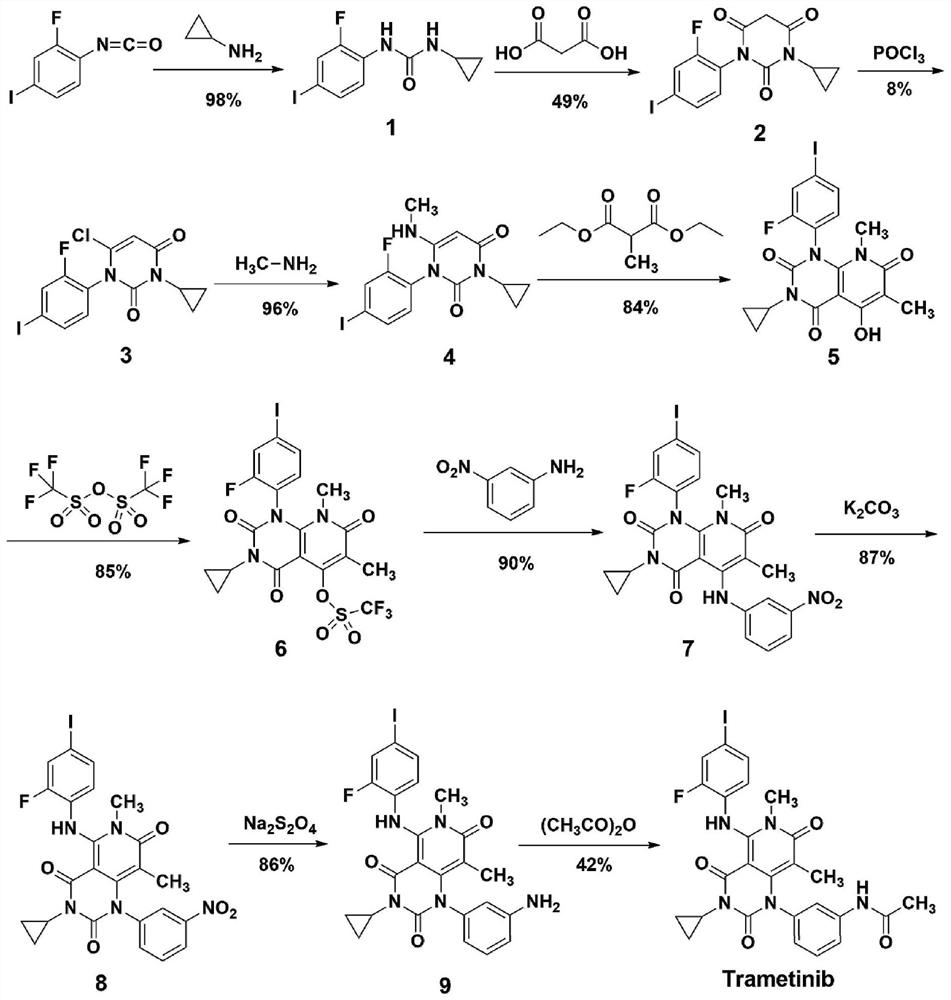
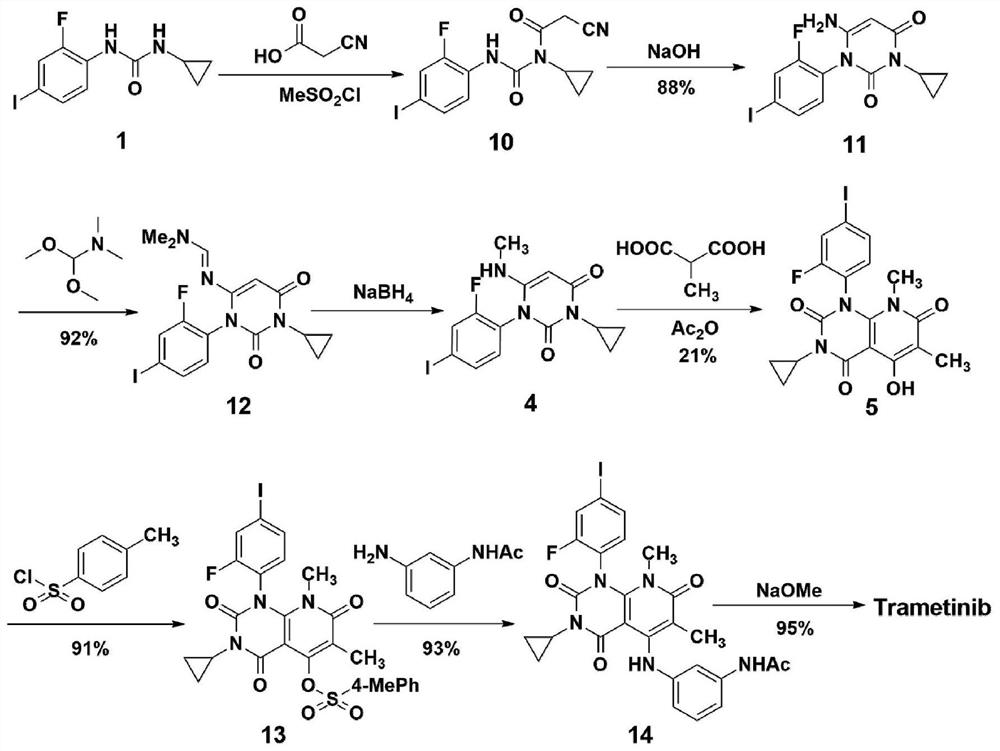
A kind of method of synthesizing trametinib
ActiveCN109320513BShort processSimple and fast operationOrganic chemistryDiethyl glutarateCarboxylic acid
Owner:安庆奇创药业有限公司
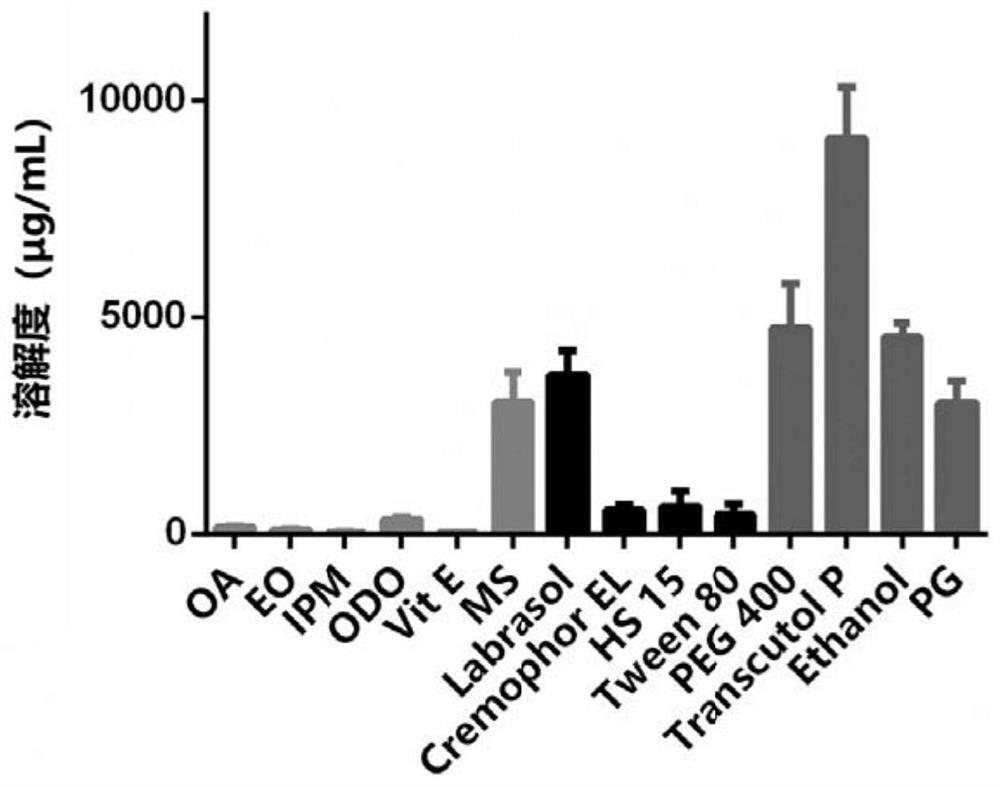
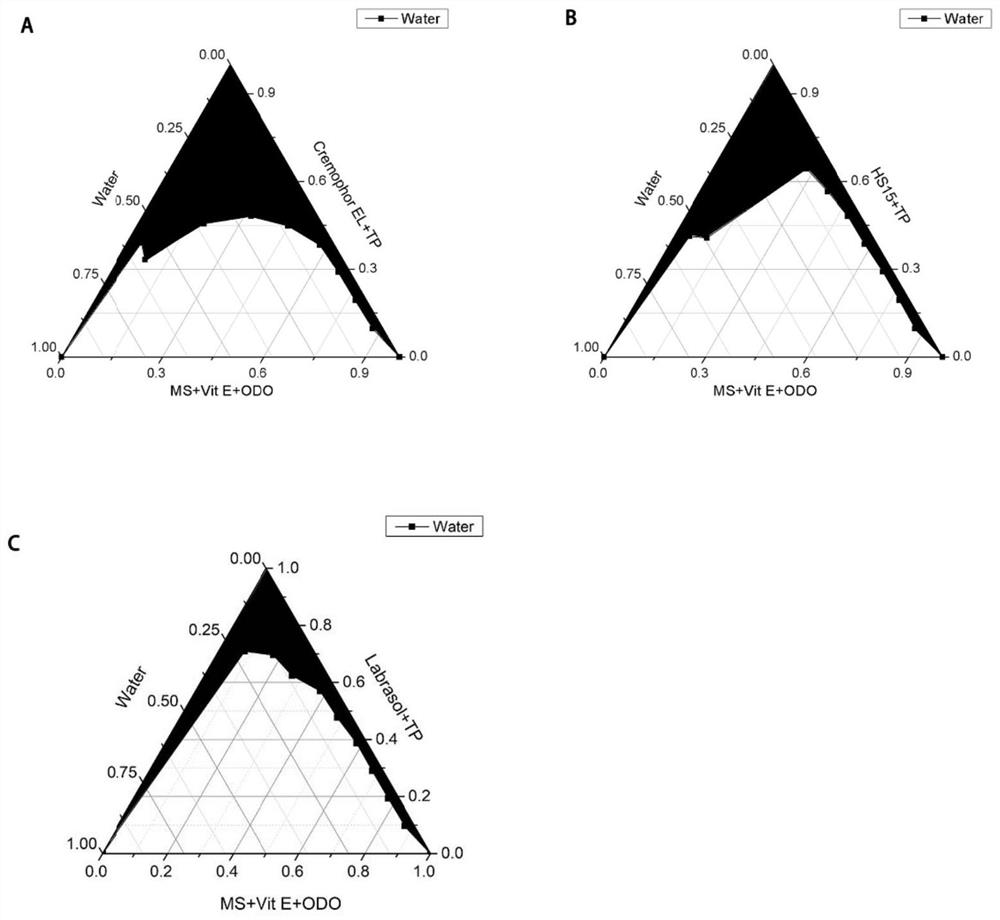
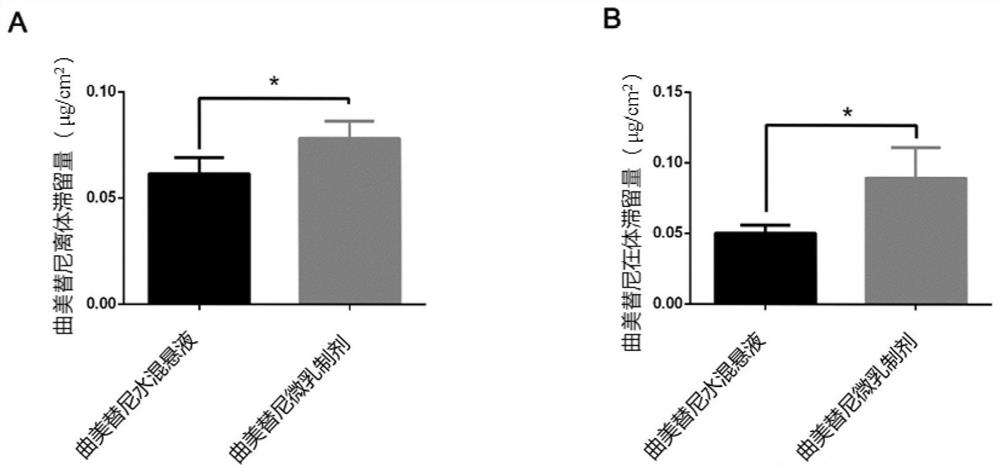
Trametinib microemulsion and application thereof
PendingCN113197857AEfficient deliveryImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsMethyl salicylateTrametinib
The invention discloses a trametinib microemulsion and application thereof. The microemulsion is prepared from trametinib, methyl salicylate, vitamin E, caprylic capric triglyceride, diethylene glycol monoethyl ether and polyoxyethylated castor oil EL. Compared with a trametinib water suspension, the trametinib microemulsion disclosed by the invention has the advantages that the retention volume is high; Compared with oral trametinib suspension, the trametinib microemulsion has more remarkable tumor inhibition efficacy, so that the trametinib micro-emulsion can effectively inhibit the melanoma and has more remarkable curative effect and advantages in the aspect of treating the melanoma. In addition, adopted instruments and equipment are simple and convenient, the preparation process is efficient, simple and controllable, and the method is suitable for industrial production.
