Application of trametinib in preparation of medicine for preventing and/or treating non-alcoholic hepatitis and/or non-alcoholic fatty liver disease
A fatty liver disease, non-alcoholic technology, applied in the field of pharmacy, to achieve the effect of less damage, less staining value, and less fat particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
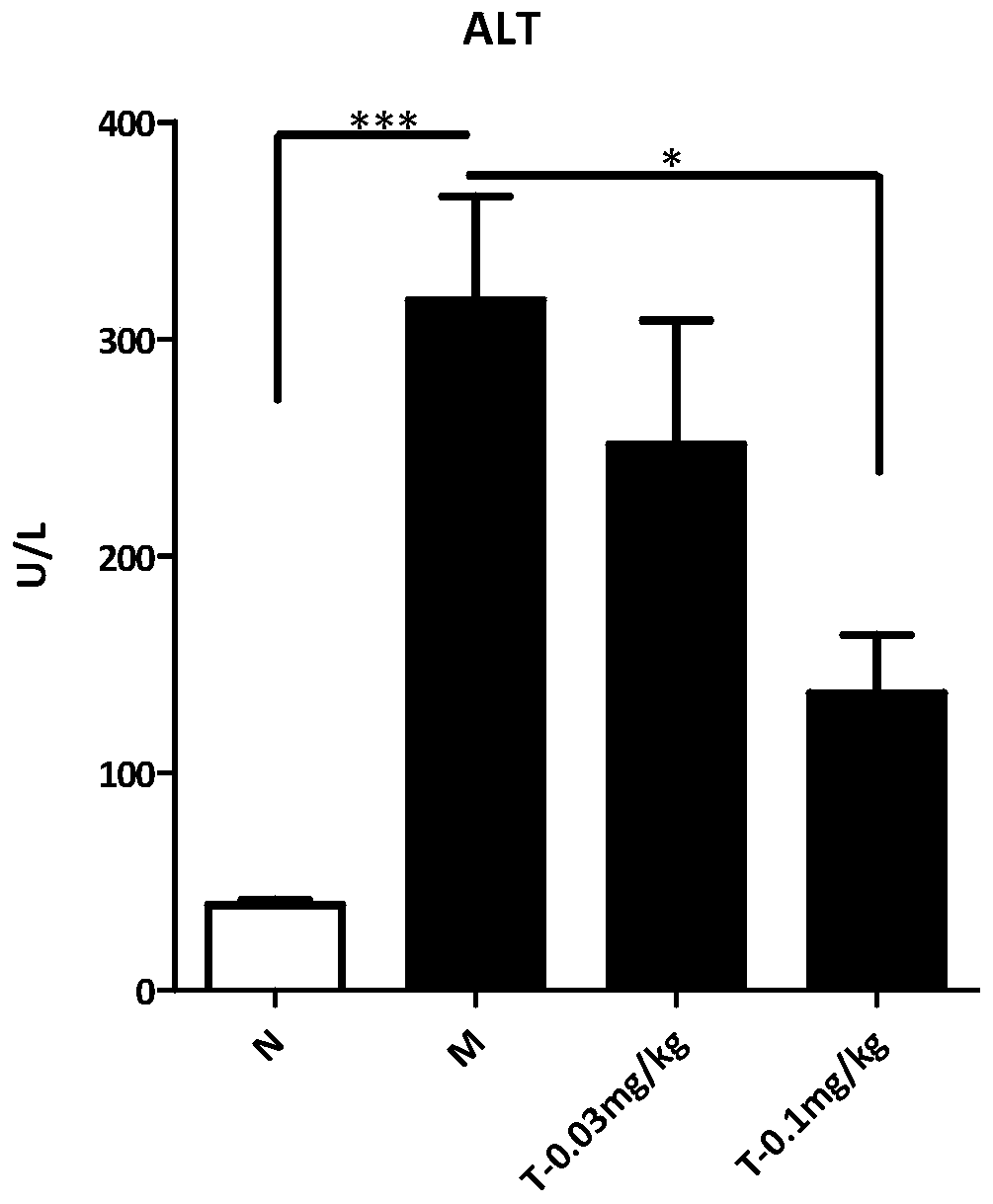
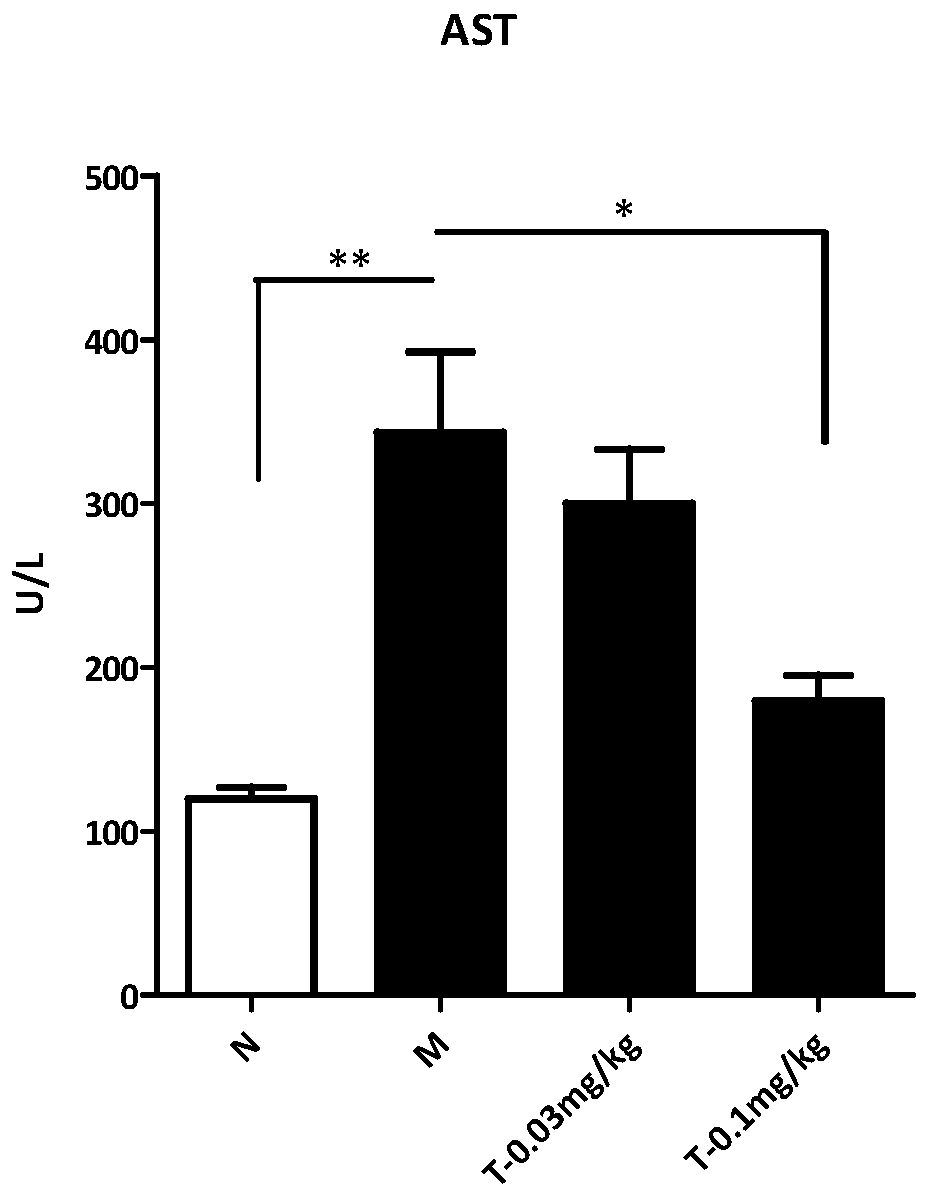
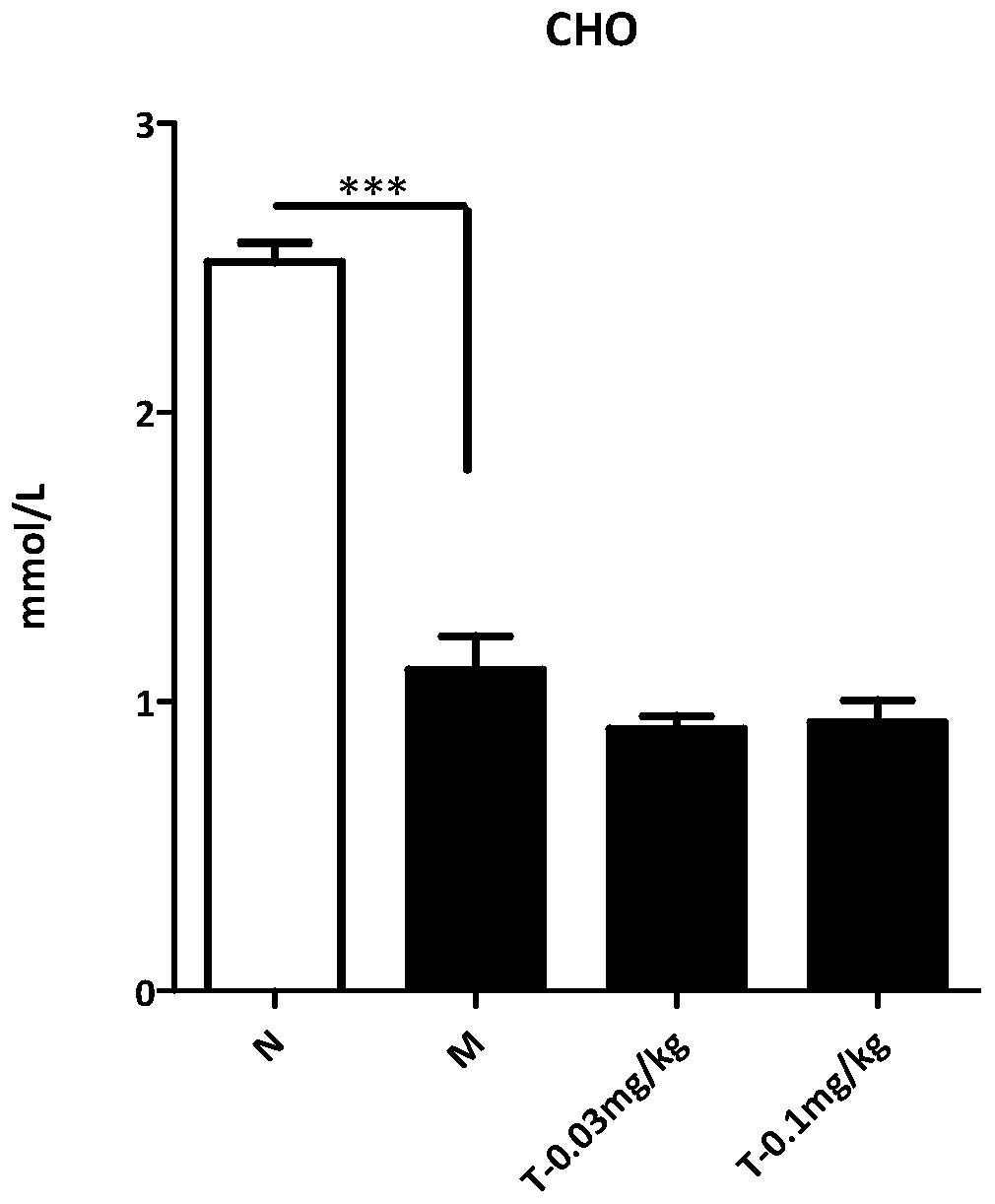
[0060] Trametinib was tested for activity in the mouse MCD model (NASH model):
[0061] Experimental methods: (1) 8-week-old C57BL / 6N were kept under environmental control, and had free access to standard feed and water. After adapting the mice for one week, they were divided into four groups. The mice in the normal group were fed with normal feed, and the