Owner:SUN YAT SEN UNIV
Compositions for treating melanoma
PendingUS20210244737A1Limited clinical benefitSlow onsetOrganic active ingredientsAntineoplastic agentsStage melanomaFibrosis
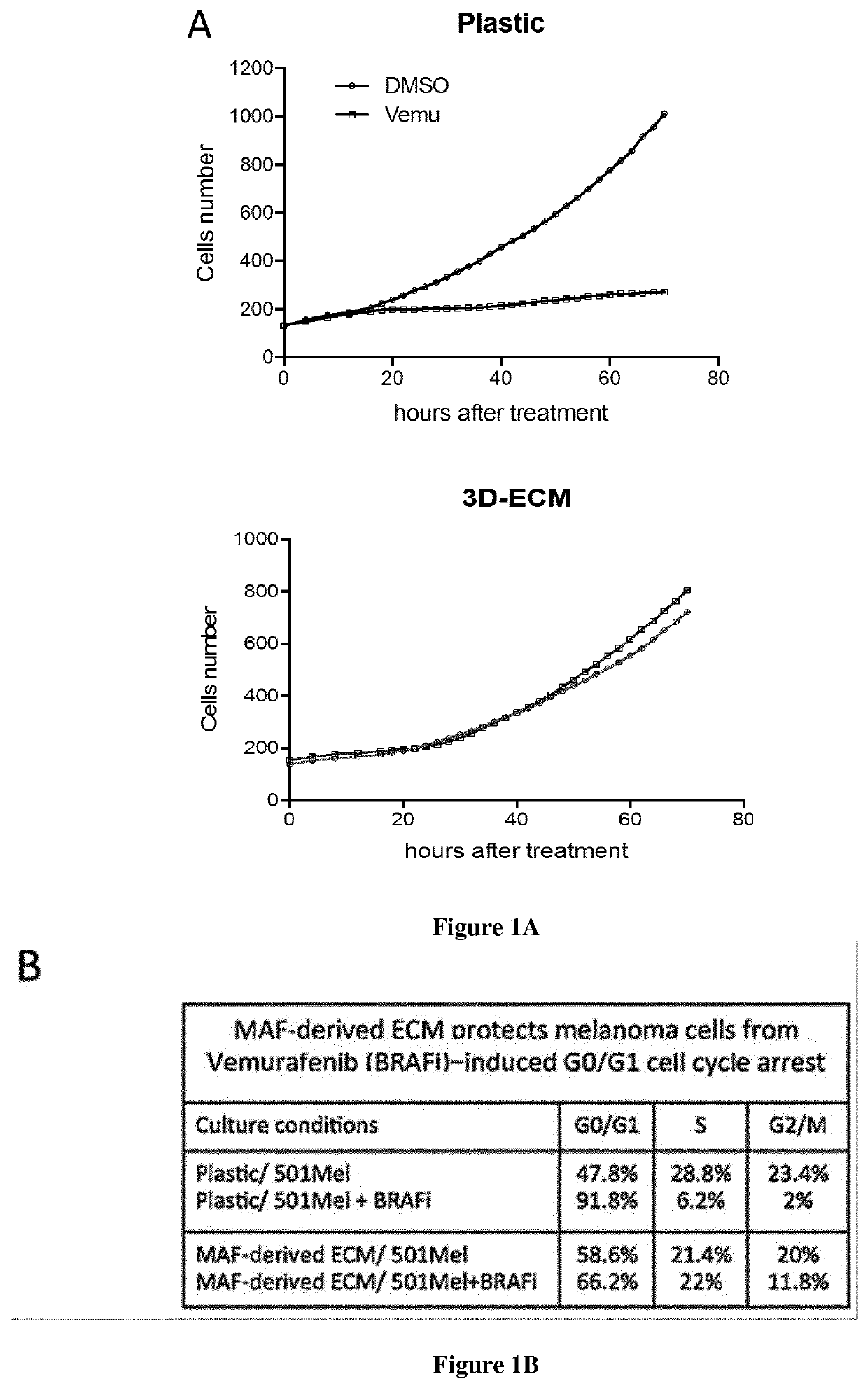
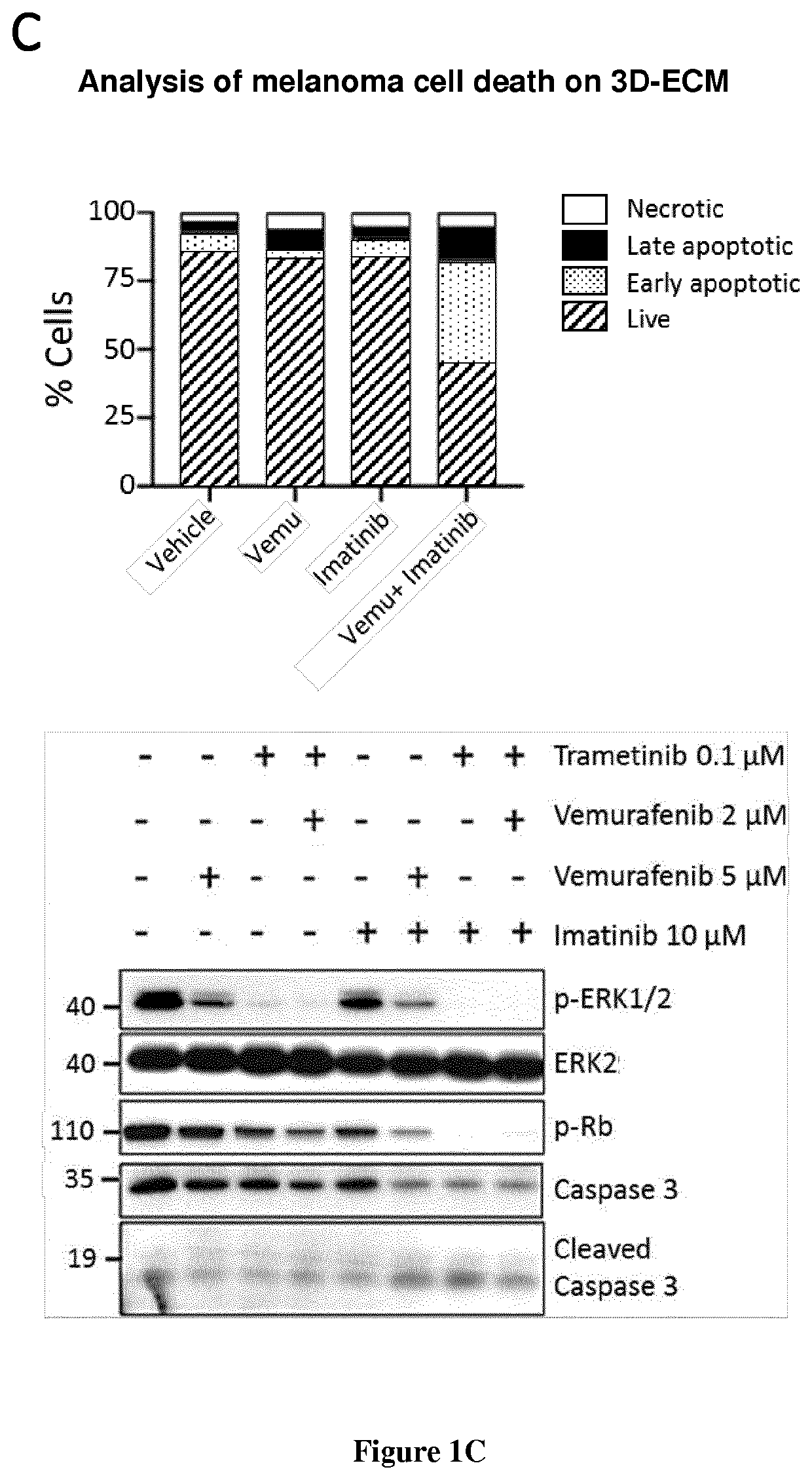
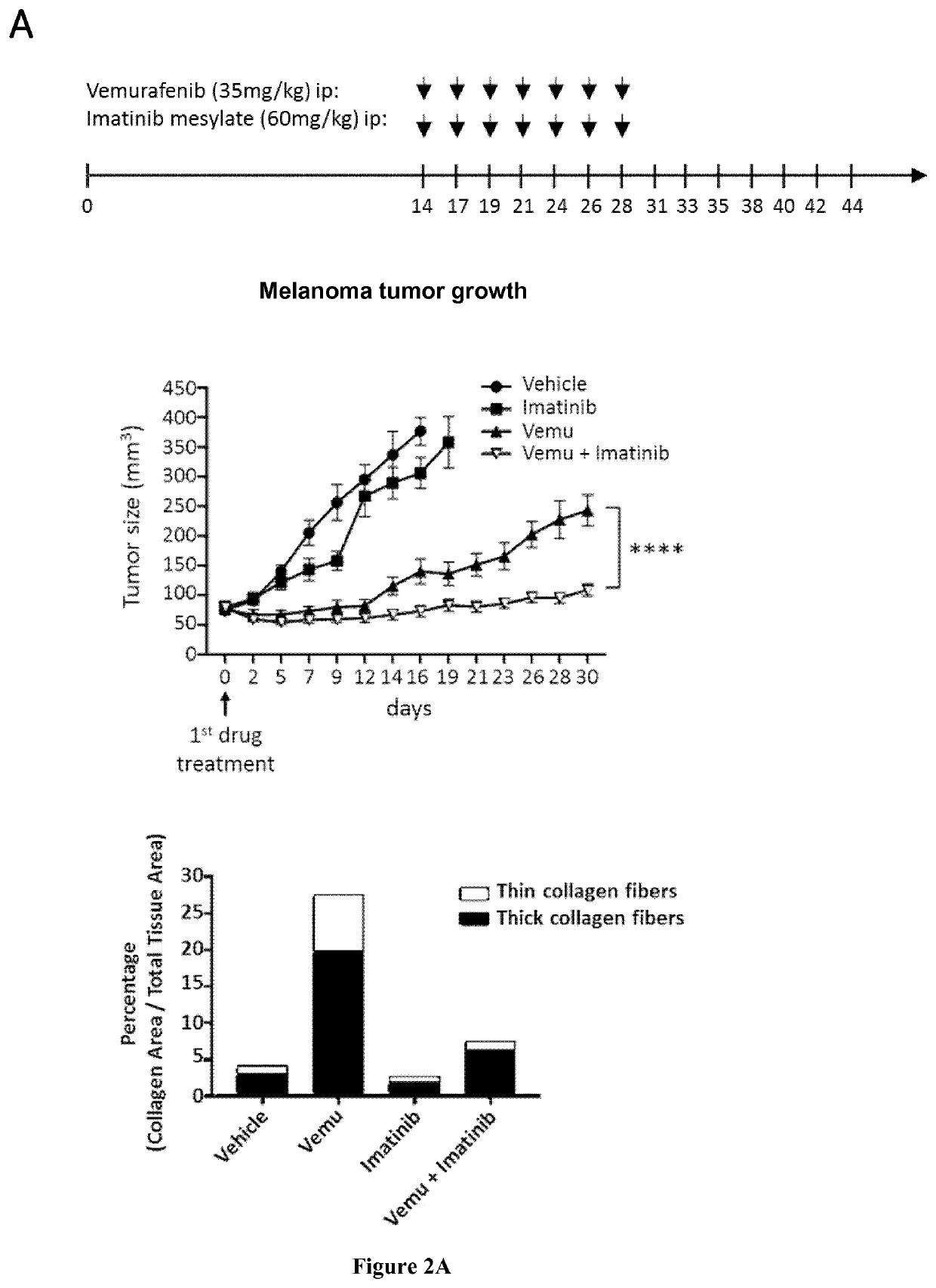
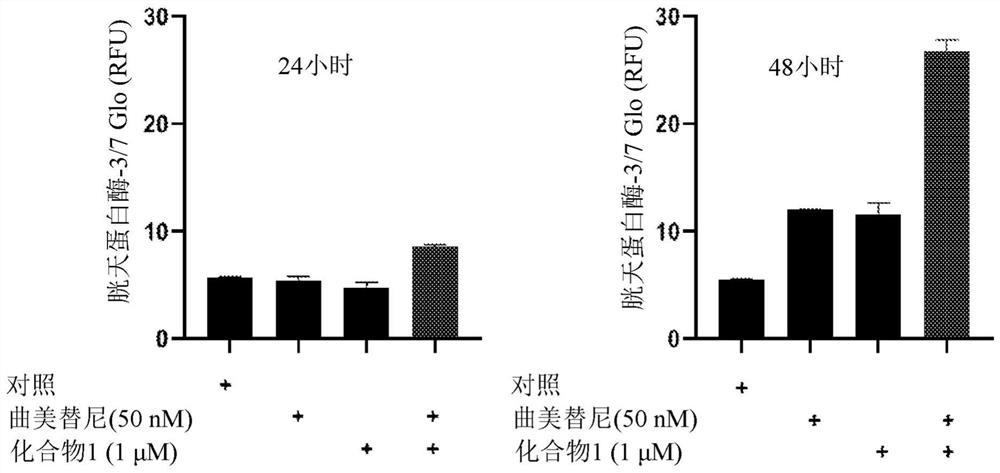
Inventors have shown that targeting DDR1 and DDR2 collagen receptors by Imatinib resensitizes melanoma tumors to BRAFV600E to targeted therapy and normalizes the fibrotic stromal reaction. These findings provide the rationale to combine Imatinib (or other DDR inhibitors) and MAPK-targeting agents to disrupt the influence of the matrix microenvironment in order to delay or prevent the emergence of therapy-resistant cells. They have shown that inhibition of DDR1 and DDR2 kinase activities by Imatinib suppressed the protection of melanoma cells against Vemurafenib (BRAFi) and Trametinib (MEKi) co-drugging and led to cell cycle arrest and cell death. Similar biochemical cell cycle and apoptotic events were promoted in presence of Nilotinib. They validated this anti-tumor activity of Imatinib combined with Vemurafenib in a pre-clinical xenograft model of melanoma and showed that targeting DDR1 / 2 signaling delays tumor relapse. Accordingly, the present invention relates to a method for treating melanoma in a subject in need thereof comprising a step of administering said subject with a therapeutically effective amount of: i) an inhibitor of BRAF, ii) an inhibitor of MEK, and iii) an inhibitor of DDR1 / 2.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Combination therapy involving diaryl macrocyclic compounds
The present disclosure relates to the use of diaryl macrocycles in conjunction with MAPK / ERK kinase-1 and 2 (MEK1 and MEK2; the present invention relates to methods and compositions for the treatment of cancer using combinations of inhibitors of MAP2K1 and MAP2K2, such as trametinib.
Owner:TURNING POINT THERAPEUTICS INC
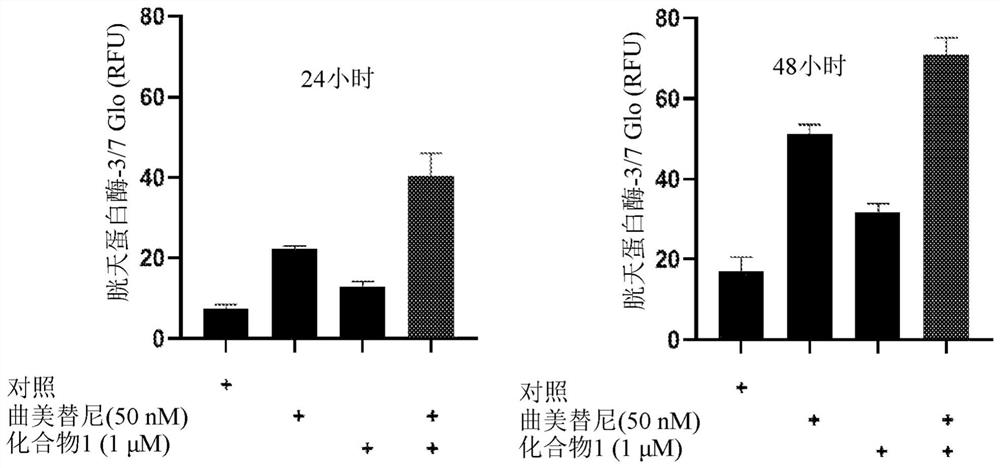
Application of trametinib in preparation of medicine for treating Parkinson's disease
ActiveCN105816461BDelay degenerative deathGood curative effectOrganic active ingredientsNervous disorderCurative effectTherapeutic effect
The invention belongs to the technical field of medicine, and discloses application of trametinib in preparation of a drug for treating Parkinson's disease, wherein trametinib is used for preparing the drug to delay the degeneration of dopaminergic neuron. The obtained product can effectively delay the degenerative death of dopaminergic neuron, to treat Parkinson's disease fundamentally; the drug can be taken orally without injection to achieve the treatment effect, has excellent curative effect for Parkinson's disease caused by a variety of reasons, and has important significance for the treatment and curing of Parkinson's disease.
Owner:FUZHOU UNIV
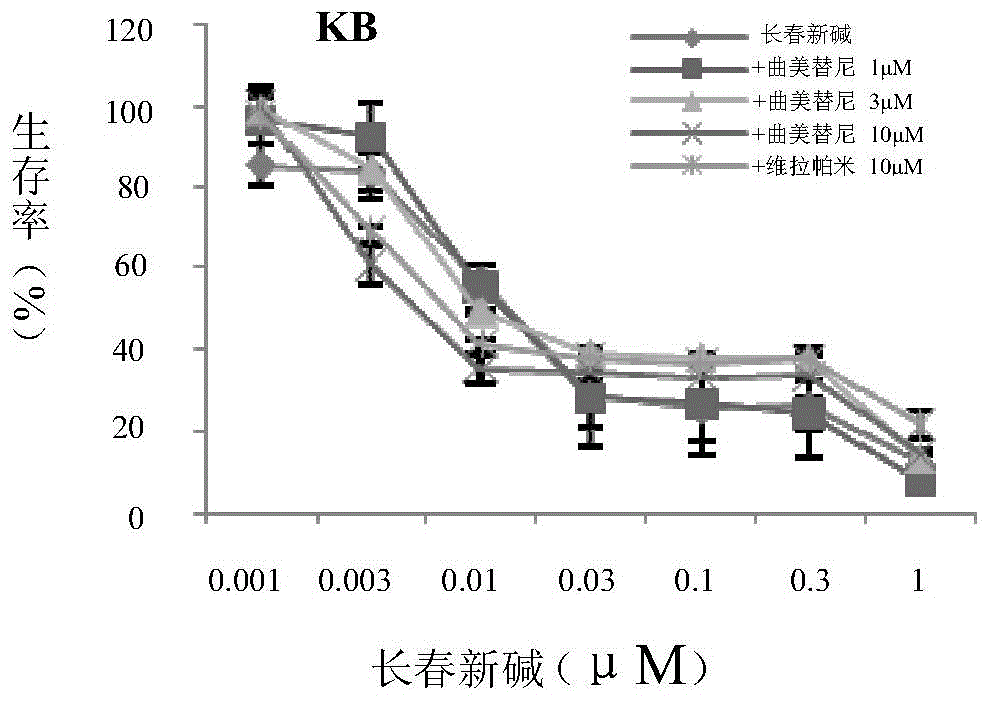
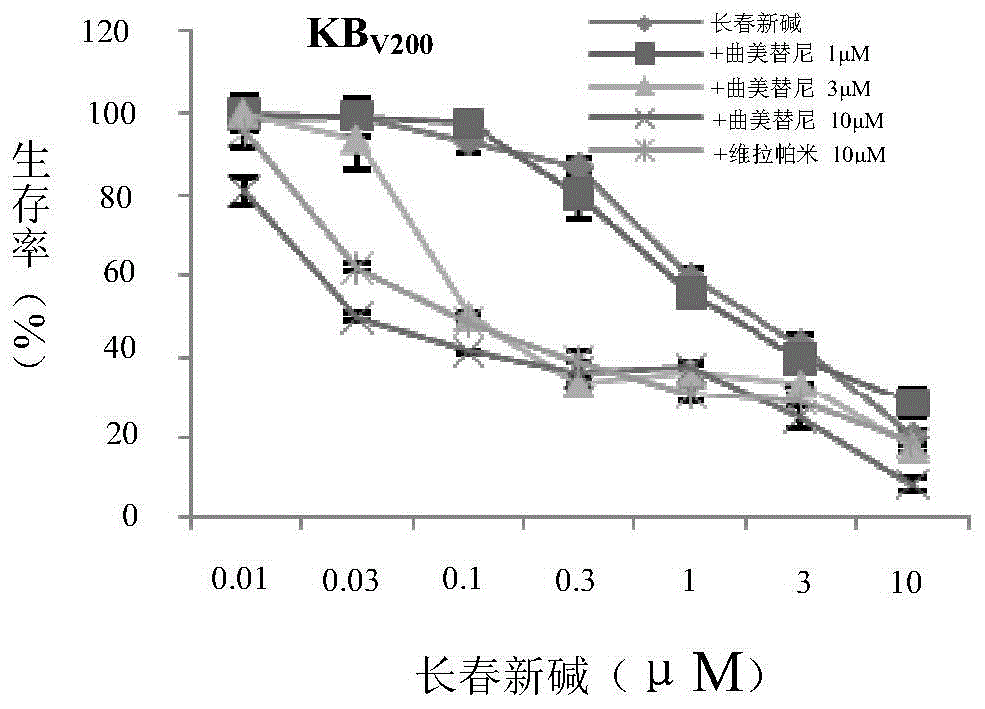
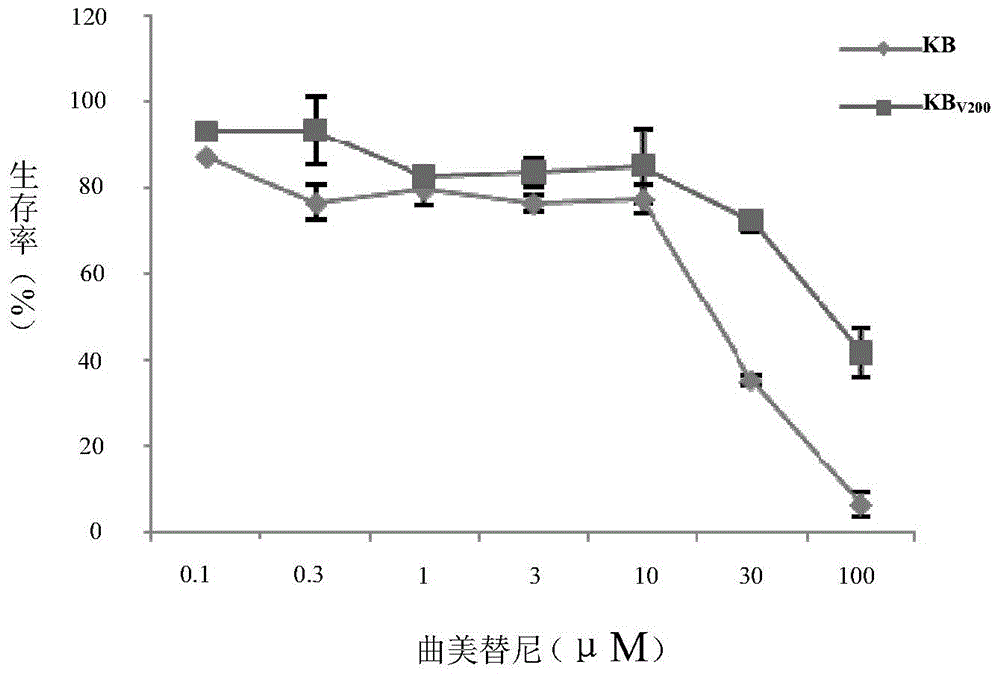
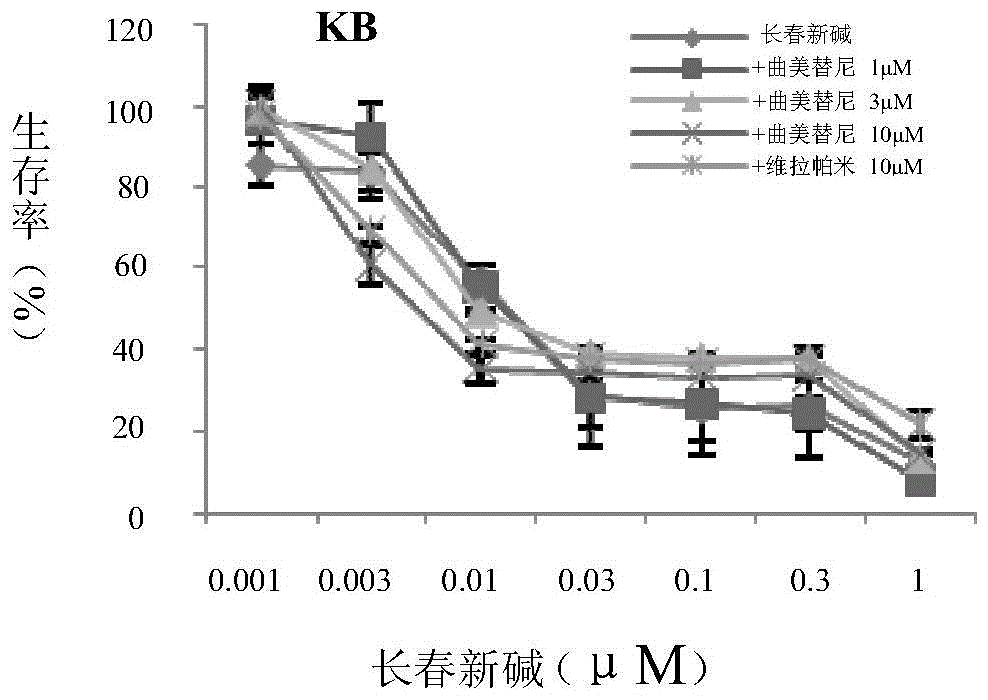
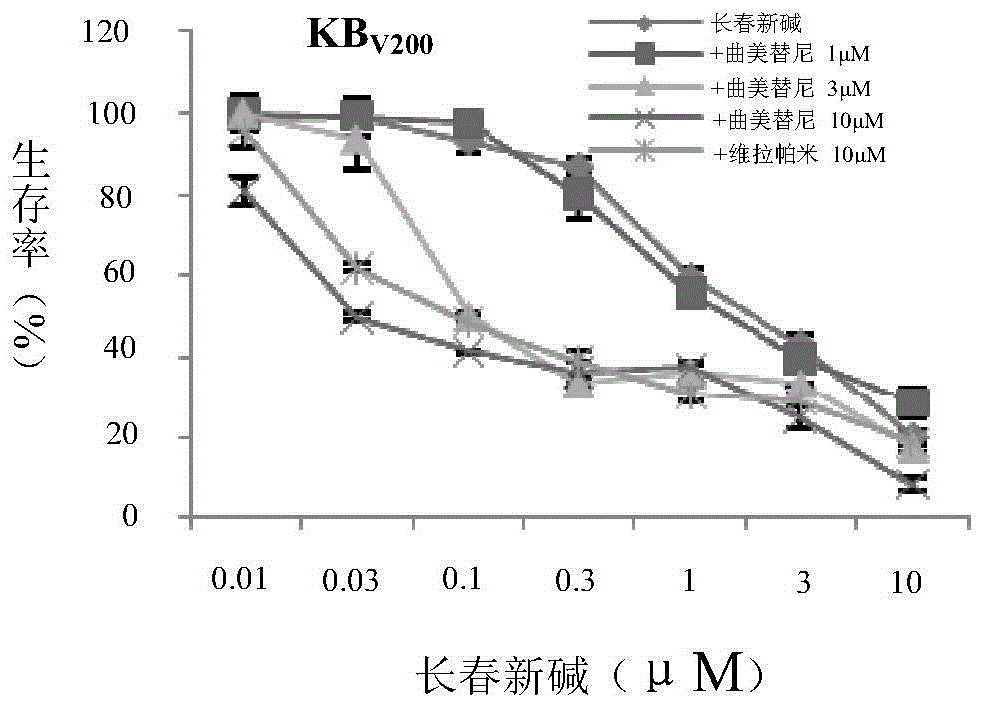
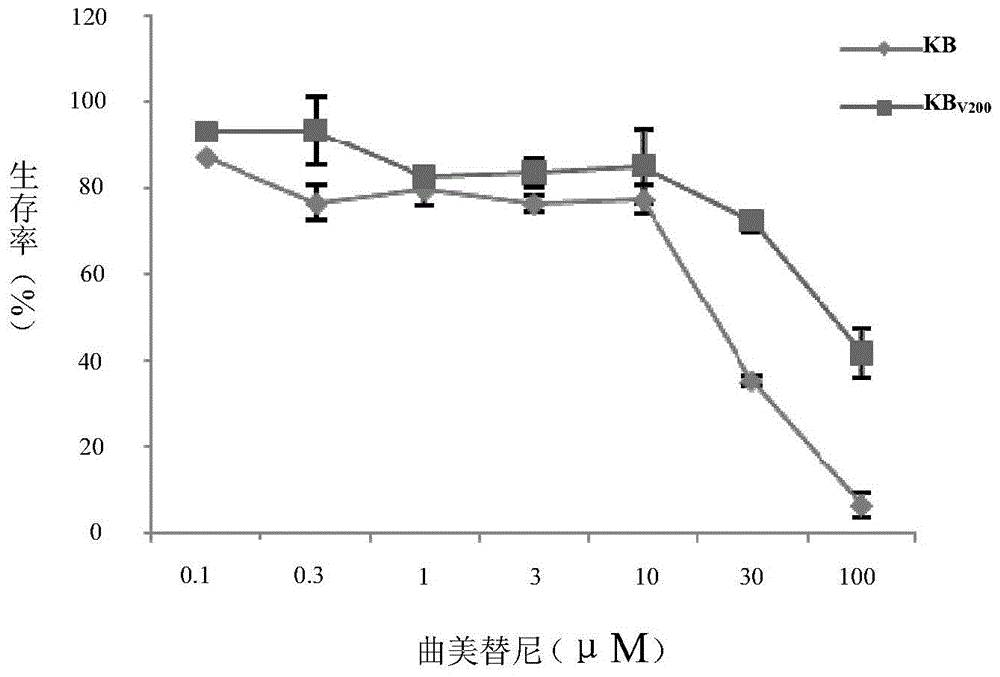
Application of trametinib in preparing medicine for reversing multi-medicine resistance of tumors
ActiveCN104434924AReverse multidrug resistanceRestore sensitivityOrganic active ingredientsAntineoplastic agentsMembrane TransportersMembrane transport protein
The invention belongs to the technical field of medicine application, and discloses an application of trametinib in preparing a medicine for reversing multi-medicine resistance of tumors. Trametinib can be used for significantly reversing the multi-medicine resistance of the tumors mediated by membrane transport protein to ensure that the sensitivity of P-glycoprotein