mice in the model group and the mice in the treatment group (low dose, high dose) were fed with methionine choline-deficient feed (MCD, A02082002B).
[0062] After feeding MCD for two weeks, mice in the administration group were injected intraperitoneally with a vehicle (0.5% sodium carboxymethylcellulose (CMC-Na, Sigma) once a day for 14 consecutive days, and mice in the model group and the normal group were small. Rats were injected vehicle with the same frequency.After 14 days, the mice were sacrificed, and the body weight and liver tissue of the mice were weighed.
[0063] Sections of individual livers were fixed with 4...
Embodiment 2
[0069] Trametinib was tested for activity on a mouse high-fat diet / fructose model (NASH model):
[0070] Experimental method: 8-week-old C57BL / 6N were divided into three groups after adapting to the new environment for one week. The normal group was fed with normal diet, and the mice in the model group and the treatment group were fed with high-fat diet (HFD) for eight weeks.
[0071] The normal control group was given normal drinking water and normal feed, the mice in the model group and the mice in the treatment group were given high fructose and high glucose drinking water (23.1g fructose and 18.9g glucose were added to 1L of water) and continued to be given high After 8 weeks of fat-feeding, mice in the administration group were injected intraperitoneally with a vehicle (0.5% carboxymethylcellulose sodium (CMC-Na, Sigma) (Trametinib) by body weight once a day for consecutive 14 days. The dosage is 0.1mg / kg), and the model group and the normal group mice are injected with v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com