high expression cells to anti-cancer medicines can be restored. According to the medicine for reversing the multi-medicine resistance of the tumors, disclosed by the invention, trametinib and the anti-cancer medicines can be combined to ensure that the effects of treating and controlling resistant tumors can be achieved by restoring the sensitivity of resistant cells to the anti-cancer medicines.
Owner:JINAN UNIVERSITY
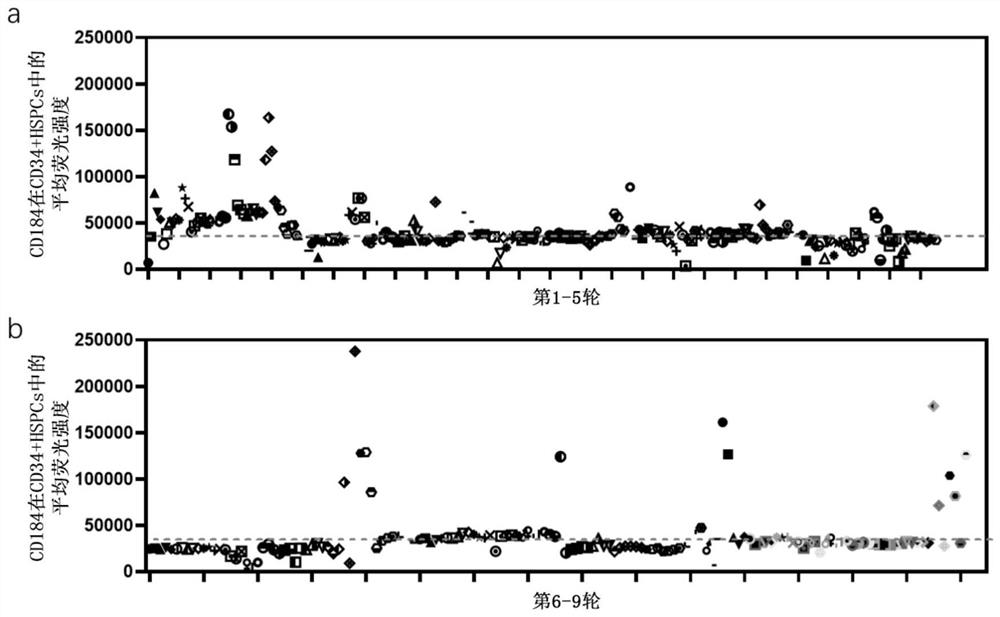
Application of compound for improving transplantation efficiency of human hematopoietic stem cells
PendingCN114246948AImprove migration abilityEnhance homing abilityAnimal cellsOrganic active ingredientsTherapeutic effectBiochemistry
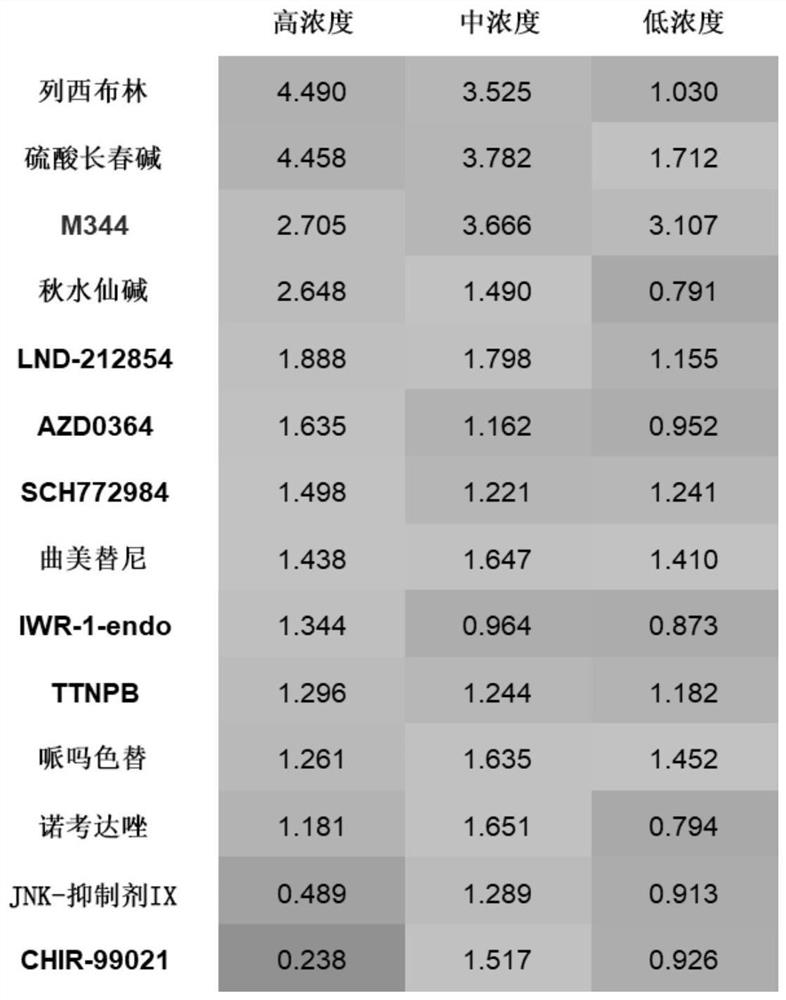
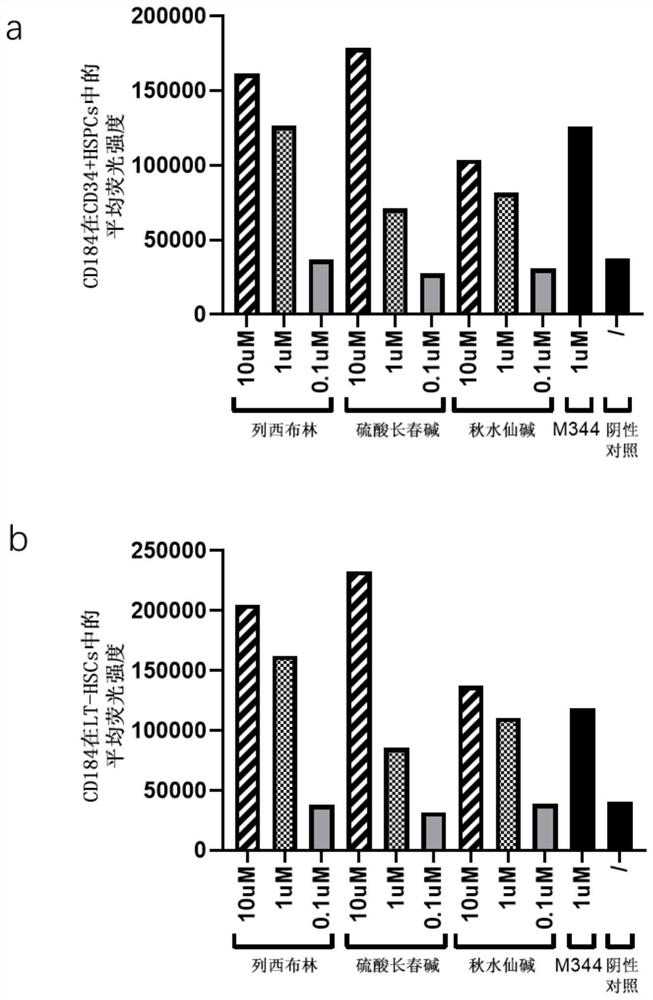
The invention discloses an application of a compound in improving the transplantation efficiency of human hematopoietic stem cells. The compound is selected from one or more than two of a microtubulin polymerization inhibitor, LND-212854, AZD0364, SCH772984, Pimsertib, Trametinib, IWR-1-endo, TTNPB, JNK-inhibitor IX and CHIR99021. The invention further discloses an application of the compound in improving the transplantation efficiency of the human hematopoietic stem cells. The compound provided by the invention can improve the expression of the hematopoietic stem cell surface protein CD184 through in-vitro short-time treatment without influencing the phenotype, motility rate and various characteristics of cells. Through the combination of the chemokine receptor CD184 expressed by the HSC and the chemokine ligand SDF1 in the bone marrow microenvironment, the migration ability of the HSC and the homing ability of the HSC in the bone marrow are improved, and the transplantation efficiency and the treatment effect of the HSC are enhanced.
Owner:EDIGENE GUANGZHOU INC
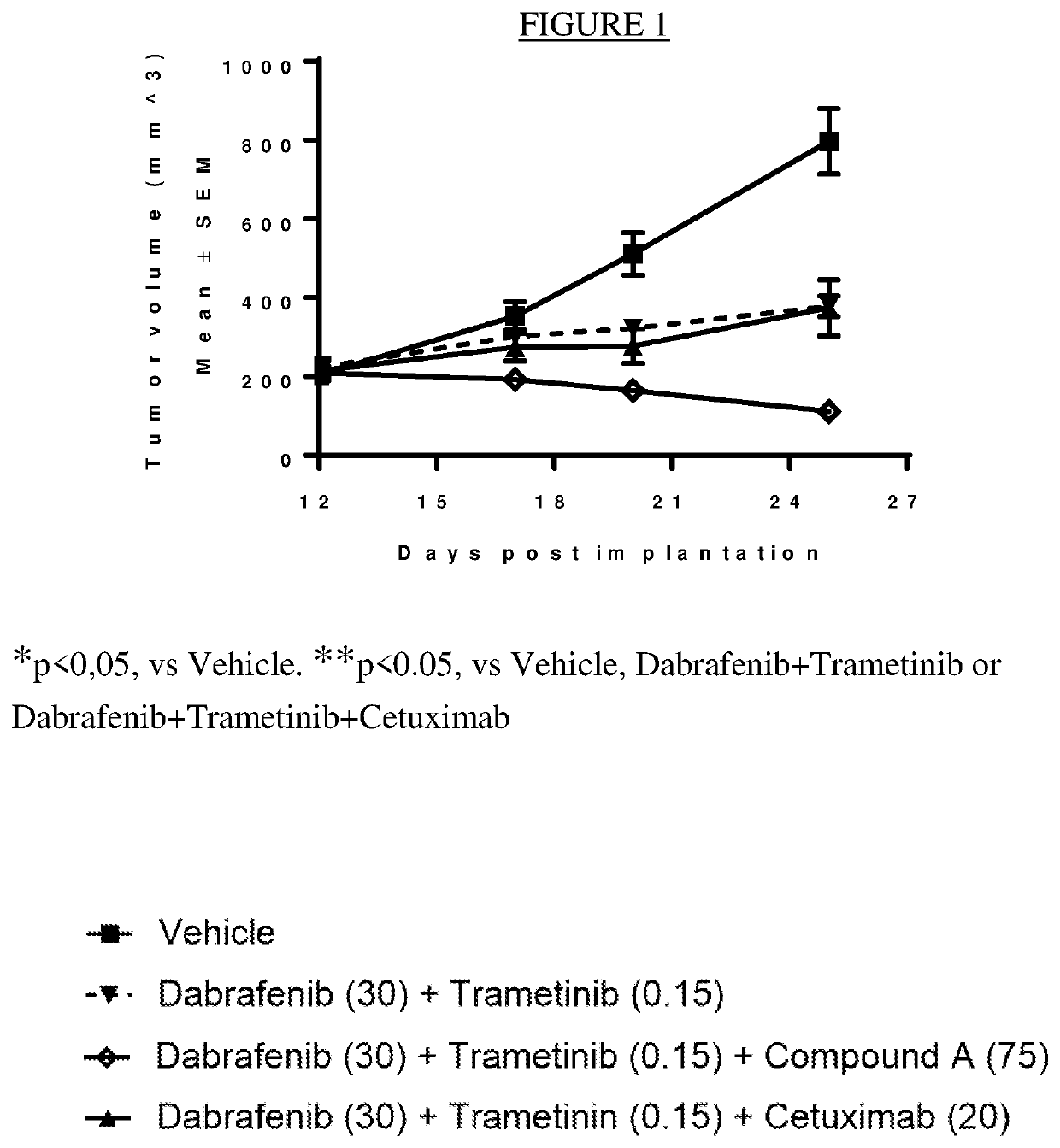
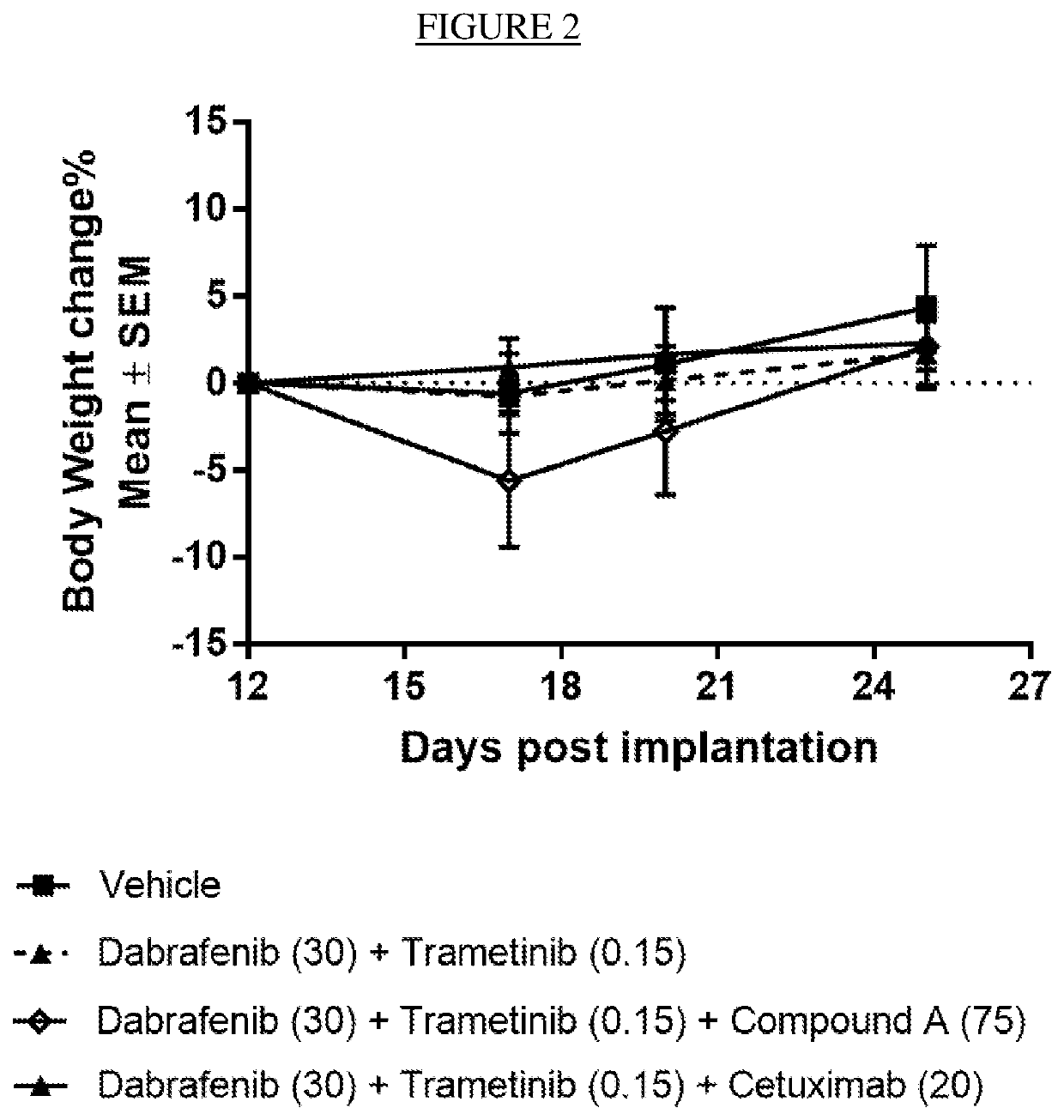
A triple pharmaceutical combination comprising dabrafenib, trametinib and an erk inhibitor
InactiveUS20210121460A1Organic active ingredientsPharmaceutical delivery mechanismDabrafenibPharmaceutical drug
The present invention relates to a pharmaceutical combination comprising dabrafenib, trametinib and an Erk-inhibitor; pharmaceutical compositions comprising the same; and methods of using such combinations and compositions in the treatment or prevention of conditions in which MAPK pathway inhibition is beneficial, for example, in the treatment of cancers.
Owner:NOVARTIS AG
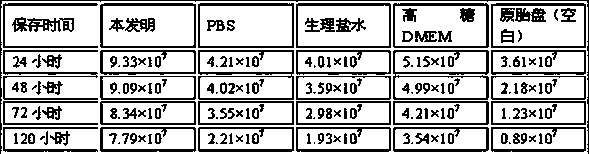
Placenta preservation solution and preparation method thereof
The invention discloses a placenta preservation solution. The placenta preservation solution is used for preserving placenta tissue samples after collection and before separation, and mainly comprisesa high-sugar DMEM dry powder culture medium, sodium bicarbonate, insulin, penicillin, streptomycin, amphotericin, a heparin sodium injection, rapamycin, trametinib and paeoniflorin. The placenta preservation solution has the advantages of simplicity in proportioning, low cost and convenience in use, keeping of the activity of stem cells in the placenta tissue sample, realization of effective preservation of the stem cells at 0-8 DEG C for at least 120 h, small influences of time on the activity of the separated stem cells, great reduction of the time limit from collection to preparation of the tissue samples, and effective reduction of the pollution probability.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
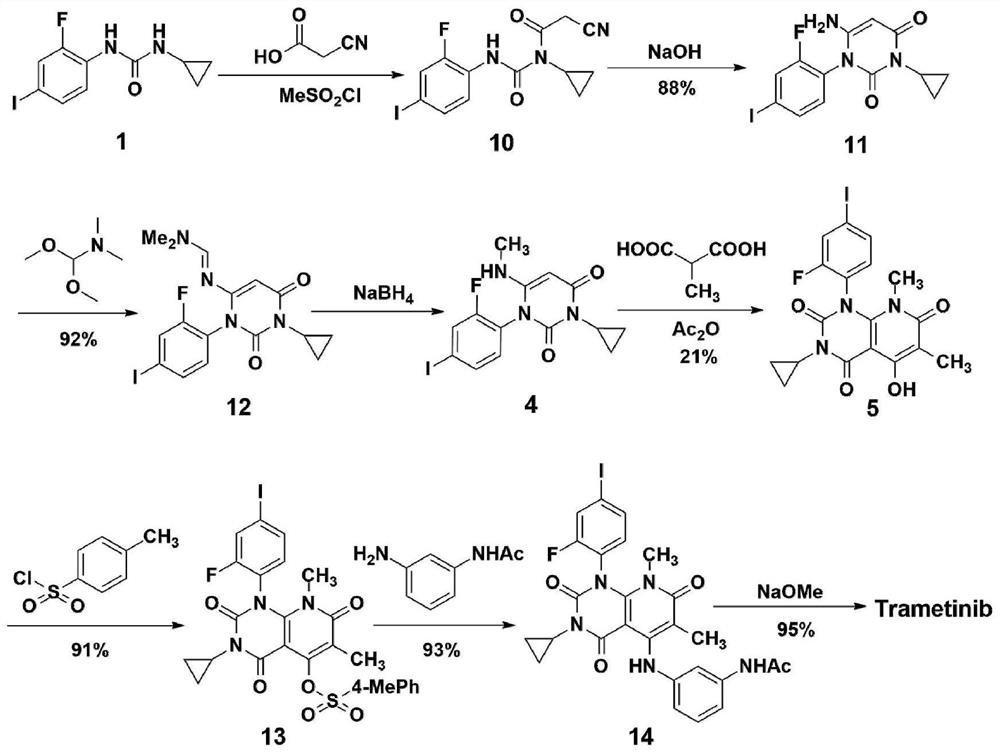
A method for synthesizing trametinib key intermediate
ActiveCN109336884BShort method stepsPotential for pilot scale-upOrganic chemistryMethylmalonic acidTrametinib
The invention discloses a method for synthesizing a key intermediate of trametinib, which uses monoformamide monoethyl malonate and methylmalonic acid to complete the cyclization reaction to obtain a crude pyridinetrione compound; the obtained pyridinetrione The crude compound is directly cyclized with N-(2-fluoro-4-iodophenyl)-N'-cyclopropylurea to obtain the key intermediate of trametinib. The present invention adopts monoethyl malonate and methylmalonic acid to complete the cyclization reaction to obtain a pyridinetrione compound, which is directly cyclized with a urea compound without purification to obtain a key intermediate for synthesizing trametinib. The method steps are short, the total yield is 47.3%, and it has the potential of scale-up in pilot scale, which provides a new scheme for the synthesis of trametinib.
Owner:安庆奇创药业有限公司
Inducer for neural differentiation of mesenchymal stem cells
InactiveCN111154722AGenetic change noNo riskCulture processNervous system cellsPancreatic hormoneNerve cells
The invention belongs to the technical field of induced differentiation of stem cells, in particular to an inducer for neural differentiation of mesenchymal stem cells. The inducer for neural differentiation of the mesenchymal stem cells is prepared from the following components by a mass concentration ratio, 2-8 mg / L of acetaminophen, 4-6 mg / L of insulin, 2-6 mg / L of glucagon-like peptide-1 (GLP-1), 200-400 [mu]g / L of trametinib, 20-100 [mu]g / L of forskolin, 2-10 [mu]g / L of keratinocyte growth factor (KGF) and 2-10 [mu]g / L of bone morphogenetic protein-4 (BMP-4). The inducer has high induction efficiency, few induction steps and short induction time; nerve cells obtained by induction have high activity, have no rejection and no ethical problem after being transplanted, and are high in safety.
Owner:宋修会
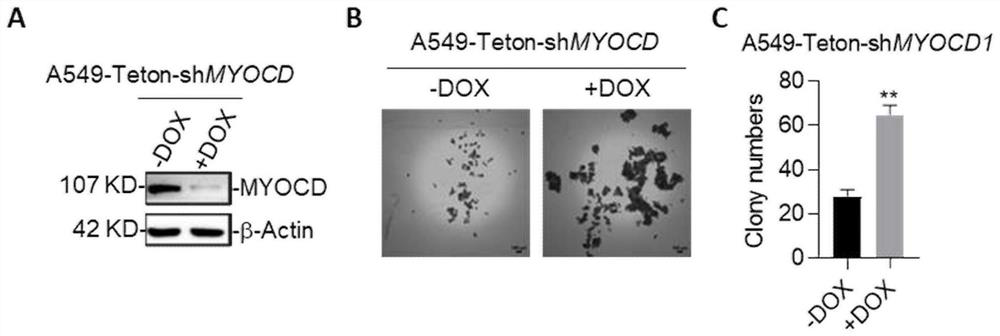
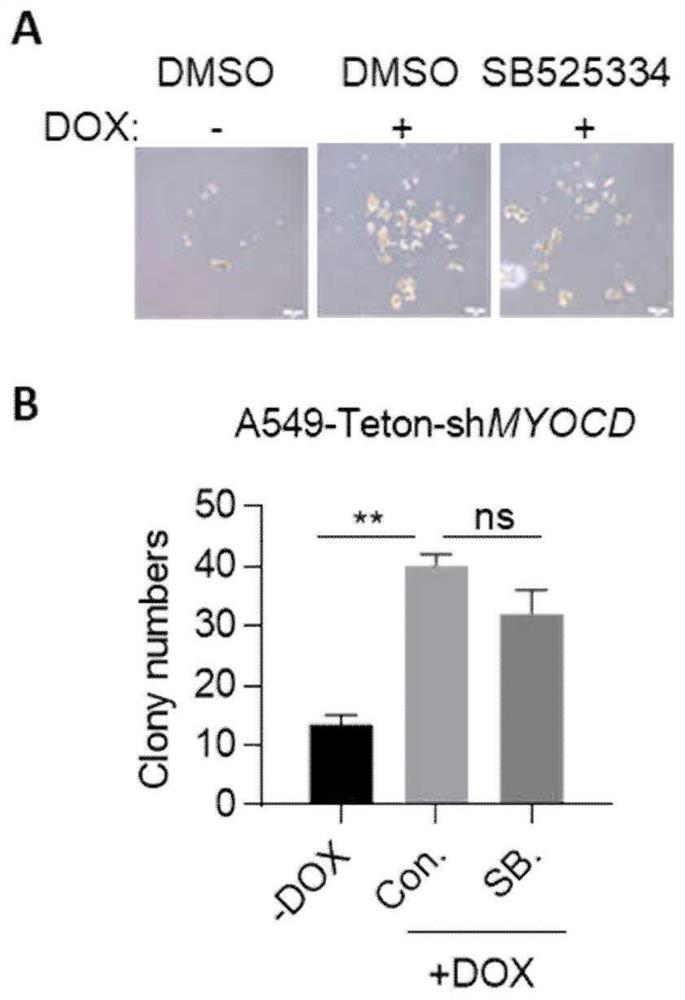
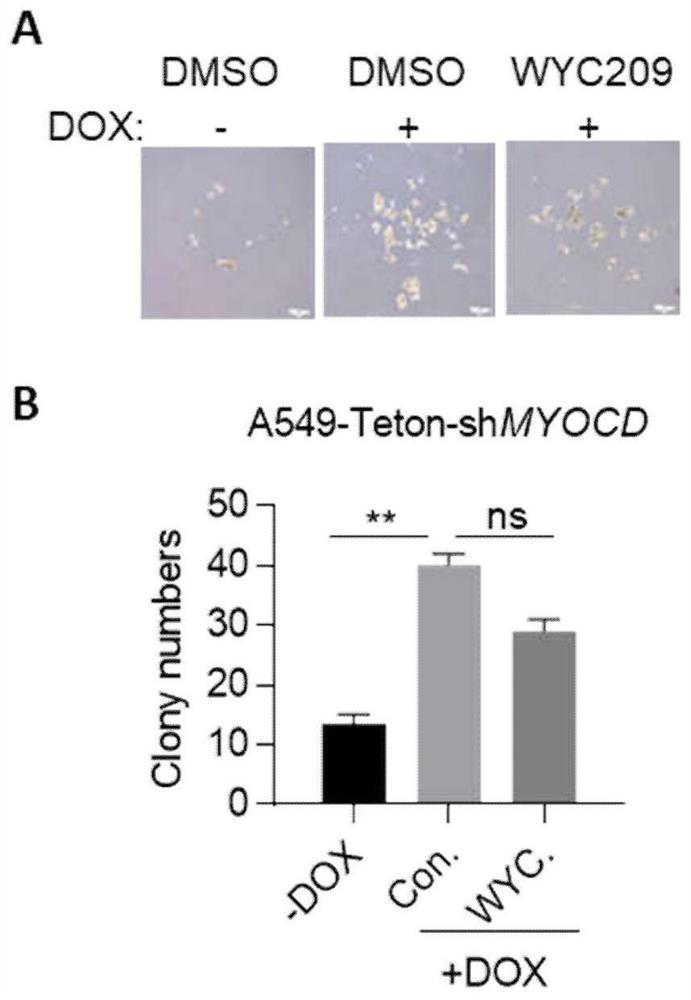
Combination drugs for the treatment of kras mutation and myocd loss of function lung cancer
ActiveCN113069457BPrevent drynessInhibit growthOrganic active ingredientsAntineoplastic agentsKras mutationIndividualized treatment
The invention discloses a combination drug for treating K+ / M-lung cancer, aiming to provide a combined treatment drug for individualized treatment of K+ / M-lung cancer patients aiming at activated signal pathways; the technical key point is transforming growth The invention consists of a factor beta receptor I inhibitor, a retinoic acid receptor RAR inhibitor and trametinib; it belongs to the field of medical biotechnology.
Owner:JINAN UNIVERSITY
Method of using mek inhibitor to prevent radiation induced fibrosis
PendingUS20210260066A1Reduce generationOrganic active ingredientsDermatological disorderMEK inhibitorRadiation induced fibrosis
A method of reducing the severity of radiation induced fibrosis (RIF) by administering to a patient at least a first dose of an MeK inhibitor such as trametinib between 0.01 mg to 2.0 mg, and after said radiation procedure, administering to said patient a further dose of the MeK inhibitor between 0.01 mg and 2.0 mg after the radiation procedure.
Owner:THOMAS JEFFERSON UNIV
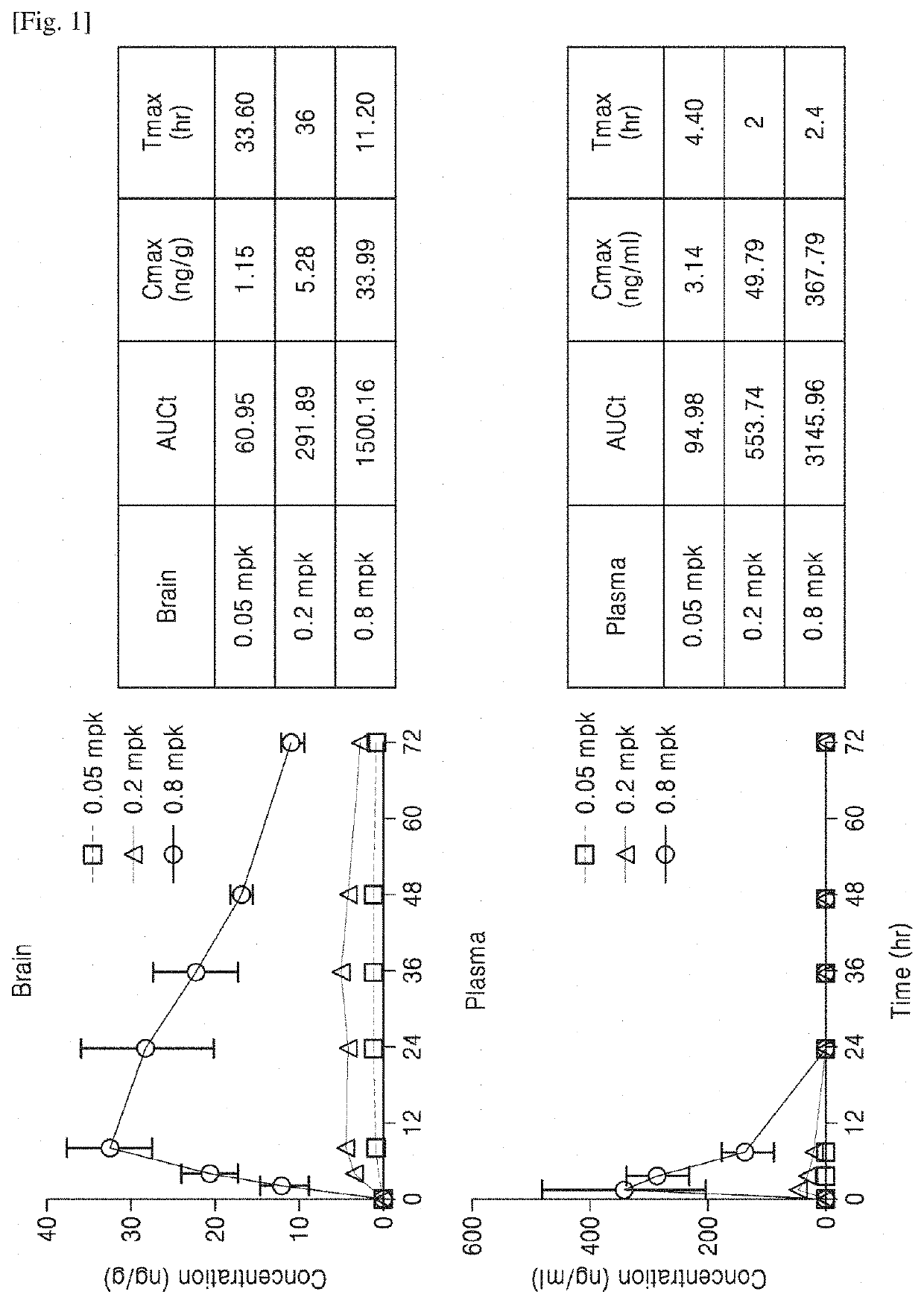
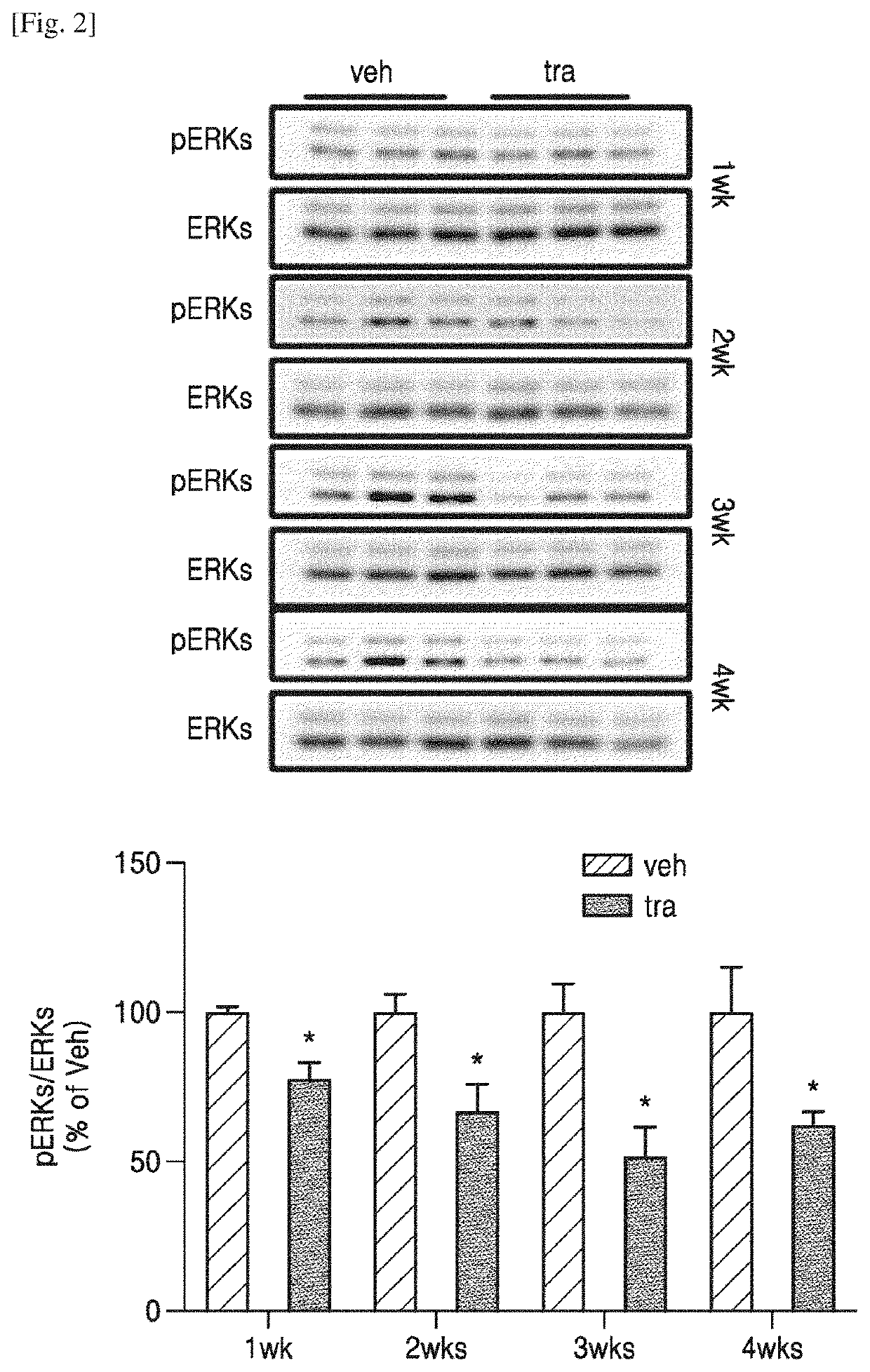
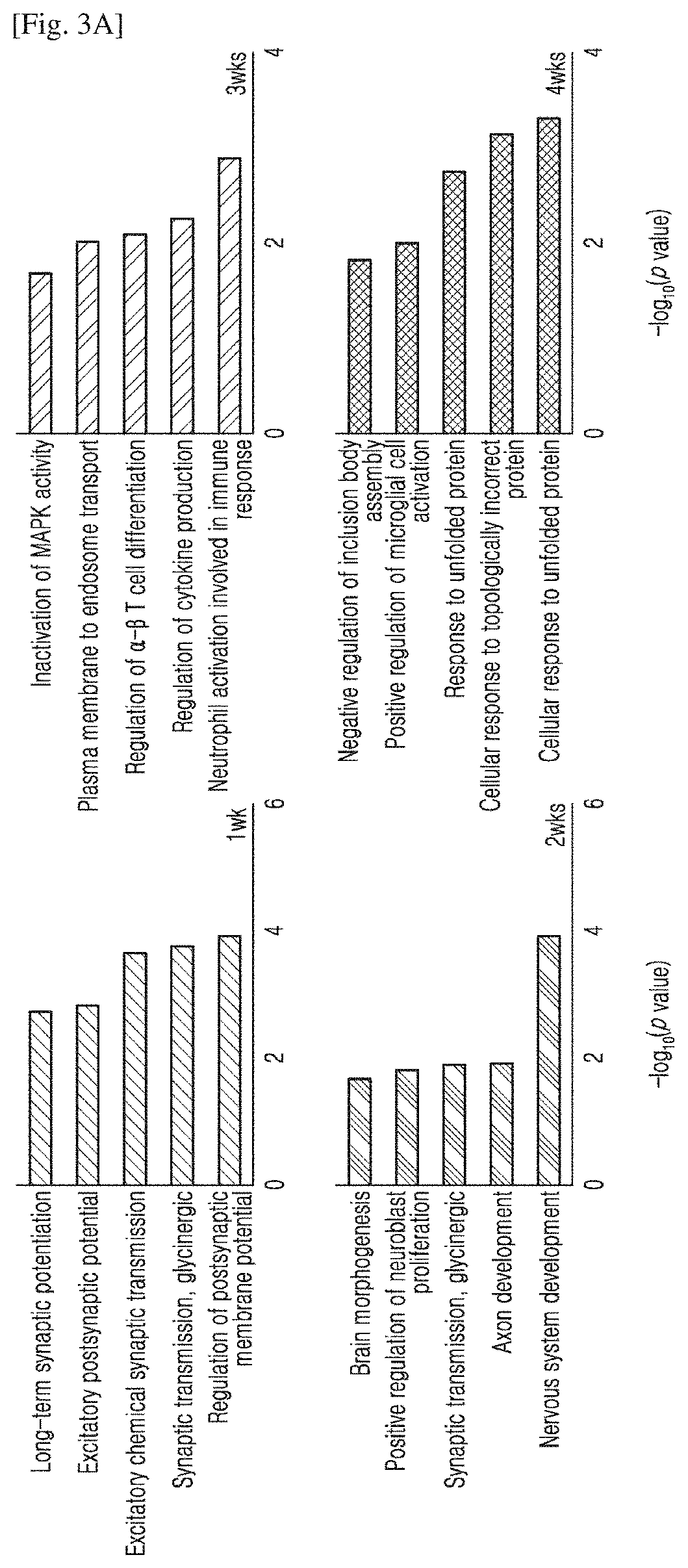
Administration method and dosage regimen for treatment of neurodegenerative diseases using trametinib and markers
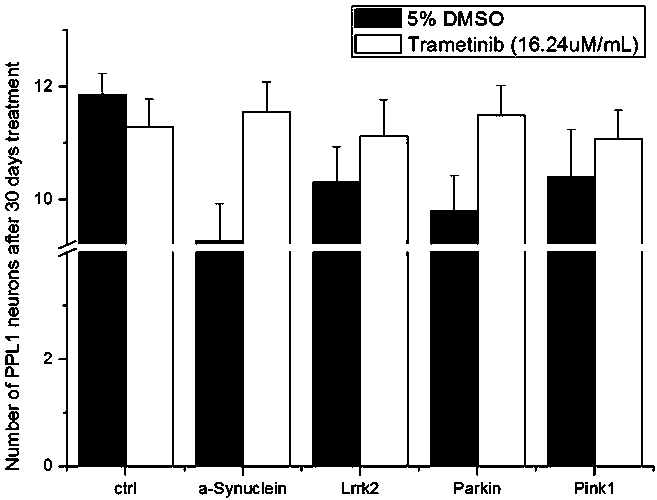
PendingUS20220265657A1Induce geneticInduce structuralOrganic active ingredientsSenses disorderDosing regimenExpression gene
The present invention relates to administration methods and dosage regimens for treatment of neurodegenerative diseases using trametinib and markers. The administration methods and dosage regimens induce neural regeneration and changes in gene expression.
Owner:GENUV INC
Application of trametinib in the preparation of drugs for reversing tumor multidrug resistance
ActiveCN104434924BReverse multidrug resistanceRestore sensitivityOrganic active ingredientsAntineoplastic agentsMembrane TransportersMembrane transport protein
Owner:JINAN UNIVERSITY
Combination therapy
The present invention relates to a pharmaceutical combination comprising (a) a Raf inhibitor as defined herein, or a pharmaceutically acceptable salt thereof and (b) a MEK inhibitor, particularly trametinib, particularly for use in the treatment of a proliferative disease. This invention also relates to uses of such combination for preparation of a medicament for the treatment of a proliferative disease; methods of treating a proliferative disease in a subject in need thereof comprising administering to said subject a jointly therapeutically effective amount of said combination; use of such combination for the treatment of proliferative disease; pharmaceutical compositions comprising such combination and commercial packages thereto.
Owner:NOVARTIS AG
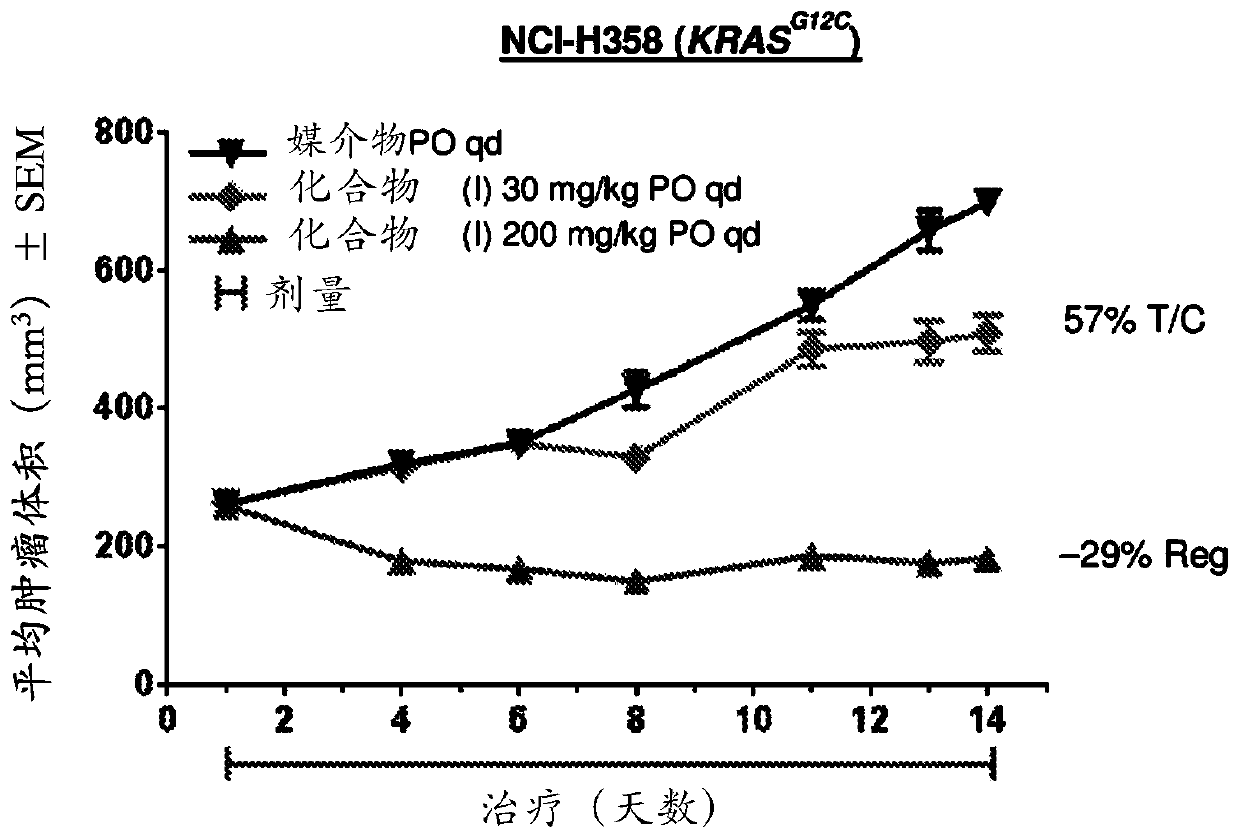
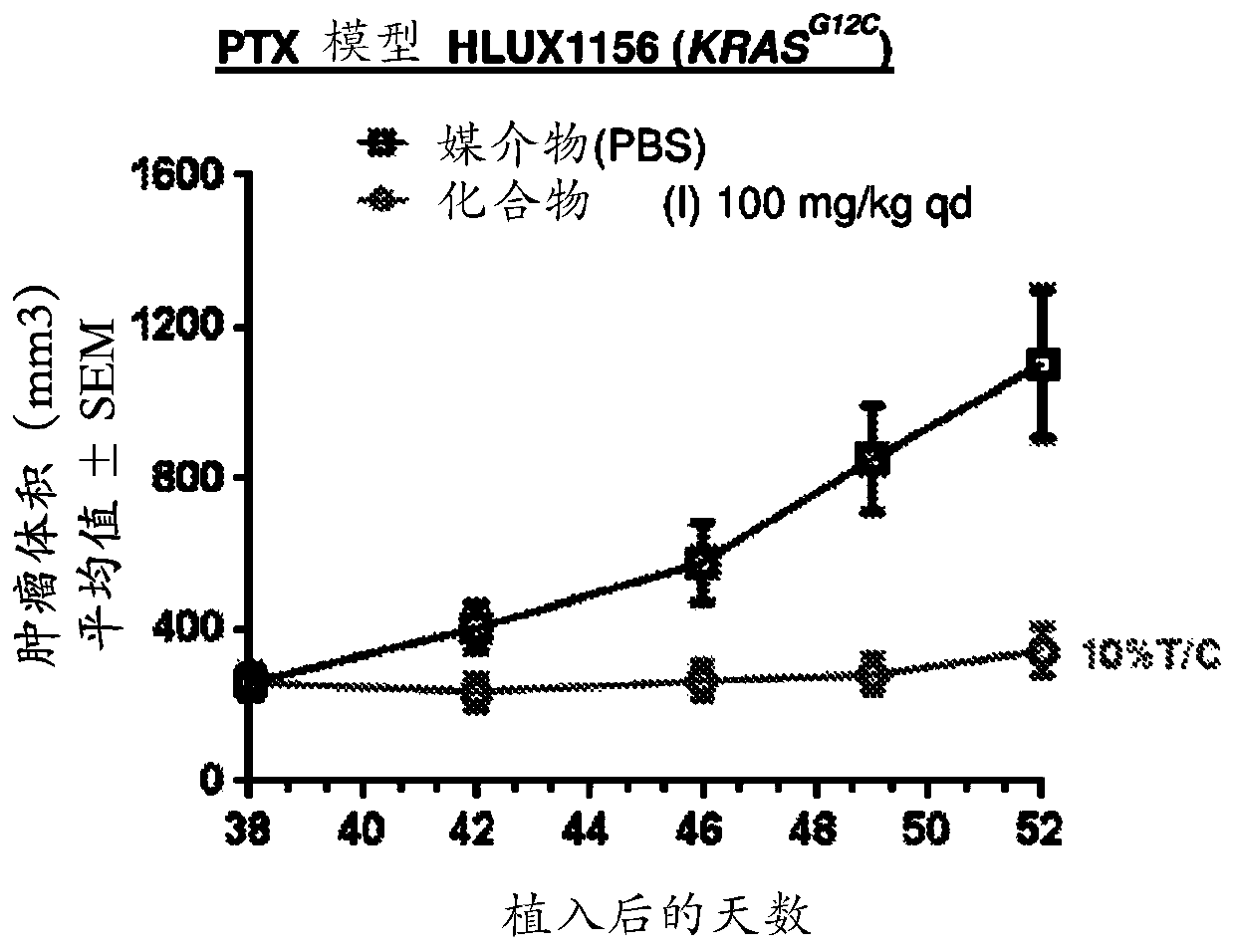
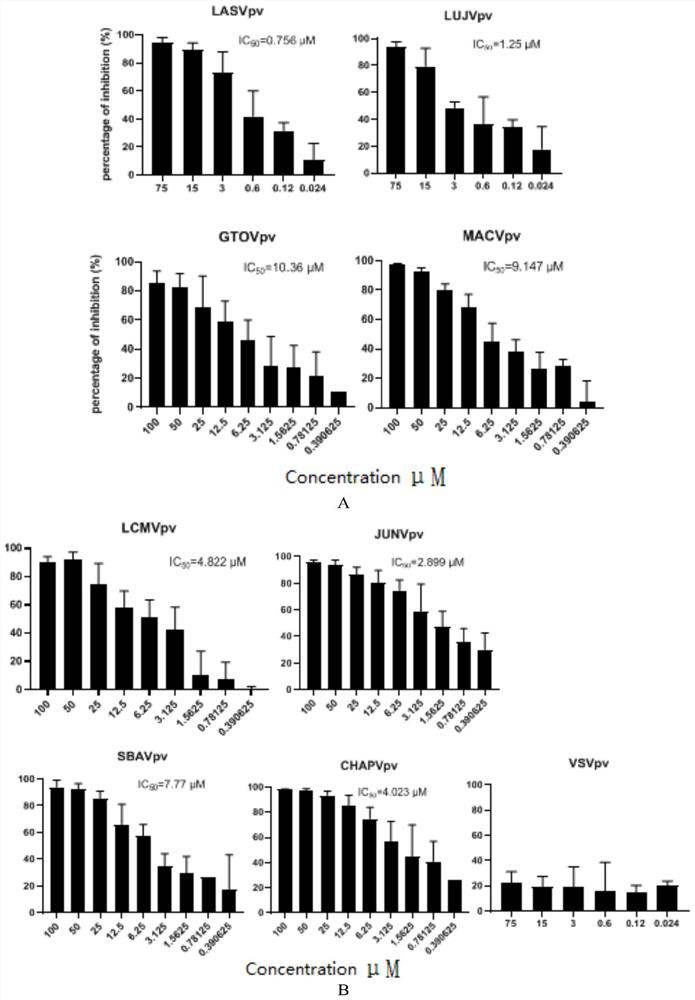
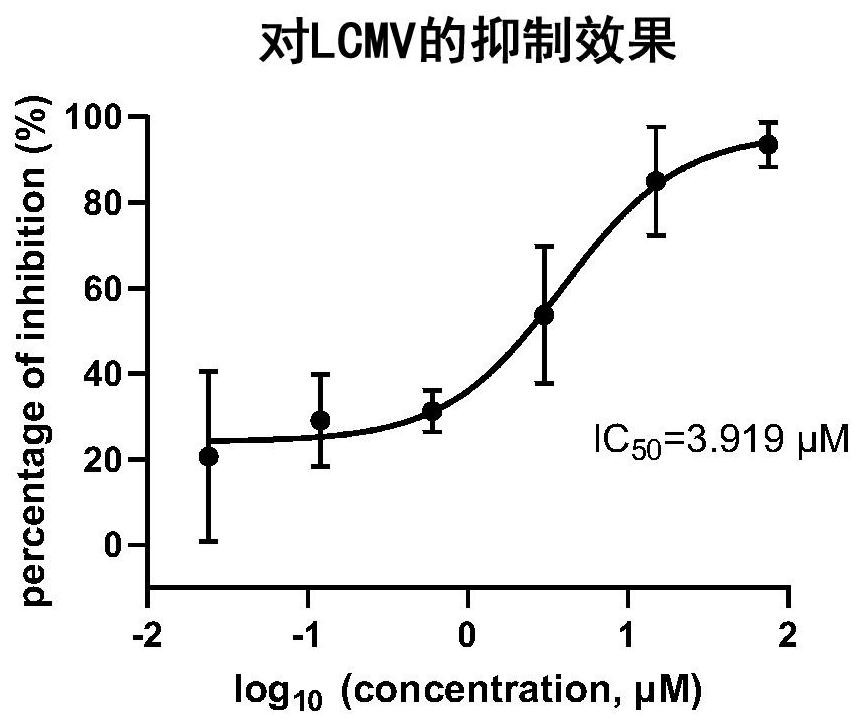
Application of trametinib in preparation of anti-arenavirus antiviral preparation
ActiveCN112957361AInhibitionGood treatment effectOrganic active ingredientsAntiviralsBiotechnologyPharmaceutical drug
The invention relates to application of trametinib in preparation of an anti-arenavirus antiviral preparation, and belongs to the technical field of medicine application. The invention particularly relates to application of trametinib in preparation of an antiviral preparation for inhibiting or killing arenavirus. The new application of trametinib is provided, the application range of trametinib is expanded, and particularly, trametinib can be used for preparing the antiviral preparation of the arenavirus and killing or inhibiting the arenavirus, and then can be used for treating arenavirus infection and diseases caused by the arenavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com